Abstract
Purpose
Immune checkpoint inhibitors (ICIs) plus tyrosine kinase inhibitors (TKIs) has become first-line therapy for metastatic renal cell carcinoma patients. This study aims to investigate the effect of tumor infiltrating B lymphocytes (TIBs) on the combination therapy.
Methods
The retrospective analysis was conducted on the clinical records of 115 metastatic clear cell renal cell carcinoma (mccRCC) patients treated with anti-PD-1 antibody plus Axitinib between March 2020 and June 2023. Observation target: objective response rate (ORR), and overall survival (OS), progression-free survival (PFS) and immune profile.
Results
Patients with high TIBs portended lower ORR of the combination therapy (p = 0.033). TIBs was an independent predictor for poorer OS (p = 0.013) and PFS (p = 0.021) in mccRCC patients with combination treatment. TIBs infiltration was associated with more CD4+T (p < 0.001), CD8+T (p < 0.001), M2 macrophages (p = 0.020) and regulatory T cells (Tregs) (p = 0.004). In TIBs high patients, the percentages of PD-1, CTLA-4 and TIM-3 positive rate were significantly increased in CD4+T (p = 0.038, 0.029 and 0.002 respectively) and CD8+T cells (p = 0.006, 0.026 and < 0.001 respectively).
Conclusions
Our study revealed TIBs infiltration predicted adverse outcomes in mccRCC patients treated with anti-PD-1 antibody plus Axitinib. As a corollary, TIBs positively associated with M2 macrophages and Tregs, leading to subsequent multiple immune checkpoints related exhaustion of T cells. Thus, only PD-1 blockade are inadequate to reverse T cells exhaustion effectively in high TIBs mccRCC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal cancer accounts for 5% of all tumors and its incidence continued to increase annually in both gender (Siegel et al. 2023). Renal cell carcinoma (RCC) is the most common type of renal cancer. Since high proportion of asymptomatic cases, 25–30% RCC patients had metastases at the time of initial diagnosis (Usher-Smith et al. 2020). The prognosis significantly deteriorated if the disease turn into metastatic renal cell carcinoma (mRCC).
Tyrosine kinase inhibitors (TKIs) were used as first-line treatment for mRCC, which had significantly improved the prognosis of these patients (Motzer et al. 2007). Recent years, clinical trials like CLEAR, CheckMate 9ER and KEYNOTE-426 study has revolutionized the therapeutic strategy (Choueiri et al. 2023; Motzer et al. 2022; Plimack et al. 2023). Immune checkpoint inhibitors (ICIs) plus TKIs has become first-line therapy for mRCC patients. The objective response rate (ORR) had reached over 50% in these studies mentioned above. Tumor mutation burden, PD-L1 expression and microsatellite instability (MSI) had been approved to be predictors for ICIs therapy (Havel et al. 2019). However, nearly all previous studies focused on the role of indicators in TKI or ICI monotherapy respectively. Little research explore predictors of the “ICI + TKI” combination therapy.
The biological and clinical relevance of T lymphocytes in anti-tumor immune response and immunotherapy has been well characterized. As another subtype of adaptive immune cells, the function of B lymphocytes is often overlooked. For a long time, B cells are recognized as the main effector cells of humoral immunity and suppress cellular immunity. Emerging data have shown that B cells play a complex role in tumor immunity. On one hand, regulatory B cells (Bregs) subsets express immune inhibitory cytokines such as IL-10, TGF-β and IL-35 (Kessel et al. 2012; Pylayeva-Gupta et al. 2016; M. Zhou et al. 2016a, b). Additionally, Bregs express a variety of suppressive ligands including PD-L1, PD-1 and LAP-TGF-β, which can suppress immune effector cells response (Lee-Chang et al. 2019; Xiao et al. 2016; Zhang et al. 2016). On the other hand, Intratumoral B cells may drive antibody-dependent cellular cytotoxicity (ADCC) and enhance antigen presentation by secreting IgG antibodies (Carmi et al. 2015). B cell can also work as anti-tumor cytokine producer and even direct tumor killer to limit tumor progression (Shi et al. 2013; Tao et al. 2015).
A recent study showed that high CD20+ B cells infiltration identifies a poor prognosis subset of RCC patients (Sjoberg et al. 2018). Whereas, another researches suggested TIBs predicted longer overall survival (OS), progression-free survival (PFS) and better therapeutic response in TKI-treated mRCC patients (Lin et al. 2018). In addition, the effect of TIBs on immunotherapy response is also contradictory. High B cells infiltration improved soft-tissue sarcomas, melanoma and RCC patients survival and predicted a high response rate to anti-PD-1 therapy (Helmink et al. 2020; Petitprez et al. 2020). However, another study showed B cell depletion or absence is disassociated with response to anti-PD-1 inhibitors in melanoma (Damsky et al. 2019). Therefore, the role of B cells in ICI therapy remains to be revealed.
Clear cell renal cell carcinoma (ccRCC) is the most common pathological subtype of RCC, accounting for 70–80% of all RCC cases. In this study, we aim to assess the prognostic value of TIBs in metastatic ccRCC (mccRCC) patients treated with anti-PD-1 antibody combined Axitinib.
Patients and methods
Patient population
RCC patients treated with anti-PD-1 antibody plus Axitinib combination therapy was retrospectively screened in the Department of Urology, Zhongshan Hospital, Fudan University. 134 RCC patients who underwent anti-PD-1 antibody (Pembrolizumab or Tislelizumab) plus Axitinib between March 2020 and June 2023 were identified as potentially relevant. Six pantients were excluded because of inapposite age (< 18 or > 80). Four pantients were excluded because of complicated by other tumors. Then, nine patients were excluded because the pathological diagnosis was non-clear cell renal cell carcinoma (nccRCC). Finally, 115 ccRCC patients were ultimately included in the analysis (Fig. 1). The median follow-up was 18 months (2–42 months) and the major characteristics were listed in Table 1. All enrolled patients had received primary tumor resection (radical or cytoreductive nephrectomy). Metastasectomy was also performed in 35 suitable patients.
Kidney Renal Clear Cell Carcinoma (KIRC) cohort from The Cancer Genome Atlas (TCGA) was downloaded from TCGA database (https://cancergenome.nih.gov/). Absolute proportion of immune cells in TCGA Cohort was obtained by CIBERSORT calculation (Chen et al. 2018). Absolute proportion of TIBs was defined as the sum of absolute proportion of B cells naïve, B cells memory and plasma cells calculated by CIBERSORT.
Immunohistochemistry (IHC) staining and evaluation
IHC staining were applied to identifiy specific cells. Tissue microarray slides were scanned on NanoZoomer-XR (Hamamatsu). Immune cell number was counted by two urologists (blinded to the clinical data) independently. The density of positive staining cells was calculated (cells/mm2) and used for grouping and analyzing. Details of IHC antibodies were shown in Table S1.
Flow cytometry (FCM)
Fresh ccRCC tissues (at least 1 cm3 away from the tumor site) were minced and digested with collagenase IV to prepare single cell suspensions, and followed incubated by RBC lysis buffer (BD Biosciences). Then, single cells were stained with appropriate monoclonal antibodies for 30 min at 4 degree centigrade. After disposed by Fixation/Permeabilization Solution Kit (BD Biosciences) according to manufacture protocol. Cell suspensions were stained with fluorochrome-labeled antibodies and preserved with cell staining buffer. FCM was performed with a BD FACScelesta, and cells were analyzed using FlowJo software (Tree Star). Details of FCM antibodies were shown in Table S2.
Statistical analyses
Baseline demographic, histological and clinical data were collected from medical records, electronic databases and follow-up information. The tumor histological type and stage according to the 2016 WHO criteria and the definitions of the 8th edition of the American Joint Committee on Cancer (AJCC) stage classification (Cornejo et al. 2020; Delahunt et al. 2019). Disease progression was identified via RECIST1.1 criteria (Eisenhauer et al. 2009). OS was defined as the time from combined therapy to death or the last observation. PFS was calculated from the date of combined therapy to disease progression, mortality or the last observation.
The cut point of TIBs density was determined at 5.0/mm2 using X-tile software version 3.4.7 (Robert Camp 2005) through minimum p value method based on the patients’ OS information (Camp et al. 2004). Statistical analyses were performed using SPSS 21.0 (SPSS Inc.) in this study. The association between cells infiltration and baseline characteristics were assessed by the chi-square test method. The Kaplan–Meier method and log rank test was applied to survival analyses. Univariate- and multivariate-Cox proportional hazard models were used to identify independent prognostic factors in sequence. Pearson’s correlation test was used to evaluate the associations between immune cells. 2-tailed p < 0.05 was considered as statistically significant.
Results
TIBs infiltration predicted lower ORR in combination therapy
TIBs expression level and localization were evaluated by IHC staining on primary RCC specimens by using anti-CD19 antibody. As shown, CD19 was mainly distributed on the membrane of TIBs (Fig. 2A). The density of TIBs varied from 0/mm2 to 46/mm2 (Fig. 2B). TIBs infiltrating status had no correlation with gender, age, TNM stage, ISUP grade or metastatic organ number. Patients with high TIBs showed lower response to the combination therapy (p = 0.033) (Fig. 2C).
TIBs present in tumor samples and predict therapeutic response in mccRCC patients. A Representative images of CD19 staining in tumor samples. Scale bar is 50 μm. B Distribution of TIBs density in tumor samples of the study cohort. C Treatment response in different TIBs groups. CR complete response; PR partial response; SD stable disease; PD progressive disease
TIBs portended shorter OS and PFS in combination treated ccRCC patients
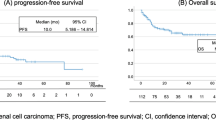
In the cohort, patients with high TIBs were linked with shorter OS by Kaplan–Meier analysis (p = 0.007) (Fig. 3A). Univariate Cox regression model revealed that ISUP grade (p = 0.007), TIBs (p = 0.01), metastatic organ number (p = 0.04) and received metastasectomy (p = 0.035) were significant prognostic factors for OS. Multivariate-Cox analysis confirmed TIBs was an independent predictor for poorer OS of combination treated ccRCC patients (p = 0.013) (Fig. 3C). The univariate- and multivariate-Cox analyses of TIBs and other clinical characteristics with OS were listed in Table S3.
At the final followup, patients with high TIBs demonstrated shorter PFS (p = 0.008) (Fig. 3B). ISUP grade (p = 0.001), TIBs (p = 0.011), IMDC risk stratification (p = 0.026) and metastatic organ number (p = 0.043) had significant relevance to PFS in univariate-Cox regression model. Besides, PD-1 expression showed moderate effects on PFS (p = 0.064) in univariate-Cox analysis, which also needs to be considered. Via multivariate-Cox analysis, we confirmed TIBs was still a significant predictor of PFS in ccRCC patients treated with combination therapy (p = 0.021) (Fig. 3D). The univariate- and multivariate-Cox analyses of TIBs and other clinical characteristics with OS and PFS were listed in Table S3.
Kaplan–Meier analysis showed no significant difference between the Tislelizumab-treated and Pembrolizumab-treated groups in OS (p = 0.944) (Fig.S1A) or PFS (p = 0.310) (Fig.S1B). The objective response rate (ORR) also showed no significant difference between the two groups (p = 0.898).
TIBs infiltration associated with immunosuppressive microenvironment
To investigate the potential mechanism of TIBs suppressive effect on combination therapy, we investigate immunocyte profiles in the cohort. IHC staining on microarray showed high TIBs associated with more infiltration of CD4+T (p < 0.001) (Fig. 4A), CD8+T (p < 0.001) (Fig. 4B), M2 macrophages (p = 0.020) (Fig. 4C) and regulatory T cells (Tregs) (p = 0.004) (Fig. 4D).
In consideration of M2 macrophages and Treg cells exhaust CD8+T cells in tumor immune microenvironment (TIME), we explored the correlation between several immune checkpoints expression (PD-1, TIM-3, CTLA-4, LAG3 and TIGIT) and TIBs in the TCGA database. Results showed TIBs positive corrected with all these immune checkpoint molecules (Fig.S2). Then, we collected 22 fresh ccRCC samples and detected these immune checkpoints in CD4+T and CD8+T cells by FCM. Results showed that in TIBs high patients, PD-1, CTLA-4 and TIM-3 positive rate were significantly increased both in CD4+T (p = 0.038, 0.029 and 0.002 respectively, Fig. 5A–C) and CD8+T cells (p = 0.006, 0.026 and < 0.001 respectively, Fig. 5F–H). However, LAG3 and TIGIT expression level showed no significant difference in either CD4+T (p = 0.179 and 0.142 respectively, Fig. 5D–E) or CD8+T cells (p = 0.212 and 0.367 respectively, Fig. 5I, J).
Comparison of immune checkpoints expression between TIBs high and low groups. A–E Comparison of PD-1 (A), TIM-3 (B), CTLA-4 (C), LAG3 (D) and TIGIT (E) positive rate in CD4+T cells between TIBs high and low groups. F–J Comparison of PD-1 (F), TIM-3 (G), CTLA-4 (H), LAG3 (I) and TIGIT (J) positive rate in CD8+T cells between TIBs high and low groups
Discussion
ICIs plus TKIs has become first-line therapy for mRCC patients. Though, there is a lot of researches explored predictors in TKI or ICI monotherapy, little study has addressed predictors to guide selection for the combination therapy (Havel et al. 2019).
Emerging evidence shows TIME acts as a dominant force on treatment resistance, which can mediate immune evasion accounting for the interaction of immune cells and tumor cells (Barker et al. 2015; Correia and Bissell. 2012). Relieving TIME immunosuppressive effect is conducive to recover and reconstruct anti-tumor immunity, thereby enhancing the comprehensive therapeutic efficacy (Pitt et al. 2016). Hence, TIME has become an ideal target for cancer therapy (Belli et al. 2018; Roma-Rodrigues et al. 2019). The biological and clinical relevance of TIBs in RCC is still conflicting. Presence of CD19+B lymphocytes predicted better therapeutic response to sunitinib and longer survival (Lin et al. 2018). CD19+B cells also positive correlated with CD8+T cells in RCC. However, in another study, high infiltration of CD20+B cells indicated poor survival in RCC patients (Sjoberg et al. 2018). There was no study had yet explored the significance of TIBs in the ICIs plus TKIs combination therapy in RCC. This study showed high TIBs infiltration portended shorter OS (p = 0.007) (Fig. 3A) and PFS (p = 0.008) (Fig. 3B) in anti-PD-1 antibody plus Axitinib combination treatment. Multivariate cox regression analysis identified TIBs was an independent prognostic factor of OS (p = 0.013) (Fig. 3C) and PFS (p = 0.021) (Fig. 3D). Patients with high TIBs showed lower response to the combination therapy (p = 0.033) (Fig. 2C).
ccRCC belongs to immune “hot” tumors, which marked by large number of immune cells infiltrated in the TIME (Xu et al. 2023). In this study, high TIBs infiltration correlated with more CD4+T (p < 0.001) (Fig. 4A) and CD8+T cells (p < 0.001) (Fig. 4B) in RCC tumor sites. Unlike most tumors, high infiltration of CD8+T cells often predicts poor outcomes in RCC patients (Remark et al. 2013). It suggests that CD8+T cells infiltrated in RCC microenvironment are mostly functionally exhausted. Moreover, two immune suppressor cells, M2 macrophages (p = 0.020) (Fig. 4C) and Treg cells (p = 0.004) (Fig. 4D) were also increased in high TIBs patients.
Previous studies showed B cells could induce M2 macrophage polarization, as well as suppress CD8+T cells and M1 macrophages, which contributed the promotion of cancer cell proliferation (Liu et al. 2015; Roghanian et al. 2016). B cell-derived GABA promotes monocyte differentiation into anti-inflammatory macrophages that secrete interleukin-10 and inhibit CD8+T cell cytotoxic function (Zhang et al. 2021). Tumor associated macrophages can also upregulate the expression of inhibitory receptors PD-1 and CTLA-4 in T cells (Yin et al. 2019). Depletion of B cells prevented generation of M2 macrophage and increased the activity of antitumor T cell response (Affara et al. 2014; Liu et al. 2015).
High intratumoral B cells were also associated with increased recruitment and proliferation of Treg cells (Zhang et al. 2013). Bregs induce CD4+T cells to Tregs in IL-10 dependent and independent mechanisms (Zhang et al. 2013; X. Zhou et al. 2016a, b). IgD(low/-) B cells promote Treg homeostatic expansion via glucocorticoid-induced tumor necrosis factor receptor ligand (Ray et al. 2019). Tregs can also facilitate inhibitory receptor expression, including PD-1, on intratumoral T cells via enrichment of IL-35 expression (Sawant et al. 2019). Elimination of Tregs couled effectively restore IFN-γ production in the CD8+T cells and enhance the antitumor response to anti-VEGF therapy (Long et al. 2020).
In consideration of M2 macrophages and Treg cells exhaust CD8+T cells in TIME, we explored the correlation between several immune checkpoints expression (PD-1, TIM-3, CTLA-4, LAG3 and TIGIT) and TIBs in the TCGA database. Result showed TIBs positive corrected with all these immune checkpoint molecules (Supplemental Fig. 1). FCM verified that in TIBs high patients, PD-1, CTLA-4 and TIM-3 expression elevated significantly both in CD4+T (p = 0.038, 0.029 and 0.002 respectively, Fig. 5A–C) and CD8+T cells (p = 0.006, 0.026 and < 0.001 respectively, Fig. 5F–H). It suggested multiple immune checkpoints related exhaustion happened in these CD4+T and CD8+T cells. Only inhibiting PD-1 may inadequate to reverse these T cells exhaustion effectively. This explains low ORR of these patients to 天the combination treatment.
Conclusions
In summary, our study revealed TIBs infiltration predicted adverse outcomes in mccRCC patients treated with anti-PD-1 antibody plus Axitinib. As a corollary, TIBs positively associated with M2 macrophages and Treg, leading to subsequent T cells exhaustion. Due to single-center and retrospective design, prospective or external validations is needed in order to strengthen evidence chain. Besides, the related mechanism have not been elaborated in this article and should be expound in-depth in future study. Nevertheless, our research is still helpful to understand the impact of TIBs in mccRCC patients treated with anti-PD-1 antibody plus Axitinib.
Data availability
The raw data used to support the findings of this study are available from the corresponding author upon reasonable request.
References
Affara NI, Ruffell B, Medler TR, Gunderson AJ, Johansson M, Bornstein S, Bergsland E, Steinhoff M, Li Y, Gong Q, Ma Y, Wiesen JF, Wong MH, Kulesz-Martin M, Irving B, Coussens LM (2014) B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell 25:809–821. https://doi.org/10.1016/j.ccr.2014.04.026
Barker HE, Paget JT, Khan AA, Harrington KJ (2015) The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer 15:409–425. https://doi.org/10.1038/nrc3958
Belli C, Trapani D, Viale G, D’Amico P, Duso BA, Della VP, Orsi F, Curigliano G (2018) Targeting the microenvironment in solid tumors. Cancer Treat Rev 65:22–32. https://doi.org/10.1016/j.ctrv.2018.02.004
Camp RL, Dolled-Filhart M, Rimm DL (2004) X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 10:7252–7259. https://doi.org/10.1158/1078-0432.CCR-04-0713
Carmi Y, Spitzer MH, Linde IL, Burt BM, Prestwood TR, Perlman N, Davidson MG, Kenkel JA, Segal E, Pusapati GV, Bhattacharya N, Engleman EG (2015) Allogeneic IgG combined with dendritic cell stimuli induce antitumour T-cell immunity. Nature 521:99–104. https://doi.org/10.1038/nature14424
Chen B, Khodadoust MS, Liu CL, Newman AM, Alizadeh AA (2018) Profiling tumor infiltrating immune cells with CIBERSORT. Methods Mol Biol 1711:243–259. https://doi.org/10.1007/978-1-4939-7493-1_12
Choueiri TK, Eto M, Motzer R, De Giorgi U, Buchler T, Basappa NS, Mendez-Vidal MJ, Tjulandin S, Hoon PS, Melichar B, Hutson T, Alemany C, McGregor B, Powles T, Grunwald V, Alekseev B, Rha SY, Kopyltsov E, Kapoor A, Alonso GT, Goh JC, Staehler M, Merchan JR, Xie R, Perini RF, Mody K, McKenzie J, Porta CG (2023) Lenvatinib plus pembrolizumab versus sunitinib as first-line treatment of patients with advanced renal cell carcinoma (CLEAR): extended follow-up from the phase 3, randomised, open-label study. Lancet Oncol 24:228–238. https://doi.org/10.1016/S1470-2045(23)00049-9
Cornejo KM, Rice-Stitt T, Wu CL (2020) Updates in staging and reporting of genitourinary malignancies. Arch Pathol Lab Med 144:305–319. https://doi.org/10.5858/arpa.2019-0544-RA
Correia AL, Bissell MJ (2012) The tumor microenvironment is a dominant force in multidrug resistance. Drug Resist Updat 15:39–49. https://doi.org/10.1016/j.drup.2012.01.006
Damsky W, Jilaveanu L, Turner N, Perry C, Zito C, Tomayko M, Leventhal J, Herold K, Meffre E, Bosenberg M, Kluger HM (2019) B cell depletion or absence does not impede anti-tumor activity of PD-1 inhibitors. J Immunother Cancer 7:153. https://doi.org/10.1186/s40425-019-0613-1
Delahunt B, Eble JN, Egevad L, Samaratunga H (2019) Grading of renal cell carcinoma. Histopathology 74:4–17. https://doi.org/10.1111/his.13735
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Havel JJ, Chowell D, Chan TA (2019) The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer 19:133–150. https://doi.org/10.1038/s41568-019-0116-x
Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han G, Gopalakrishnan V, Xi Y, Zhao H, Amaria RN, Tawbi HA, Cogdill AP, Liu W, LeBleu VS, Kugeratski FG, Patel S, Davies MA, Hwu P, Lee JE, Gershenwald JE, Lucci A, Arora R, Woodman S, Keung EZ, Gaudreau PO, Reuben A, Spencer CN, Burton EM, Haydu LE, Lazar AJ, Zapassodi R, Hudgens CW, Ledesma DA, Ong S, Bailey M, Warren S, Rao D, Krijgsman O, Rozeman EA, Peeper D, Blank CU, Schumacher TN, Butterfield LH, Zelazowska MA, McBride KM, Kalluri R, Allison J, Petitprez F, Fridman WH, Sautes-Fridman C, Hacohen N, Rezvani K, Sharma P, Tetzlaff MT, Wang L, Wargo JA (2020) B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577:549–555. https://doi.org/10.1038/s41586-019-1922-8
Kessel A, Haj T, Peri R, Snir A, Melamed D, Sabo E, Toubi E (2012) Human CD19(+)CD25(high) B regulatory cells suppress proliferation of CD4(+) T cells and enhance Foxp3 and CTLA-4 expression in T-regulatory cells. Autoimmun Rev 11:670–677. https://doi.org/10.1016/j.autrev.2011.11.018
Lee-Chang C, Rashidi A, Miska J, Zhang P, Pituch KC, Hou D, Xiao T, Fischietti M, Kang SJ, Appin CL, Horbinski C, Platanias LC, Lopez-Rosas A, Han Y, Balyasnikova IV, Lesniak MS (2019) Myeloid-derived suppressive cells promote B cell-mediated immunosuppression via transfer of PD-L1 in glioblastoma. Cancer Immunol Res 7:1928–1943. https://doi.org/10.1158/2326-6066.CIR-19-0240
Lin Z, Liu L, Xia Y, Chen X, Xiong Y, Qu Y, Wang J, Bai Q, Guo J, Xu J (2018) Tumor infiltrating CD19(+) B lymphocytes predict prognostic and therapeutic benefits in metastatic renal cell carcinoma patients treated with tyrosine kinase inhibitors. Oncoimmunology 7:e1477461. https://doi.org/10.1080/2162402X.2018.1477461
Liu RX, Wei Y, Zeng QH, Chan KW, Xiao X, Zhao XY, Chen MM, Ouyang FZ, Chen DP, Zheng L, Lao XM, Kuang DM (2015) Chemokine (C-X-C motif) receptor 3-positive B cells link interleukin-17 inflammation to protumorigenic macrophage polarization in human hepatocellular carcinoma. Hepatology 62:1779–1790. https://doi.org/10.1002/hep.28020
Long Y, Tao H, Karachi A, Grippin AJ, Jin L, Chang YE, Zhang W, Dyson KA, Hou AY, Na M, Deleyrolle LP, Sayour EJ, Rahman M, Mitchell DA, Lin Z, Huang J (2020) Dysregulation of glutamate transport enhances treg function that promotes VEGF blockade resistance in glioblastoma. Cancer Res 80:499–509. https://doi.org/10.1158/0008-5472.CAN-19-1577
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356:115–124. https://doi.org/10.1056/NEJMoa065044
Motzer RJ, Powles T, Burotto M, Escudier B, Bourlon MT, Shah AY, Suarez C, Hamzaj A, Porta C, Hocking CM, Kessler ER, Gurney H, Tomita Y, Bedke J, Zhang J, Simsek B, Scheffold C, Apolo AB, Choueiri TK (2022) Nivolumab plus cabozantinib versus sunitinib in first-line treatment for advanced renal cell carcinoma (CheckMate 9ER): long-term follow-up results from an open-label, randomised, phase 3 trial. Lancet Oncol 23:888–898. https://doi.org/10.1016/S1470-2045(22)00290-X
Petitprez F, de Reynies A, Keung EZ, Chen TW, Sun CM, Calderaro J, Jeng YM, Hsiao LP, Lacroix L, Bougouin A, Moreira M, Lacroix G, Natario I, Adam J, Lucchesi C, Laizet YH, Toulmonde M, Burgess MA, Bolejack V, Reinke D, Wani KM, Wang WL, Lazar AJ, Roland CL, Wargo JA, Italiano A, Sautes-Fridman C, Tawbi HA, Fridman WH (2020) B cells are associated with survival and immunotherapy response in sarcoma. Nature 577:556–560. https://doi.org/10.1038/s41586-019-1906-8
Pitt JM, Marabelle A, Eggermont A, Soria JC, Kroemer G, Zitvogel L (2016) Targeting the tumor microenvironment: removing obstruction to anticancer immune responses and immunotherapy. Ann Oncol 27:1482–1492. https://doi.org/10.1093/annonc/mdw168
Plimack ER, Powles T, Stus V, Gafanov R, Nosov D, Waddell T, Alekseev B, Pouliot F, Melichar B, Soulieres D, Borchiellini D, McDermott RS, Vynnychenko I, Chang YH, Tamada S, Atkins MB, Li C, Perini R, Molife LR, Bedke J, Rini BI (2023) Pembrolizumab plus axitinib versus sunitinib as first-line treatment of advanced renal cell carcinoma: 43-month follow-up of the phase 3 KEYNOTE-426 study. Eur Urol 84:449–454. https://doi.org/10.1016/j.eururo.2023.06.006
Pylayeva-Gupta Y, Das S, Handler JS, Hajdu CH, Coffre M, Koralov SB, Bar-Sagi D (2016) IL35-Producing B cells promote the development of pancreatic neoplasia. Cancer Discov 6:247–255. https://doi.org/10.1158/2159-8290.CD-15-0843
Ray A, Khalil MI, Pulakanti KL, Burns RT, Gurski CJ, Basu S, Wang D, Rao S, Dittel BN (2019) Mature IgD(low/-) B cells maintain tolerance by promoting regulatory T cell homeostasis. Nat Commun 10:190. https://doi.org/10.1038/s41467-018-08122-9
Remark R, Alifano M, Cremer I, Lupo A, Dieu-Nosjean MC, Riquet M, Crozet L, Ouakrim H, Goc J, Cazes A, Flejou JF, Gibault L, Verkarre V, Regnard JF, Pages ON, Oudard S, Mlecnik B, Sautes-Fridman C, Fridman WH, Damotte D (2013) Characteristics and clinical impacts of the immune environments in colorectal and renal cell carcinoma lung metastases: influence of tumor origin. Clin Cancer Res 19:4079–4091. https://doi.org/10.1158/1078-0432.CCR-12-3847
Roghanian A, Fraser C, Kleyman M, Chen J (2016) B cells promote pancreatic tumorigenesis. Cancer Discov 6:230–232. https://doi.org/10.1158/2159-8290.CD-16-0100
Roma-Rodrigues C, Mendes R, Baptista PV, Fernandes AR (2019) Targeting tumor microenvironment for cancer therapy. Int J Mol Sci 20:840. https://doi.org/10.3390/ijms20040840
Sawant DV, Yano H, Chikina M, Zhang Q, Liao M, Liu C, Callahan DJ, Sun Z, Sun T, Tabib T, Pennathur A, Corry DB, Luketich JD, Lafyatis R, Chen W, Poholek AC, Bruno TC, Workman CJ, Vignali D (2019) Adaptive plasticity of IL-10(+) and IL-35(+) T(reg) cells cooperatively promotes tumor T cell exhaustion. Nat Immunol 20:724–735. https://doi.org/10.1038/s41590-019-0346-9
Shi JY, Gao Q, Wang ZC, Zhou J, Wang XY, Min ZH, Shi YH, Shi GM, Ding ZB, Ke AW, Dai Z, Qiu SJ, Song K, Fan J (2013) Margin-infiltrating CD20(+) B cells display an atypical memory phenotype and correlate with favorable prognosis in hepatocellular carcinoma. Clin Cancer Res 19:5994–6005. https://doi.org/10.1158/1078-0432.CCR-12-3497
Siegel RL, Miller KD, Wagle NS, Jemal A (2023) Cancer statistics, 2023. CA Cancer J Clin 73:17–48. https://doi.org/10.3322/caac.21763
Sjoberg E, Frodin M, Lovrot J, Mezheyeuski A, Johansson M, Harmenberg U, Egevad L, Sandstrom P, Ostman A (2018) A minority-group of renal cell cancer patients with high infiltration of CD20+B-cells is associated with poor prognosis. Br J Cancer 119:840–846. https://doi.org/10.1038/s41416-018-0266-8
Tao H, Lu L, Xia Y, Dai F, Wang Y, Bao Y, Lundy SK, Ito F, Pan Q, Zhang X, Zheng F, Shu G, Fang B, Jiang J, Xia J, Huang S, Li Q, Chang AE (2015) Antitumor effector B cells directly kill tumor cells via the Fas/FasL pathway and are regulated by IL-10. Eur J Immunol 45:999–1009. https://doi.org/10.1002/eji.201444625
Usher-Smith J, Simmons RK, Rossi SH, Stewart GD (2020) Current evidence on screening for renal cancer. Nat Rev Urol 17:637–642. https://doi.org/10.1038/s41585-020-0363-3
Xiao X, Lao XM, Chen MM, Liu RX, Wei Y, Ouyang FZ, Chen DP, Zhao XY, Zhao Q, Li XF, Liu CL, Zheng L, Kuang DM (2016) PD-1hi identifies a novel regulatory B-cell population in human hepatoma that promotes disease progression. Cancer Discov 6:546–559. https://doi.org/10.1158/2159-8290.CD-15-1408
Xu S, Ma B, Feng X, Yao C, Jian Y, Chen Y, Wang X, Xie H, Li L (2023) EZH2-regulated immune risk score prognostic model predicts outcome of clear cell renal cell carcinoma. Transl Androl Urol 12:71–82. https://doi.org/10.21037/tau-22-817
Yin C, Han Q, Xu D, Zheng B, Zhao X, Zhang J (2019) SALL4-mediated upregulation of exosomal miR-146a-5p drives T-cell exhaustion by M2 tumor-associated macrophages in HCC. Oncoimmunology 8:1601479. https://doi.org/10.1080/2162402X.2019.1601479
Zhang Y, Eliav Y, Shin SU, Schreiber TH, Podack ER, Tadmor T, Rosenblatt JD (2013) B lymphocyte inhibition of anti-tumor response depends on expansion of Treg but is independent of B-cell IL-10 secretion. Cancer Immunol Immunother 62:87–99. https://doi.org/10.1007/s00262-012-1313-6
Zhang Y, Morgan R, Chen C, Cai Y, Clark E, Khan WN, Shin SU, Cho HM, Al BA, Pimentel A, Rosenblatt JD (2016) Mammary-tumor-educated B cells acquire LAP/TGF-beta and PD-L1 expression and suppress anti-tumor immune responses. Int Immunol 28:423–433. https://doi.org/10.1093/intimm/dxw007
Zhang B, Vogelzang A, Miyajima M, Sugiura Y, Wu Y, Chamoto K, Nakano R, Hatae R, Menzies RJ, Sonomura K, Hojo N, Ogawa T, Kobayashi W, Tsutsui Y, Yamamoto S, Maruya M, Narushima S, Suzuki K, Sugiya H, Murakami K, Hashimoto M, Ueno H, Kobayashi T, Ito K, Hirano T, Shiroguchi K, Matsuda F, Suematsu M, Honjo T, Fagarasan S (2021) B cell-derived GABA elicits IL-10(+) macrophages to limit anti-tumour immunity. Nature 599:471–476. https://doi.org/10.1038/s41586-021-04082-1
Zhou M, Wen Z, Cheng F, Ma J, Li W, Ren H, Sheng Y, Dong H, Lu L, Hu HM, Wang LX (2016a) Tumor-released autophagosomes induce IL-10-producing B cells with suppressive activity on T lymphocytes via TLR2-MyD88-NF-kappaB signal pathway. Oncoimmunology 5:e1180485. https://doi.org/10.1080/2162402X.2016.1180485
Zhou X, Su YX, Lao XM, Liang YJ, Liao GQ (2016b) CD19(+)IL-10(+) regulatory B cells affect survival of tongue squamous cell carcinoma patients and induce resting CD4(+) T cells to CD4(+)Foxp3(+) regulatory T cells. Oral Oncol 53:27–35. https://doi.org/10.1016/j.oraloncology.2015.11.003
Funding
Sailing Project of “Scientifc and Technological Innovation Action Plan” of Shanghai Science and Technology Commission, 20YF1406100, Youth Fund of Zhongshan Hospital, Fudan University, 2020ZSQN18, 2021ZSQN28, National Natural Science Foundation of China, 82102967.
Author information
Authors and Affiliations
Contributions
ZL: IHC evaluation, statistical analyses and writing of original draft. SX: carrying out the experiments of FCM and bioinformatics analyses. YQ: fresh tumor collection, IHC staining and evaluation. JG: study design and supervision. LL: modifying the final manuscript and carrying out the experiments of FCM. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
432_2024_5803_MOESM4_ESM.tif
Supplementary file4 Fig.S2 Pearson’s correlation between TIBs and immune checkpoints expression from TCGA data. (A) Pearson’s correlation between TIBs and PD-1 expression. (B) Pearson’s correlation between TIBs and TIM-3 expression. (C) Pearson’s correlation between TIBs and CTLA-4 expression. (D) Pearson’s correlation between TIBs and LAG3 expression. (E) Pearson’s correlation between TIBs and TIGIT expression (TIF 204 KB)
432_2024_5803_MOESM5_ESM.tif
Supplementary file5 Fig.S1 The Kaplan-Meier analysis of OS and PFS between Tislelizumab-treated and Pembrolizumab-treated patients. (A,B) Kaplan–Meier survival analysis of OS (A) and PFS (B) between Tislelizumab-treated and Pembrolizumab-treated patients (TIF 406 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, Z., Xiao, S., Qi, Y. et al. Tumor infiltrating B lymphocytes (TIBs) associate with poor clinical outcomes, unfavorable therapeutic benefit and immunosuppressive context in metastatic clear cell renal cell carcinoma (mccRCC) patients treated with anti-PD-1 antibody plus Axitinib. J Cancer Res Clin Oncol 150, 262 (2024). https://doi.org/10.1007/s00432-024-05803-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00432-024-05803-5