Abstract
Introduction
For HR-positive/HER2-negative patients who can undergo breast-conserving surgery (BCS) but have a tumor size of 2–5 cm or 1–3 lymph node metastases, neoadjuvant chemotherapy (NAC) is still controversial.
Methods
Patients with T2N0-1M0 HR-positive/HER2-negative BC who underwent BCS between 2010 and 2017 were selected from the SEER database. Propensity score matching (PSM) was used to minimize the influence of confounding factors. The overall survival (OS) and breast cancer-specific survival (BCSS) of patients were estimated by Kaplan‒Meier curves and Cox proportional hazard models. Independent prognostic factors were included to construct a nomogram prediction model.
Results
A total of 6475 BC patients were enrolled, of whom 553 received NAC and 5922 received adjuvant chemotherapy (AC). In the T2N0-1M0 population and T2N1M0 subgroup, AC patients before PSM had better OS and BCSS than NAC patients. After PSM, there was no significant difference in OS or BCSS between the two groups. However, in the T2N0M0 subgroup, there was no difference in survival between the AC and NAC groups before and after PSM. Stratified analysis revealed that for complete response (CR) patients, survival was roughly equivalent between the NAC and AC groups. However, the survival of no response (NR) and partial response (PR) patients was significantly worse than that of AC patients. Cox analysis revealed that radiotherapy after BCS was an independent protective factor for OS. NAC is an independent risk factor for NR and PR patients. The nomogram has good prediction efficiency.
Conclusion
NAC before BCS is not necessary for T2N0-1M0 HR-positive/HER2-negative BC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to recent cancer statistics, breast cancer (BC) is still the most common cancer and the second most common cause of cancer-related mortality in women worldwide(Siegel et al. 2024). The incidence of BC in women continues to increase annually(Giaquinto et al. 2022), so more precise individual treatment is becoming essential. BC is classified into hormone receptor (HR)-positive/HER2-negative, HER2-positive, and triple-negative tumor subtypes based on estrogen or progesterone receptor expression and HER2 gene amplification (Burstein et al. 2021; Waks and Winer 2019). HR-positive/HER2-negative BC is the most common subtype of BC (Giaquinto et al. 2022; Waks and Winer 2019). The molecular subtype of BC is the key to guiding the formulation of the best individualized treatment plan (Goldhirsch et al. 2013; Waks and Winer 2019).
Although most patients with early-stage BC receive surgery combined with postoperative adjuvant therapy, the use of neoadjuvant chemotherapy (NAC) is increasing (Murphy et al. 2018). NAC refers to adjuvant chemotherapy prior to surgery and was initially reserved for the treatment of inoperable advanced patients (Rubens et al. 1980). However, with advancements in research, NAC indications have been extended to operable BC (Bear et al. 2003). It can not only reduce the total resection rate and improve the breast-conserving surgery (BCS) rate (Golshan et al. 2015, 2016; Kim et al. 2019) but also reduce the scope of axillary surgery(King and Morrow 2015; Mamtani et al. 2016; Morrow and Khan 2020), which plays an important role in the treatment of BC. In particular, the treatment response is significant in HER2-positive and triple-negative breast cancer (TNBC) patients (Cortazar et al. 2014; Murphy et al. 2018). The guidelines recommend neoadjuvant systemic therapy for patients with HER2-positive BC or TNBC (Korde et al. 2021). However, for patients with HR-positive/HER2-negative tumors, NAC is mainly used to shrink the primary tumor for smaller breast surgery (Torrisi et al. 2021). The pathological complete response (pCR) rate is an important indicator of the efficacy of NAC. HER2-positive BC and TNBC can achieve pCR rates of up to 45% (Boughey et al. 2014; Rouzier et al. 2005), but patients with HR-positive/HER2-negative tumors have PCR rates of only 0–18% after NAC (Torrisi et al. 2021), which is much lower than those of patients with the other two subtypes. However, pCR was strongly associated with survival in patients with HER2-positive BC and TNBC but not in HR-positive/HER2-negative BC patients (von Minckwitz et al. 2012). Despite the low pCR rate, HR-positive/HER2-negative BC patients generally have an improved long-term prognosis (Torrisi et al. 2021) and good locoregional recurrence-free survival (Caudle et al. 2012). Moreover, some studies have reported that there is no significant difference in the BCS conversion rate and tumor response rate between HR-positive/HER2-negative patients and patients with other types of BC (Kim et al. 2019). Thus, the use of NAC in the HR-positive/HER2-negative population showed an increasing trend (Murphy et al. 2018), even if the pCR rates were not comparable to those in the other two subtypes of breast cancer.
NAC is mainly used for BC patients whose tumor size is > 5 cm, who have axillary lymph node metastasis, who are HER2-positive BC, or who are TNBC (Jiang et al. 2022). However, it is controversial whether to perform NAC in HR-positive/HER2-negative BC patients who are eligible for BCS but with a tumor size of 2–5 cm or 1–3 lymph node metastases (Jiang et al. 2022; Spring et al. 2022). Therefore, our study focused on T2N0-1M0 HR-positive/HER2-negative BC patients who could undergo BCS, aiming to explore whether NAC could improve survival in this patient population where BCS is feasible.
Materials and methods
Selection of study subjects
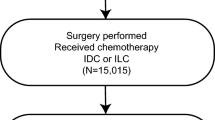
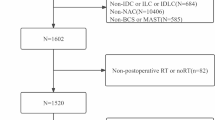
Patients who were diagnosed with T2N0-1M0 HR-positive/HER2-negative BC and treated with BCS were enrolled from the Surveillance, Epidemiology, and End Results (SEER) database (17regs, 2022nov sub). The inclusion criteria for patients were as follows: (1) had T2N0-1M0 BC diagnosed by pathology between 2010 and 2017 and (2) had a single primary tumor. The exclusion criteria were as follows: (1) had a BC subtype other than HR-positive/HER2-negative; (2) underwent surgery other than BCS; (3) had a survival time < 3 months; (4) received preoperative radiotherapy; (5) did not receive chemotherapy; (6) had uncertain or unknown information, such as the degree of tissue differentiation. The specific screening procedure is shown in Fig. 1. The extracted clinicopathological data included year of diagnosis, age, race, marital status, histological type, differentiation degree, T-N-M stage, BC subtype, surgery, radiotherapy, chemotherapy, neoadjuvant treatment response, and survival data.
Ethical information
The SEER database has an open-access policy, and all personal information has been de-identified. Therefore, no additional ethical approval or informed consent was required.
Statistical analysis
Patients were divided into two groups according to whether they had received NAC. Descriptive statistics were used for baseline characteristics, and the Pearson χ2 test was used to test the balance between groups. To improve the comparability between groups, 1:1 propensity score matching (PSM, method = "nearest", caliper = 0.02) was used to reduce the influence of confounding factors. The endpoints analysed were overall survival (OS) and breast cancer-specific survival (BCSS). Kaplan–Meier curves and log-rank tests were used to compare the effect of NAC on survival. Cox proportional hazards models were used to evaluate the independent prognostic factors of patients. The adjusted hazard ratio (AHR) was calculated based on multivariate Cox regression analysis. Independent prognostic factors identified by Cox analysis were included in the construction of the nomogram, and the c-index and calibration curves were used to evaluate the predictive performance of the nomogram. Two-sided P < 0.05 was considered to indicate a statistically significant difference in this study. All analyses and plots were performed using R software (4.2.3) and related R packages.
Results
Baseline characteristics of T2N0-1M0 HR-positive/HER2-negative BC patients
A total of 6475 patients with T2N0-1M0 HR-positive/HER2-negative BC who underwent BCS were included in this study. Of these patients, 553 received NAC, and 5922 received adjuvant chemotherapy (AC). Most of the patients were > 50 years old (65.9%), white (76.9%), married (61.9%), ductal (85.2%), poorly or undifferentiated (47.9%) and treated with radiotherapy (84.9%). The NAC and AC groups differed in terms of age, race, marital status, histological type, and degree of differentiation. Among the patients receiving NAC, the proportion of patients under 50 years old and patients with poor differentiation increased. After PSM to balance confounding factors, the χ2 test suggested balance and comparability between the two groups (Table 1).
Survival analysis of the overall study subjects
The Kaplan‒Meier method was used to construct survival curves for T2N0–1M0 BC patients. Before PSM, the AC group demonstrated better OS (p = 0.0019) and BCSS (p = 0.00064) than did the NAC group (Fig. 2A and B). However, after PSM, there was no significant difference in OS (p = 0.28) and BCSS (p = 0.29) between the NAC and AC groups (Fig. 2C and D). Stratified analysis of the response to NAC suggested that for complete response (CR) patients, the survival effects of NAC and AC were approximately the same. However, NAC conferred worse survival in the no response (NR) and partial response (PR) populations (Fig. 3). The results of the subsequent Cox proportional hazards regression analysis showed that all the variables analysed were independent prognostic factors in this population. Radiation after BCS is an independent protective factor for OS in this population but is not related to BCSS. For NR and PR patients, NAC was considered to be an independent risk factor for OS [NR, (HR 3.72; 95% CI 2.32–5.95; P < 0.001) PR, (HR 1.39; 95% CI 1.05–1.84; P = 0.023)] and BCSS [NR, (HR 4.51; 95% CI 2.69–7.56; P < 0.001) PR, (HR 1.54; 95% CI 1.13–2.11; P = 0.007)] (Table 2). Overall, NAC followed by BCS did not confer a survival benefit compared with BCS followed by AC in the T2N0-1M0 population. NAC even demonstrated worse survival in the NR and PR populations.
Survival analysis of the T2N0M0 and T2N1M0 subgroups
To further investigate the effect of NAC on survival, the population was divided into T2N0M0 and T2N1M0 subgroups according to N stage, and baseline statistics and PSM were performed for each subgroup (Supplementary Table 1). In the T2N0M0 group, there were no statistically significant differences in OS and BCSS between the AC and NAC groups before and after PSM (Fig. 4A–D). After stratification of patients according to their response to NAC, except for the NR population, the CR and PR populations both showed no difference in survival between the two groups (Fig. 5). In the T2N1M0 subgroup, before PSM, the NAC group had worse OS and BCSS than did the AC group (Fig. 4E–F). However, after PSM, there was no significant difference in OS and BCSS between the AC and NAC groups (Fig. 4G–H). When stratified according to the response to NAC, there was no significant difference in OS and BCSS between the two groups of CR patients. However, both NR and PR patients who received NAC still had worse survival (Fig. 6).
Kaplan‒Meier survival curves for patients with the T2N0M0 subgroup and T2N1M0 subgroup. A T2N0M0, before PSM, overall survival; B T2N0M0, before PSM, breast cancer-specific survival; C T2N0M0, after PSM, overall survival; D T2N0M0, after PSM, breast cancer-specific survival; E T2N1M0, before PSM, overall survival; F T2N1M0, before PSM, breast cancer-specific survival; G T2N1M0, after PSM, overall survival; H T2N1M0, after PSM, breast cancer-specific survival
Age was strongly associated with treatment choice and treatment efficacy in the HR-positive/HER2-negative population, so we calculated age-AHR for the two subgroups based on multivariate Cox regression (Table 3). In the T2N0M0 subgroup, NAC did not improve survival compared with the AC group, regardless of menopausal status. However, after stratification, receiving NAC was a risk factor in the < 50 years NR population.
Nomograms
According to the results of the Cox regression analysis, age, race, marital status, histological type, differentiation grade, postoperative radiotherapy and neoadjuvant chemotherapy were determined to be independent prognostic factors. Independent prognostic factors were included in the construction of the nomogram. Different prognostic factor variables were assigned their specific scores, and cumulative scores were compared with linear predictors to yield probabilistic predictions of OS and BCSS at 1, 3, and 5 years (Fig. 7A and B). The c-index of the nomograms for predicting OS and BCSS were 0.646 and 0.662, respectively. Calibration curves further demonstrated the robust predictive performance of the model (Fig. 7C and D).
Discussion
NAC has the advantages of shrinking the primary tumor, reducing surgical trauma and monitoring the response to chemotherapy, but it has the disadvantages of delaying surgical treatment, inducing chemotherapy resistance and increasing the risk of disease progression (Hönig et al. 2004; Ikeda et al. 2002). Therefore, the use of NAC is controversial for some chemotherapy-insensitive tumor types.
For stage II BC patients, NAC can provide the clinical advantage of tumor downstaging, especially in HER2-positive and TNBC patients (Burstein et al. 2021). According to the 2019 St. Gallen expert consensus, preoperative systemic therapy is preferred for stage II HER2-positive patients and TNBC patients (Burstein et al. 2019). For HR-positive/HER2-negative patients, surgical resection is often the first choice, followed by NAC, neoadjuvant endocrine therapy, and systemic adjuvant therapy based on clinicopathological characteristics (Cantini et al. 2024). The distribution of our included baseline population was the same, with 91.5% of patients choosing BCS combined with AC and only 8.5% choosing NAC combined with BCS. Although the pCR rate of NAC in HR-positive/HER2-negative patients is very low, its clinical response rate can reach 70% (Alba et al. 2012), and evidence supports that NAC can achieve a 60% BCS conversion rate in this population (Torrisi et al. 2021), thus NAC is recommended only for patients who require downsizing for BCS (Torrisi et al. 2021). Our study revealed that for patients with stage T2N0-1M0 disease who could have undergone BCS, preoperative NAC did not confer a significant survival benefit. The survival of patients treated with NAC was comparable to or slightly worse than that of patients treated with AC. Interestingly, no significant difference in survival was observed between CR patients and those who received AC, whereas NR and PR patients demonstrated worse survival than those who received AC. A previous study reported the same efficacy of NAC and AC for patients with stage II BC (Fisher et al. 2023), which may be the reason why the survival of CR patients is roughly the same as that of patients who achieved AC. However, PR and NR patients are not sensitive to chemotherapy drugs. For patients who can undergo direct surgery, NAC actually prolongs the ineffective treatment time and delays the effective surgical treatment. During this period of NAC treatment, it may not only induce clonal expansion of drug-resistant cells but also promote disease progression and ultimately lead to worse survival (Hönig et al. 2004). In conclusion, NAC does not result in better survival than AC in patients with T2N0-1M0 HR-positive/HER2-negative BC for whom BCS is feasible; therefore, NAC is not recommended for this population.
Several studies have suggested that the efficacy of NAC is closely related to age (Boughey et al. 2022; Loibl et al. 2015; Verdial et al. 2022). Although the survival of younger breast cancer patients is worse, the pCR rate of NAC in the younger HR-positive/HER2-negative population was significantly greater than that in older patients (Loibl et al. 2015). Moreover, younger women who underwent NAC had a greater nodal pCR rate and greater axillary downstaging (Boughey et al. 2022; Verdial et al. 2022). However, our findings suggest that NAC is not recommended for chemotherapy-insensitive patients of all ages. This result could also be attributed to the apparent effect of NAC in delaying effective treatment in a chemotherapy-insensitive population. In the era of precision medicine, it is essential to identify biomarkers or predictive models that can predict the survival of early HR-positive/HER2-negative patients (Cantini et al. 2024). The nomogram model robustly predicted the 1-, 3-, and 5-year OS and BCSS of T2N0-1M0 HR-positive/HER2-negative patients who underwent BCS. Cox analysis revealed that postoperative radiotherapy was an independent protective factor for OS in patients with T2N0-1M0 HR-positive/HER2-negative BC, but it was not associated with BCSS. HR-positive/HER2-negative BC generally has a more favourable outcome after radiotherapy than other types of breast cancer (He et al. 2019; Hung et al. 2023). This may be related to the increase in radiosensitivity caused by the interaction between estrogen receptors and androgen receptors (Michmerhuizen et al. 2022). In addition, radiotherapy after BCS can also reduce the possibility of tumor metastasis (Hung et al. 2023) and improve the effect of immunotherapy (Yu et al. 2019). These factors may account for the overall survival advantage of radiotherapy after BCS in our study population.
We must acknowledge and accept the limitations of our study. First, we cannot avoid the bias caused by the retrospective nature of the study, but we balanced group differences through PSM to enhance comparability and minimize the impact of bias. Second, information on Ki67 was missing in the SEER database, so we could not perform an analysis of this critical factor. Finally, more detailed treatment information is not available in the SEER database, and we plan to obtain more comprehensive data to validate our results in future clinical work.
In general, NAC before BCS is not necessary for T2N0-1M0 HR-positive/HER2-negative BC patients.
Data availability
The datasets analysed during the current study are available in the Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov). SEER*Stat Database: Incidence—SEER Research Data, 17 Registries, Nov 2022 Sub (2000–2020).
References
Alba E, Calvo L, Albanell J, De la Haba JR, Arcusa Lanza A, Chacon JI, Sanchez-Rovira P, Plazaola A, Lopez Garcia-Asenjo JA, Bermejo B, Carrasco E, Lluch A (2012) Chemotherapy (CT) and hormonotherapy (HT) as neoadjuvant treatment in luminal breast cancer patients: results from the GEICAM/2006-03, a multicenter, randomized, phase-II study. Ann Oncol 23(12):3069–3074. https://doi.org/10.1093/annonc/mds132
Bear HD, Anderson S, Brown A, Smith R, Mamounas EP, Fisher B, Margolese R, Theoret H, Soran A, Wickerham DL, Wolmark N (2003) The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 21(22):4165–4174
Boughey JC, Hoskin TL, Goetz MP (2022) Neoadjuvant chemotherapy and nodal response rates in luminal breast cancer: effects of age and tumor Ki67. Ann Surg Oncol 29(9):5747–5756. https://doi.org/10.1245/s10434-022-11871-z
Boughey JC, McCall LM, Ballman KV, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, Leitch AM, Flippo-Morton T, Hunt KK (2014) Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann Surg. https://doi.org/10.1097/SLA.0000000000000924
Burstein HJ, Curigliano G, Loibl S, Dubsky P, Gnant M, Poortmans P, Colleoni M, Denkert C, Piccart-Gebhart M, Regan M, Senn HJ, Winer EP, Thurlimann B (2019) Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann Oncol 30(10):1541–1557. https://doi.org/10.1093/annonc/mdz235
Burstein HJ, Curigliano G, Thürlimann B, Weber WP, Poortmans P, Regan MM, Senn HJ, Winer EP, Gnant M (2021) Customizing local and systemic therapies for women with early breast cancer: the St. Gallen International Consensus Guidelines for treatment of early breast cancer. Ann Oncol. 32(10):1216–1235. https://doi.org/10.1016/j.annonc.2021.06.023
Cantini L, Trapani D, Guidi L, Boscolo Bielo L, Scafetta R, Koziej M, Vidal L, Saini KS, Curigliano G (2024) Neoadjuvant therapy in hormone receptor-positive/HER2-Negative breast cancer. Cancer Treat Rev 123:102669. https://doi.org/10.1016/j.ctrv.2023.102669
Caudle AS, Yu T-K, Tucker SL, Bedrosian I, Litton JK, Gonzalez-Angulo AM, Hoffman K, Meric-Bernstam F, Hunt KK, Buchholz TA, Mittendorf EA (2012) Local-regional control according to surrogate markers of breast cancer subtypes and response to neoadjuvant chemotherapy in breast cancer patients undergoing breast conserving therapy. Breast Cancer Res. 14(3):R83
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, Swain SM, Prowell T, Loibl S, Wickerham DL, Bogaerts J, Baselga J, Perou C, Blumenthal G, Blohmer J, Mamounas EP, Bergh J, Semiglazov V, Justice R, Eidtmann H, Paik S, Piccart M, Sridhara R, Fasching PA, Slaets L, Tang S, Gerber B, Geyer CE, Pazdur R, Ditsch N, Rastogi P, Eiermann W, von Minckwitz G (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet (London, England) 384(9938):164–172. https://doi.org/10.1016/S0140-6736(13)62422-8
Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, Wickerham DL, Begovic M, DeCillis A, Robidoux A, Margolese RG, Cruz AB, Hoehn JL, Lees AW, Dimitrov NV, Bear HD (2023) Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 41(10):1795–1808. https://doi.org/10.1200/JCO.22.02571
Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A, Jemal A, Siegel RL (2022) Breast Cancer Statistics, 2022. CA Cancer J Clin 72(6):524–541. https://doi.org/10.3322/caac.21754
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, Senn HJ (2013) Personalizing the treatment of women with early breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 24(9):2206–2223. https://doi.org/10.1093/annonc/mdt303
Golshan M, Cirrincione CT, Sikov WM, Berry DA, Jasinski S, Weisberg TF, Somlo G, Hudis C, Winer E, Ollila DW (2015) Impact of neoadjuvant chemotherapy in stage II-III triple negative breast cancer on eligibility for breast-conserving surgery and breast conservation rates: surgical results from CALGB 40603 (Alliance). Ann Surg. https://doi.org/10.1097/SLA.0000000000001417
Golshan M, Cirrincione CT, Sikov WM, Carey LA, Berry DA, Overmoyer B, Henry NL, Somlo G, Port E, Burstein HJ, Hudis C, Winer E, Ollila DW (2016) Impact of neoadjuvant therapy on eligibility for and frequency of breast conservation in stage II-III HER2-positive breast cancer: surgical results of CALGB 40601 (Alliance). Breast Cancer Res Treat 160(2):297–304
He L, Lv Y, Song Y, Zhang B (2019) The prognosis comparison of different molecular subtypes of breast tumors after radiotherapy and the intrinsic reasons for their distinct radiosensitivity. Cancer Manag Res 11:5765–5775. https://doi.org/10.2147/CMAR.S213663
Hönig A, Rieger L, Sutterlin M, Dietl J, Solomayer E-F (2004) Preoperative chemotherapy and endocrine therapy in patients with breast cancer. Clin Breast Cancer 5(3):198–207
Hung S-K, Yang H-J, Lee M-S, Liu D-W, Chen L-C, Chew C-H, Lin C-H, Lee C-H, Li S-C, Hong C-L, Yu C-C, Yu B-H, Hsu F-C, Chiou W-Y, Lin H-Y (2023) Molecular subtypes of breast cancer predicting clinical benefits of radiotherapy after breast-conserving surgery: a propensity-score-matched cohort study. Breast Cancer Res 25(1):149. https://doi.org/10.1186/s13058-023-01747-9
Ikeda T, Jinno H, Matsu A, Masamura S, Kitajima M (2002) The role of neoadjuvant chemotherapy for breast cancer treatment. Breast Cancer 9(1):8–14
Jiang ZF, Li JB, Chen JY, Liu YP, Wang K, Nie JY, Wang XJ, Hao CF, Yin YM, Wang SS, Yan M, Wang T, Yan Y, Chen XY, Song EW, Grp CBGW (2022) Chinese Society of Clinical Oncology (CSCO) Breast Cancer Guidelines 2022. Trans Breast Cancer Res. https://doi.org/10.21037/tbcr-22-21
Kim HS, Yoo TK, Park WC, Chae BJ (2019) Potential benefits of neoadjuvant chemotherapy in clinically node-positive luminal subtype- breast cancer. J Breast Cancer 22(3):412–424. https://doi.org/10.4048/jbc.2019.22.e35
King TA, Morrow M (2015) Surgical issues in patients with breast cancer receiving neoadjuvant chemotherapy. Nat Rev Clin Oncol 12(6):335–343. https://doi.org/10.1038/nrclinonc.2015.63
Korde LA, Somerfield MR, Carey LA, Crews JR, Denduluri N, Hwang ES, Khan SA, Loibl S, Morris EA, Perez A, Regan MM, Spears PA, Sudheendra PK, Symmans WF, Yung RL, Harvey BE, Hershman DL (2021) Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. Journal of Clinical Oncol 39(13):1485–1505. https://doi.org/10.1200/JCO.20.03399
Loibl S, Jackisch C, Lederer B, Untch M, Paepke S, Kümmel S, Schneeweiss A, Huober J, Hilfrich J, Hanusch C, Gerber B, Eidtmann H, Denkert C, Costa SD, Blohmer J-U, Nekljudova V, Mehta K, von Minckwitz G (2015) Outcome after neoadjuvant chemotherapy in young breast cancer patients: a pooled analysis of individual patient data from eight prospectively randomized controlled trials. Breast Cancer Res Treat 152(2):377–387. https://doi.org/10.1007/s10549-015-3479-z
Mamtani A, Barrio AV, King TA, Van Zee KJ, Plitas G, Pilewskie M, El-Tamer M, Gemignani ML, Heerdt AS, Sclafani LM, Sacchini V, Cody HS, Patil S, Morrow M (2016) How often does neoadjuvant chemotherapy avoid axillary dissection in patients with histologically confirmed nodal metastases? Results of a prospective study. Ann Surg Oncol 23(11):3467–3474. https://doi.org/10.1245/s10434-016-5246-8
Michmerhuizen AR, Lerner LM, Ward C, Pesch AM, Zhang A, Schwartz R, Wilder-Romans K, Eisner JR, Rae JM, Pierce LJ, Speers CW (2022) Androgen and oestrogen receptor co-expression determines the efficacy of hormone receptor-mediated radiosensitisation in breast cancer. Br J Cancer 127(5):927–936. https://doi.org/10.1038/s41416-022-01849-9
Morrow M, Khan AJ (2020) Locoregional management after neoadjuvant chemotherapy. J Clin Oncol 38(20):2281–2289. https://doi.org/10.1200/JCO.19.02576
Murphy BL, Day CN, Hoskin TL, Habermann EB, Boughey JC (2018) Neoadjuvant chemotherapy use in breast cancer is greatest in excellent responders: triple-negative and HER2+ subtypes. Ann Surg Oncol 25(8):2241–2248. https://doi.org/10.1245/s10434-018-6531-5
Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P, Morandi P, Fan C, Rabiul I, Ross JS, Hortobagyi GN, Pusztai L (2005) Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res 11(16):5678–5685
Rubens RD, Sexton S, Tong D, Winter PJ, Knight RK, Hayward JL (1980) Combined chemotherapy and radiotherapy for locally advanced breast cancer. Eur J Cancer 16(3):351–356
Siegel RL, Giaquinto AN, Jemal A (2024) Cancer statistics, 2024. CA A Cancer J Clin 74(1):12–49. https://doi.org/10.3322/caac.21820
Spring LM, Bar Y, Isakoff SJ (2022) The evolving role of neoadjuvant therapy for operable breast cancer. J Natl Compr Cancer Netw 20(6):723–734. https://doi.org/10.6004/jnccn.2022.7016
Torrisi R, Marrazzo E, Agostinetto E, De Sanctis R, Losurdo A, Masci G, Tinterri C, Santoro A (2021) Neoadjuvant chemotherapy in hormone receptor-positive/HER2-negative early breast cancer: when, why and what? Crit Rev Oncol Hematol 160:103280. https://doi.org/10.1016/j.critrevonc.2021.103280
Verdial FC, Mamtani A, Pawloski KR, Sevilimedu V, D’Alfonso TM, Zhang H, Gemignani ML, Barrio AV, Morrow M, Tadros AB (2022) The effect of age on outcomes after neoadjuvant chemotherapy for breast cancer. Ann Surg Oncol 29(6):3810–3819. https://doi.org/10.1245/s10434-022-11367-w
von Minckwitz G, Untch M, Blohmer J-U, Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich J, Huober J, Jackisch C, Kaufmann M, Konecny GE, Denkert C, Nekljudova V, Mehta K, Loibl S (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30(15):1796–1804. https://doi.org/10.1200/JCO.2011.38.8595
Waks AG, Winer EP (2019) Breast cancer treatment: a review. JAMA 321(3):288–300. https://doi.org/10.1001/jama.2018.19323
Yu W-D, Sun G, Li J, Xu J, Wang X (2019) Mechanisms and therapeutic potentials of cancer immunotherapy in combination with radiotherapy and/or chemotherapy. Cancer Lett 452:66–70. https://doi.org/10.1016/j.canlet.2019.02.048
Acknowledgements
We are grateful for the Surveillance, Epidemiology, and End Results database and all our colleagues in the Department of Oncology, The Second Affiliated Hospital of Xi’an Jiaotong University.
Funding
This study was supported by the National Natural Science Foundation of China (No. 52203186), the Basic Research Program of the Natural Science Foundation of Shaanxi Province (No. 2021JQ-422), and the Natural Science Foundation of Shaanxi Provincial Department of Education (No. 2022JM-101).
Author information
Authors and Affiliations
Contributions
SL, HK and HW proposed the main principles and guided the research design. DL, LC, QH and XR retrieved and analysed the raw data. PL, XL, YW and MW interpreted the results. DL wrote the first edition of the paper, and then LC and QH modified it. The findings were discussed among all the authors, and the manuscript was revised accordingly. All contributors reviewed and approved the submission of the final paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, D., Chang, L., Hao, Q. et al. Is neoadjuvant chemotherapy necessary for T2N0-1M0 hormone receptor-positive/HER2-negative breast cancer patients undergoing breast-conserving surgery?. J Cancer Res Clin Oncol 150, 285 (2024). https://doi.org/10.1007/s00432-024-05810-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00432-024-05810-6