Abstract
Purpose
Neoadjuvant chemotherapy (NCT) is the standard preoperative treatment for resectable locally advanced esophageal squamous cell carcinoma (ESCC). Some studies reported neoadjuvant immunochemotherapy (NICT) could improve pathological response with manageable safety. However, few studies have compared the efficacy and safety of NICT and NCT, especially survival outcomes. In this study, we compared the efficacy and safety of NICT and NCT after a median follow-up of 36.0 months.
Methods
This was a retrospective study with a 1:1 propensity score matching (PSM). Locally advanced ESCC patients treated with neoadjuvant sintilimab plus chemotherapy or chemotherapy followed by esophagectomy were reviewed. The primary outcome was recurrence-free survival (RFS).
Results
Forty-five patients were identified in each group by PSM. The pathological complete response (pCR) rate in NICT and NCT group were 28.9% and 8.9% (P = 0.02). The hazard ratio (HR) was 0.396 (95% CI 0.171–0.919, p = 0.025) for RFS and 0.377 (95% CI 0.145–0.981, p = 0.038) for overall survival (OS), 3-year RFS was 80.6% and 62.1%, 3-year OS was 86.2% and 68.1%. Patients with pCR, MPR or downstaging had better 3-year RFS and 3-year OS. The incidences of postoperative complications and treatment-related adverse events (TRAEs) were similar.
Conclusion
This trial preliminarily shows that NICT improves pathological and survival outcomes over NCT for resectable locally advanced ESCC, with acceptable and manageable safety.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal cancer (EC) is a common malignant tumor, which ranks the seventh leading cause of cancer incidence and fifth leading cause of cancer mortality in China (Sung et al. 2021; Chen et al. 2023a; Zheng et al. 2024). China accounts for about half of the burden of EC worldwide (Sung et al. 2021; Chen et al. 2023a; Zheng et al. 2024). It was estimated about 224,000 new cases and 187,500 new deaths of EC occurred in China in 2022 (Zheng et al. 2024). Esophageal squamous cell carcinoma (ESCC) is the predominant histological type, accounting about 86% of all cases (Zheng et al. 2024). For the locally advanced resectable ESCC, multidisciplinary comprehensive treatment of neoadjuvant chemotherapy (NCT) or neoadjuvant chemoradiotherapy (NCRT) followed by esophagectomy is an effective strategy, which was confirmed to improve the survival of patients compared to surgery alone (Medical Research Council Oesophageal Cancer Working Group. 2002; Allum et al. 2009; Ando et al. 2012; van et al. 2012; Yang et al. 2018, 2021; Eyck et al. 2021). NCRT improve local pathological response compared to NCT, but more safety concerns affected its clinical application (Kumagai et al. 2014; Chan et al. 2018; Wang et al. 2021a; Zhang et al. 2022a). Many studies have shown that the overall survival (OS) of NCRT was not significantly improved compared to NCT (Zhang et al. 2022a; Tang et al. 2023; Kato et al. 2022). Thus, the novel neoadjuvant strategy of locally advanced ESCC is urgent to explore. However, the long-term survival of NCT or NCRT followed by esophagectomy for ESCC is still not promising.
Programmed cell death protein 1 (PD-1) inhibitors combined with chemotherapy have demonstrated promising antitumor effects and become the first-line standard care of advanced esophageal and gastroesophageal junction (GEJ) carcinoma (Lu et al. 2022; Luo et al. 2021; Wang et al. 2022; Xu et al. 2023. Sun et al. 2021; Li et al. 2021; Doki et al. 2022; Song et al. 2023). Neoadjuvant immunochemotherapy (NICT) is also considered to have great prospects and caused extensive concern. Several studies have demonstrated that NICT produced a higher pathological complete response (pCR) rate ranging from 21.7% to 50% with manageable toxicity profile (Zhang et al. 2021; Yang et al. 2022; Liu et al. 2022a, b. Lv et al. 2022, 2023; Yan et al. 2022; Liu et al.2022a, b; Zhang et al. 2022b; Chen et al. 2023b; Zhang et al. 2023a, b; Yang et al 2023; Yin et al. 2022; Wang et al.2023; Shen et al. 2021). However, few studies have compared the efficacy and safety of NICT and NCT alone, especially survival outcomes. In this study, we compared the efficacy and safety of NICT and NCT followed by esophagectomy in patients with resectable locally advanced ESCC after a median follow-up of 36.0 months in a retrospective consecutive cohort.
Methods
Study design and patient selection
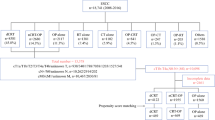
This is a retrospective consecutive cohort study with propensity score matching (PSM) to compared the efficacy and safety of NICT and NCT followed by esophagectomy in patients with locally advanced resectable ESCC. The study was approved by the Ethics Committee of the hospital. Consecutive locally advanced ESCC patients treated with NICT or NCT alone followed by esophagectomy at The Fourth Hospital of Hebei Medical University between July 2019 and October 2021 were reviewed. The inclusion criteria were: an age of 18 years or older, both sexes, histologically confirmed ESCC, clinically staged as II-IVA, treated with neoadjuvant sintilimab combined with chemotherapy (albumin-bound paclitaxel and nedaplatin) or chemotherapy alone followed by esophagectomy. Patients with unresectable tumors or receiving other antitumor treatments before esophagectomy were excluded. Diagnosis and clinical stage were determined by chest-abdominal contrast enhanced computed tomography scan and/or enhanced magnetic resonance imaging, endoscopic ultrasound, cervical ultrasound. Position emission tomography-computed tomography was performed if necessary.
Treatment
The eligible patients received 2–4 cycles of neoadjuvant sintilimab (200 mg, I.V, D1, Q3W) combined with chemotherapy (albumin-bound paclitaxel 260 mg/m2 and nedaplatin 80 mg/m2, I.V, D1, Q3W) or chemotherapy alone. Radiographic responses and restaging were assessed every 2 cycles by the same imaging means of the baseline. All patients suitable for radical esophagectomy underwent McKeown esophagectomy. The esophagectomy was usually performed within 4–8 weeks after neoadjuvant treatment. Pathological examination was carried out by two experienced pathologists according to the standard protocols. The survival follow-up was conducted according to the latest clinical guidelines, every 3 months during the first 2 years, and then every 6 months.
Outcomes
The primary outcome was recurrence-free survival (RFS). RFS was defined as the time from the date of neoadjuvant treatment to the first documentation of recurrence or death. The secondary outcomes included the pCR rate, major pathological response (MPR) rate, tumor downstaging rate, OS and safety. The pCR was defined as no evidence of residual tumor in the primary tumor and resected lymph nodes. The MPR was defined as less than10% residual tumor in the primary tumor. Tumor downstaging was defined as a decrease in T stage or/and N stage. OS was defined as the time from the date of neoadjuvant treatment to death from any cause.
Statistical analysis
R software (version 4.0.0) and SPSS software (IBM SPSS Statistics, version 26.0) were used for all statistical analyses. The continuous variables were presented as median and range, the comparison between two groups used Mann–Whitney U test. The categorical variables were presented as number and percentage, the comparison between two groups used Chi-square test or Fisher’s exact test. The 95% confidence intervals (CI) of pCR, MPR and tumor downstaging rate with was calculated using the Clopper–Pearson exact method. Median follow-up time was estimated using reverse Kaplan–Meier method. RFS and OS and the corresponding 95% CI were estimated using the Kaplan–Meier method, and the comparison between two groups used a log-rank test. A 1:1 PSM (caliper = 0.01) was conducted between NICT group and NCT group to minimize the bias of confounding variables. The propensity score was estimated by logistic regression models with following confounding variables: age, sex, smoking history, drinking history, Eastern Cooperative Oncology Group (ECOG) performance status (PS) Score, tumor location, clinical TNM stage, clinical T stage, and clinical N stage. The effect of neoadjuvant treatment among subgroups according to baseline characteristics were estimated using Univariable and multivariable Cox regression models. All statistical testing is two-tailed and performed at the 5% significance level.
Results
Baseline characteristics
A total of 181 eligible locally advanced ESCC patients completed NICT or NCT and underwent esophagectomy between July 2019 and October 2021 were included. In these patients, 131 patients received NICT and 50 received NCT. The NICT group had more clinical T3 stage patients (P = 0.033) than the NCT group before PSM (Table 1). After a one-to-one PSM, the final analysis included 45 patients in the NICT group and 45 patients in the NCT group, respectively. The baseline clinical characteristics were well balanced after PSM between two groups (Table 1).
Pathological and survival outcomes
All patients completed McKeown esophagectomy in the NICT and NCT group. The R0 resection rate in the NICT group was comparable to NCT group. The pCR rate, MPR rate and tumor downstaging rate in the NICT group were significantly higher than those NCT group both in the original cohort and PSM cohort. (Table 2). The median number of removed lymph nodes were similar in both groups, with 30 (range 20–64) in the NICT group and 27 (range 20–50) in the NCT group. The adjuvant treatment was decided by multidisciplinary team according to the efficacy and safety of neoadjuvant treatment, postoperative recovery and patient's informed willingness. In the NICT group, 21 (46.7%) patients received adjuvant therapy, including 17 (37.8%) patients receiving adjuvant immunochemotherapy, 2 (4.4%) patients receiving adjuvant immunotherapy, and 2 (4.4%) patient receiving adjuvant chemotherapy. The median cycle of immunotherapy was 7 (range, 1–17), the median cycle of chemotherapy was 2 (range, 1–4). In the NCT group, 17 (37.8%) patients received adjuvant chemotherapy, The median cycle of chemotherapy was 2 (range, 1–4).
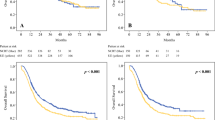
In the original cohort before matching, the median follow-up time was 34.3 (95% CI 33.2–35.7) months, with 45.0 (95% CI 40.8–50.3) months in the NCT group and 3.1 (95% CI 32.7–34.8) months in the NICT group. 25 (19.2%) patients of the NICT group and 18 (35.3%) patients of the NCT group experienced RFS events. The median RFS and median OS in both groups had not been reached yet (Fig. 1). The hazard ratio (HR) was 0.477 (95% CI 0.260–0.875, p = 0.014) for RFS and 0.394 (95% CI 0.197–0.789, p = 0.0065) for OS in the NICT group versus the NCT group. 2-year, 3-year RFS rate were 83.1% (95% CI 76.9–89.8%) and 79.8% (95% CI 72.9–87.4%) in the NICT group and 66.7% (95% CI 54.9–80.9%) and 64.6% (95% CI 52.8–79.2%) in the NCT group. 2-year, 3-year OS rate were 89.2% (95% CI 84.0–94.7%) and 86.3% (95% CI 80.3–92.7%) in the NICT group and 76.5% (95% CI 65.7–89.0%) and 70.0% (95% CI 58.3–84.0%) in the NCT group.
After PSM, the median follow-up time was 36.0 (95% CI 33.1–42.7) months, with 45.4 (95% CI 42.9–57.6) in the NCT group and 31.7 (95% CI 29.7–36.0) in the NICT group. 8 (17.8%) patients of the NICT group and 17 (37.8%) patients of the NCT group experienced RFS events. The median RFS and median OS in both groups had not been reached yet (Fig. 1). The hazard ratio (HR) was 0.396 (95% CI 0.171–0.919, p = 0.025) for RFS and 0.377 (95% CI 0.145–0.981, p = 0.038) for OS in the NICT group versus the NCT group. 2-year, 3-year RFS rate were 86.7% (95% CI 77.3–97.2%) and 80.6% (95% CI 68.9–94.1%) in the NICT group and 64.4% (95% CI 51.9–80.1%) and 62.1% (95% CI 49.5–78.1%) in the NCT group. 2-year, 3-year OS rate were 91.1% (95% CI 83.1–99.8%) and 86.2% (95% CI 76.6–97.1%) in the NICT group and 75.6% (95% CI 64.0–89.2%) and 68.1% (95% CI 55.6–83.5%) in the NCT group. The pCR, MPR, tumor down-staging patients have the significantly better survival outcomes (Table 3, Fig. 2). The univariable and multivariable Cox regression analysis identified the baseline factors including clinical N stage, ECOG PS score, neoadjuvant therapy regimen as independent predictors associated with RFS (Table 4).
Kaplan–Meier estimates of RFS and OS stratified by pathological responses of the PSM cohort. A RFS of the pCR group and the non-PCR group. B OS of the pCR group and the non-PCR group. C RFS of the MPR group and the non-MPR group. D OS of the MPR group and the non-MPR group. E RFS of the tumor downstaging group and not achieving tumor downstaging group. F OS of the tumor downstaging group and not achieving tumor downstaging group
Safety profile
The treatment-related adverse events (TRAEs) were comparable between two groups (Supplementary Table 1). In the PSM cohort, twenty (44.4%) and 6 (13.3%) patients of the NICT group developed grade 1–2 and grade 3–4 TRAEs, respectively. Twenty-one (46.7%) and 5 (11.1%) patients developed grade 1–2 and grade 3–4 TRAEs in the NCT group. However, the most common grade 3–4 TRAEs were neutropenia (6.7%, 6.7%), leukopenia (6.7%, 4.4%) in both groups.
No patients reported intraoperative complications. Postoperative complications were also comparable between both groups (Supplementary Table 2). In the PSM cohort, twenty-one (46.7%) and 21 (44.4%) patients developed grade 1–2 postoperative complications in the NICT group and NCT group, respectively. Two (4.4%) patients in the NICT group occurred grade 3–4 postoperative complications, with one patient had both grade 3 pulmonary infection and acute respiratory failure, and one had both grade 3 pulmonary infection. One (2.2%) patient in the NCT group had grade 4 pulmonary infection. No surgical mortality was reported in both groups.
Discussion
This is the first 3-year follow-up outcomes of neoadjuvant immunochemotherapy versus chemotherapy for ESCC. The results preliminarily show sintilimab combined with chemotherapy have pathological and survival benefit comparable to chemotherapy alone, without increasing the postoperative complications.
To date, the majority of previous studies on NICT just released the pathological outcomes. The first phase 3 ESCORT-NEO study compared NICT with NCT alone in resectable locally advanced ESCC. An early look at the data showed the pCR rate was significantly higher in the camrelizumab plus chemotherapy arms (28.0% in albumin-bound paclitaxel and 15.4% in paclitaxel arm) compared with chemotherapy alone (4.7%) (Li et al. 2024). In our study, the pCR rate was 28.9% in the sintilimab combined with chemotherapy group and 8.9% in the chemotherapy group in the PSM cohort, which were consistent with the results of ESCORT-NEO study and showed pathological outcomes benefit of NICT compared with the NCT.
The follow-up data was not matured of ESCORT-NEO study. The other prospective studies that released survival outcomes were all single-arm studies and the follow-up period was relatively short. A phase 2 study showed that the 1-year DFS and OS of neoadjuvant sintilimab and chemotherapy were 68.3% and 90.8% after a median follow-up of 14.6 months (Zhang et al. 2022b). The KEEP-G 03 study showed that the 1-year DFS of neoadjuvant sintilimab and chemotherapy was 78.9% after a median follow-up of 17.3 months (Chen et al. 2023b). Two-year outcomes from phase 2 NICE study showed that the 2-year OS and RFS rates were 78.1% and 67.9% after a median follow-up of 27.4 months (Yang et al. 2024). A previous 1:1 PSM analysis shown that the 2-year DFS rates of the NICT group and in NCT groups were 80.7% and 63.8% (HR, 0.448, P = 0.046), the 2-year OS rates in the NICT group was 83.2% and 72.3% in the NCT group (HR, 0.564, P = 0.189) (Jing et al. 2022). In our study, after a followed-up time of 3 years, the results showed the 3-year RFS and OS rate of NICT were 80.6% and 86.2% compared to 62.1% and 68.1% of NCT. Overall, all these survival outcomes preliminarily showed the survival benefit when combined with PD-1 inhibitor and chemotherapy in the neoadjuvant setting.
Currently, NCRT is another important standard treatment choice for locally advanced ESCC based on the CROSS study and NEOCRTEC5010 study, the pCR rate was more than 40% (van et al. 2012; Yang et al. 2018, 2021; Eyck et al. 2021). However, the improvements in pathological response did not translate into survival benefit. The long-term survival results demonstrated no significant differences between the NCRT and NCT (Zhang et al. 2022a; Kato et al. 2022). The poor control of occult systemic metastasis was believed one of the top most reasons (Yang et al. 2021; Nakashima et al. 2018; Pasini et al. 2005; Shapiro et al. 2015). The CROSS and NEOCRTEC5010 study showed the decrease in distant progression of the NCRT was mainly during the first 24 months (Yang et al. 2021; Shapiro et al. 2015). An inverse probability of treatment weighting (IPTW) analysis showed NICT and NCRT had the comparable R0 resection rate and pCR rate. However, the patients received NICT exhibited a better prognosis than NCRT patients, the 3-year OS rates were 91.7% and 79.8% (P = 0.032) and the 3-year DFS rates were 87.4% and 72.8% (P = 0.039) (Yu et al. 2024). The DFS and OS rate of NICT in this study during the first 36 months increased numerically compared with NCRT in previous studies (Yang et al. 2021; Shapiro et al. 2015; Wang et al. 2021b; Yu et al. 2024). Moreover, the presence of the whole tumor allows more immunogenic cell death induced by chemotherapy and broader T cell response, establish systemic immune surveillance (Versluis et al. 2020; Emens et al. 2015; Topalian et al. 2020). On the other hand, NCRT might increase the risk of severe adverse events, the postoperative complications and mortality (Kumagai et al.2014), but no increase in postoperative complications and mortality were observed with NICT in this study. Therefore, NICT might be the more optimized clinical strategy and could achieve greater clinical benefits.
Further analysis revealed that the pCR, MPR, tumor downstaging patients have significantly better survival outcomes. The result from a single-arm prospective study also revealed patients who achieved MPR had improved DFS and OS (Wang et al. 2023). The results preliminarily indicate that pCR and MPR might be used as alternative survival indicators for NICT, which is consistent with previous findings in NCT and NCRT (Rizvi et al. 2014; Blum et al. 2017; CHIU et al. 2020).
There are several limitations in this study. First, this retrospective study could potentially lead to bias. We tried our best to improve the comparability through PSM method, but the sample size is limited. Therefore, these findings required further validation by prospective head-to-head comparison studies. Second, the use of adjuvant therapy in the groups may potentially affect the outcomes. Third, longer follow-up is necessary to validate the long-term benefits of NICT compared to NCT for locally advanced ESCC.
Conclusions
This trial preliminarily shows that NICT followed by esophagectomy improves pathological and survival outcomes over NCT among patients with resectable locally advanced ESCC, with acceptable and manageable safety. Long term survival validation is still needed and prospective randomized or head-to-head comparison studies are warranted.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE (2009) Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 27:5062–5067
Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, Nakamura T, Yabusaki H, Aoyama N, Kurita A, Ikeda K, Kanda T, Tsujinaka T, Nakamura K, Fukuda H (2012) A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 19:68–74
Blum MM, Xiao L, Patel VR, Maru DM, Correa AM, Amlashi GF, Liao Z, Komaki R, Lin SH, Skinner HD, Vaporciyan A, Walsh GL, Swisher SG, Sepesi B, Lee JH, Bhutani MS, Weston B, Hofstetter WL, Ajani JA (2017) Pathological complete response in patients with esophageal cancer after the trimodality approach: the association with baseline variables and survival-The University of Texas MD Anderson Cancer Center experience. Cancer 123:4106–4113
Chan KKW, Saluja R, Delos Santos K, Lien K, Shah K, Cramarossa G, Zhu X, Wong RKS (2018) Neoadjuvant treatments for locally advanced, resectable esophageal cancer: a network meta-analysis. Int J Cancer 143:430–437
Chen R, Zheng R, Zhang S, Wang S, Sun K, Zeng H, Li L, Wei W, He J (2023a) Patterns and trends in esophageal cancer incidence and mortality in China: an analysis based on cancer registry data. J Natl Cancer Cent 3:21–27
Chen X, Xu X, Wang D, Liu J, Sun J, Lu M, Wang R, Hui B, Li X, Zhou C, Wang M, Qiu T, Cui S, Sun N, Li Y, Wang F, Liu C, Shao Y, Luo J, Gu Y (2023b) Neoadjuvant sintilimab and chemotherapy in patients with potentially resectable esophageal squamous cell carcinoma (KEEP-G 03): an open-label, single-arm, phase 2 trial. J Immunother Cancer 11:e005830
Chiu CH, Zhang P, Chang AC, Derstine BA, Ross BE, Enchakalody B, Shah NV, Wang SC, Chao YK, Lin J (2020) Morphomic factors associated with complete response to neoadjuvant therapy in esophageal carcinoma. Ann Thorac Surg 109:241–248
Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, Ogata T, Kawakami H, Hsu CH, Adenis A, El Hajbi F, di Bartolomeo M, Braghiroli MI, Holtved E, Ostoich SA, Kim HR, Ueno M, Mansoor W, Yang WC, Liu T, Bridgewater J, Makino T, Xynos I, Liu X, Lei M, Kondo K, Patel A, Gricar J, Chau I, Kitagawa Y, CheckMate 648 Trial Investigators (2022) Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med 386:449–462
Emens LA, Middleton G (2015) The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res 3:436–443
Eyck BM, van Lanschot JJB, Hulshof MCCM, van der Wilk BJ, Shapiro J, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, van Laarhoven HWM, Nieuwenhuijzen GAP, Hospers GAP, Bonenkamp JJ, Cuesta MA, Blaisse RJB, Busch OR, Creemers GM, Punt CJA, Plukker JTM, Verheul HMW, Spillenaar Bilgen EJ, van der Sangen MJC, Rozema T, Ten Kate FJW, Beukema JC, Piet AHM, van Rij CM, Reinders JG, Tilanus HW, Steyerberg EW, van der Gaast A, CROSS Study Group (2021) Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: the randomized controlled CROSS trial. J Clin Oncol 39:1995–2004
Jing SW, Zhai C, Zhang W, He M, Liu QY, Yao JF, Wang R, Tian ZQ, Wang J, Liu JF (2022) Comparison of neoadjuvant immunotherapy plus chemotherapy versus chemotherapy alone for patients with locally advanced esophageal squamous cell carcinoma: a propensity score matching. Front Immunol 7(13):970534
Kato K, Ito Y, Daiko H, Ozawa S, Ogata T, Hara H, Kojima T, Abe T, Bamba T, Watanabe M, Kawakubo H, Shibuya Y, Tsubosa Y, Takegawa N, Kajiwara T, Baba H, Ueno M, Machida R, Nakamura K, Kitagawa Y (2022) A randomized controlled phase III trial comparing two chemotherapy regimen and chemoradiotherapy regimen as neoadjuvant treatment for locally advanced esophageal cancer, JCOG1109 NExT study. J Clin Oncol 40(suppl 4):238–238
Kumagai K, Rouvelas I, Tsai JA, Mariosa D, Klevebro F, Lindblad M, Ye W, Lundell L, Nilsson M (2014) Meta-analysis of postoperative morbidity and perioperative mortality in patients receiving neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal and gastro-oesophageal junctional cancers. Br J Surg 101:321–338
Li Z, Sun Y, Ye F, Ma D, Yin X, Zhuang W, Yuan X, Qin S, Zhang Y, Gu K, Zhao K, Xiao J, Cheng Y, Bai Y, Luo S, Wang L, Wang C, Cui Y, Mei L, Shen L (2021) First-line pembrolizumab plus chemotherapy versus chemotherapy in patients with advanced esophageal cancer: Chinese subgroup analysis of KEYNOTE-590. J Clin Oncol 39(suppl 15):4049
Li Y, Qin J, Xue L, Hao A, Jiang T, Liu S, Jiang H, Kang M, Li H, Tian H, Fu J, Ma J, Fu M, Han Y, Chen L, Tan L, Dai T, Liao Y, Zhang W, Li B (2024) Chemotherapy plus camrelizumab versus chemotherapy alone as neoadjuvant treatment for resectable esophageal squamous cell carcinoma (ESCORT-NEO): a multi-center, randomized phase III trial. J Clin Oncol 42(suppl 3). https://doi.org/10.1200/JCO.2024.42.3_suppl.LBA244
Liu J, Yang Y, Liu Z, Fu X, Cai X, Li H, Zhu L, Shen Y, Zhang H, Sun Y, Chen H, Yu B, Zhang R, Shao J, Zhang M, Li Z (2022a) Multicenter, single-arm, phase II trial of camrelizumab and chemotherapy as neoadjuvant treatment for locally advanced esophageal squamous cell carcinoma. J Immunother Cancer 10:e004291
Liu J, Li J, Lin W, Shao D, Depypere L, Zhang Z, Li Z, Cui F, Du Z, Zeng Y, Jiang S, He P, Gu X, Chen H, Zhang H, Lin X, Huang H, Lv W, Cai W, Liang W, Liang H, Jiang W, Wang W, Xu K, Cai W, Wu K, Lerut T, Fu J, He J (2022b) Neoadjuvant camrelizumab plus chemotherapy for resectable, locally advanced esophageal squamous cell carcinoma (NIC-ESCC2019): a multicenter, phase 2 study. Int J Cancer 151:128–137
Lu Z, Wang J, Shu Y, Liu L, Kong L, Yang L, Wang B, Sun G, Ji Y, Cao G, Liu H, Cui T, Li N, Qiu W, Li G, Hou X, Luo H, Xue L, Zhang Y, Yue W, Liu Z, Wang X, Gao S, Pan Y, Galais MP, Zaanan A, Ma Z, Li H, Wang Y, ORIENT-15 study group (2022) Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial. BMJ 377:e068714
Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q, Zhang Y, Zhao K, Chen Z, Gao S, Li J, Fu Z, Gu K, Liu Z, Wu L, Zhang X, Feng J, Niu Z, Ba Y, Zhang H, Liu Y, Zhang L, Min X, Huang J, Cheng Y, Wang D, Shen Y, Yang Q, Zou J, Xu RH (2021) Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA 326:916–925
Lv H, Tian Y, Li J, Huang C, Sun B, Gai C, Li Z, Tian Z (2022) Neoadjuvant sintilimab plus chemotherapy in resectable locally advanced esophageal squamous cell carcinoma. Front Oncol 12:864533
Lv H, Huang C, Li J, Zhang F, Gai C, Liu Z, Xu S, Wang M, Li Z, Tian Z (2023) The survival outcomes of neoadjuvant sintilimab combined with chemotherapy in patients with locally advanced esophageal squamous cell carcinoma. Front Immunol 13:1100750
Medical Research Council Oesophageal Cancer Working Group (2002) Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 359:1727–1733
Nakashima Y, Saeki H, Hu Q, Tsuda Y, Hisamatsu Y, Ando K, Oki E, Maehara Y (2018) Neoadjuvant chemotherapy versus chemoradiotherapy for patients with esophageal squamous cell carcinoma. Anticancer Res 38:6809–6814
Pasini PF, de Manzoni G, Pedrazzani C, Grandinetti A, Durante E, Gabbani M, Tomezzoli A, Griso C, Guglielmi A, Pelosi G, Maluta S, Cetto GL, Cordiano C (2005) High pathological response rate in locally advanced esophageal cancer after neoadjuvant combined modality therapy: dose finding of a weekly chemotherapy schedule with protracted venous infusion of 5-fluorouracil and dose escalation of cisplatin, docetaxel and concurrent radiotherapy. Ann Oncol 16:1133–1139
Rizvi FH, Syed AA, Khattak S, Rizvi SS, Kazmi SA, Khan MQ (2014) Complete pathological response after neoadjuvant treatment in locally advanced esophageal cancer predicts long term survival: a retrospective cohort study. Int J Surg 12:621–625
Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, van Laarhoven HWM, Nieuwenhuijzen GAP, Hospers GAP, Bonenkamp JJ, Cuesta MA, Blaisse RJB, Busch ORC, Ten Kate FJW, Creemers GM, Punt CJA, Plukker JTM, Verheul HMW, Bilgen EJS, van Dekken H, van der Sangen MJC, Rozema T, Biermann K, Beukema JC, Piet AHM, van Rij CM, Reinders JG, Tilanus HW, Steyerberg EW, van der Gaast A (2015) Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 16:1090–1098
Shen D, Chen Q, Wu J, Li J, Tao K, Jiang Y (2021) The safety and efficacy of neoadjuvant PD-1 inhibitor with chemotherapy for locally advanced esophageal squamous cell carcinoma. J Gastrointest Oncol 12:1–10
Song Y, Zhang B, Xin D, Kou X, Tan Z, Zhang S, Sun M, Zhou J, Fan M, Zhang M, Song Y, Li S, Yuan Y, Zhuang W, Zhang J, Zhang L, Jiang H, Gu K, Ye H, Ke Y, Li J, Wang Q, Zhu J, Huang J, ASTRUM-007 investigators (2023) First-line serplulimab or placebo plus chemotherapy in PD-L1-positive esophageal squamous cell carcinoma: a randomized, double-blind phase 3 trial. Nat Med 29(2):473–482
Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, Kojima T, Metges JP, Li Z, Kim SB, Cho BC, Mansoor W, Li SH, Sunpaweravong P, Maqueda MA, Goekkurt E, Hara H, Antunes L, Fountzilas C, Tsuji A, Oliden VC, Liu Q, Shah S, Bhagia P, Kato K (2021) Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 398:759–771
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249
Tang H, Wang H, Fang Y, Zhu JY, Yin J, Shen YX, Zeng ZC, Jiang DX, Hou YY, Du M, Lian CH, Zhao Q, Jiang HJ, Gong L, Li ZG, Liu J, Xie DY, Li WF, Chen C, Zheng B, Chen KN, Dai L, Liao YD, Li K, Li HC, Zhao NQ, Tan LJ (2023) Neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy followed by minimally invasive esophagectomy for locally advanced esophageal squamous cell carcinoma: a prospective multicenter randomized clinical trial. Ann Oncol 34:163–172
Topalian SL, Taube JM, Pardoll DM (2020) Neoadjuvant checkpoint blockade for cancer immunotherapy. Science 367:eaax0182
van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, ten Kate FJ, Creemers GJ, Punt CJ, Plukker JT, Verheul HM, Spillenaar Bilgen EJ, van Dekken H, van der Sangen MJ, Rozema T, Biermann K, Beukema JC, Piet AH, van Rij CM, Reinders JG, Tilanus HW, van der Gaast A, CROSS Group (2012) Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 366:2074–2084
Versluis JM, Long GV, Blank CU (2020) Learning from clinical trials of neoadjuvant checkpoint blockade. Nat Med 26:475–484
Wang HH, de Heer EC, Hulshoff JB, Kats-Ugurlu G, Burgerhof JGM, van Etten B, Plukker JTM, Hospers GAP, Dutch Upper Gastrointestinal Cancer Audit (DUCA) Group (2021a) Effect of extending the original CROSS criteria on tumor response to neoadjuvant chemoradiotherapy in esophageal cancer patients: a national multicenter cohort analysis. Ann Surg Oncol 28:3951–3960
Wang H, Tang H, Fang Y, Tan L, Yin J, Shen Y, Zeng Z, Zhu J, Hou Y, Du M, Jiao J, Jiang H, Gong L, Li Z, Liu J, Xie D, Li W, Lian C, Zhao Q, Chen C, Zheng B, Liao Y, Li K, Li H, Wu H, Dai L, Chen KN (2021b) Morbidity and mortality of patients who underwent minimally invasive esophagectomy after neoadjuvant chemoradiotherapy vs neoadjuvant chemotherapy for locally advanced esophageal squamous cell carcinoma: a randomized clinical trial. JAMA Surg 156:444–451
Wang ZX, Cui C, Yao J, Zhang Y, Li M, Feng J, Yang S, Fan Y, Shi J, Zhang X, Shen L, Shu Y, Wang C, Dai T, Mao T, Chen L, Guo Z, Liu B, Pan H, Cang S, Jiang Y, Wang J, Ye M, Chen Z, Jiang D, Lin Q, Ren W, Wang J, Wu L, Xu Y, Miao Z, Sun M, Xie C, Liu Y, Wang Q, Zhao L, Li Q, Huang C, Jiang K, Yang K, Li D, Liu Y, Zhu Z, Chen R, Jia L, Li W, Liao W, Liu HX, Ma D, Ma J, Qin Y, Shi Z, Wei Q, Xiao K, Zhang Y, Zhang Y, Chen X, Dai G, He J, Li J, Li G, Liu Y, Liu Z, Yuan X, Zhang J, Fu Z, He Y, Ju F, Liu Z, Tang P, Wang T, Wang W, Zhang J, Luo X, Tang X, May R, Feng H, Yao S, Keegan P, Xu RH, Wang F (2022) Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): a multi-center phase 3 trial. Cancer Cell 40:277-288.e3
Wang H, Jiang Z, Wang Q, Wu T, Guo F, Xu Z, Yang W, Yang S, Feng S, Wang X, Chen S, Cheng C, Chen W (2023) Pathological response and prognostic factors of neoadjuvant PD-1 blockade combined with chemotherapy in resectable oesophageal squamous cell carcinoma. Eur J Cancer 186:196–210
Xu J, Kato K, Raymond E, Hubner RA, Shu Y, Pan Y, Park SR, Ping L, Jiang Y, Zhang J, Wu X, Yao Y, Shen L, Kojima T, Gotovkin E, Ishihara R, Wyrwicz L, Van Cutsem E, Jimenez-Fonseca P, Lin CY, Wang L, Shi J, Li L, Yoon HH (2023) Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first-line treatment for advanced or metastatic oesophageal squamous cell carcinoma (RATIONALE-306): a global, randomised, placebo-controlled, phase 3 study. Lancet Oncol 24:483–495
Yan X, Duan H, Ni Y, Zhou Y, Wang X, Qi H, Gong L, Liu H, Tian F, Lu Q, Sun J, Yang E, Zhong D, Wang T, Huang L, Wang J, Wang C, Wang Y, Wan Z, Lei J, Zhao J, Jiang T (2022) Tislelizumab combined with chemotherapy as neoadjuvant therapy for surgically resectable esophageal cancer: a prospective, single-arm, phase II study (TD-NICE). Int J Surg 103:106680
Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, Mao W, Xiang J, Han Y, Chen Z, Yang H, Wang J, Pang Q, Zheng X, Yang H, Li T, Lordick F, D’Journo XB, Cerfolio RJ, Korst RJ, Novoa NM, Swanson SJ, Brunelli A, Ismail M, Fernando HC, Zhang X, Li Q, Wang G, Chen B, Mao T, Kong M, Guo X, Lin T, Liu M, Fu J, AME Thoracic Surgery Collaborative Group (2018) Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol 36:2796–2803
Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, Mao W, Xiang J, Han Y, Chen Z, Yang H, Wang J, Pang Q, Zheng X, Yang H, Li T, Zhang X, Li Q, Wang G, Chen B, Mao T, Kong M, Guo X, Lin T, Liu M, Fu J (2021) Long-term efficacy of neoadjuvant chemoradiotherapy plus surgery for the treatment of locally advanced esophageal squamous cell carcinoma: the NEOCRTEC5010 randomized clinical trial. JAMA Surg 156:721–729
Yang W, Xing X, Yeung SJ, Wang S, Chen W, Bao Y, Wang F, Feng S, Peng F, Wang X, Chen S, He M, Zhang N, Wang H, Zeng B, Liu Z, Kidane B, Seder CW, Koyanagi K, Shargall Y, Luo H, Peng S, Cheng C (2022) Neoadjuvant programmed cell death 1 blockade combined with chemotherapy for resectable esophageal squamous cell carcinoma. J Immunother Cancer 10:e003497
Yang Y, Li H, Chen X, Qin J, Li Y, Shen Y, Zhang R, Kang X, Wang Z, Zheng Q, Luo P, Li Y, He J (2023) Comparison of neoadjuvant nab-paclitaxel plus immunotherapy versus paclitaxel plus immunotherapy for esophageal squamous cell carcinoma. Thorac Cancer 14:700–708
Yang Y, Liu J, Liu Z, Zhu L, Chen H, Yu B, Zhang R, Shao J, Zhang M, Li C, Li Z (2024) Two-year outcomes of clinical N2–3 esophageal squamous cell carcinoma after neoadjuvant chemotherapy and immunotherapy from the phase 2 NICE study. J Thorac Cardiovasc Surg 167(3):838-847.e1
Yin GQ, Li ZL, Li D (2022) The safety and efficacy of neoadjuvant camrelizumab plus chemotherapy in patients with locally advanced esophageal squamous cell carcinoma: a retrospective study. Cancer Manag Res 14:2133–2141
Yu YK, Meng FY, Wei XF, Chen XK, Li HM, Liu Q, Li CJ, Xie HN, Xu L, Zhang RX, Xing W, Li Y (2024) Neoadjuvant chemotherapy combined with immunotherapy versus neoadjuvant chemoradiotherapy in patients with locally advanced esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg S0022–5223(24):00008–00014
Zhang Z, Hong ZN, Xie S, Lin W, Lin Y, Zhu J, Yang X, Lin Z, Lin J, Kang M (2021) Neoadjuvant sintilimab plus chemotherapy for locally advanced esophageal squamous cell carcinoma: a single-arm, single-center, phase 2 trial (ESONICT-1). Ann Transl Med 9:1623
Zhang G, Zhang C, Sun N, Xue L, Yang Z, Fang L, Zhang Z, Luo Y, Gao S, Xue Q, Mu J, Gao Y, Tan F, He J (2022a) Neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy for the treatment of esophageal squamous cell carcinoma: a propensity score-matched study from the national cancer center in China. J Cancer Res Clin Oncol 148:943–954
Zhang Z, Ye J, Li H, Gu D, Du M, Ai D, Chen W, Fang Y, Xu X, Bai C, Zhao K, Zhou G (2022b) Neoadjuvant sintilimab and chemotherapy in patients with resectable esophageal squamous cell carcinoma: a prospective, single-arm, phase 2 trial. Front Immunol 13:1031171
Zhang G, Yuan J, Pan C, Xu Q, Cui X, Zhang J, Liu M, Song Z, Wu L, Wu D, Luo H, Hu Y, Jiao S, Yang B (2023a) Multi-omics analysis uncovers tumor ecosystem dynamics during neoadjuvant toripalimab plus nab-paclitaxel and S-1 for esophageal squamous cell carcinoma: a single-center, open-label, single-arm phase 2 trial. EBioMedicine 90:104515
Zhang B, Zhao H, Wu X, Gong L, Yang D, Li X, Chen X, Li J, Wang W, Wu J, Xiao Q (2023b) Perioperative outcomes of neoadjuvant chemotherapy plus camrelizumab compared with chemotherapy alone and chemoradiotherapy for locally advanced esophageal squamous cell cancer. Front Immunol 14:1066527
Zheng RS, Chen R, Han BF, Wang SM, Li L, Sun KX, Zeng HM, Wei WW, He J (2024) Cancer incidence and mortality in China, 2022. Zhonghua Zhong Liu Za Zhi 46(3):221–231
Funding
This work was supported by the Natural Science Foundation of Hebei Province (Grant number: H2022206443) and government-funded clinical medical talent training project of Hebei Province (Grant number: ZF2024118).
Author information
Authors and Affiliations
Contributions
Huilai Lv: Conceptualization, Data curation, Investigation, Methodology, Funding acquisition, Writing – original draft, Writing – review & editing. Fan Zhang: Data curation, Formal Analysis, Writing – original draft. Chao Huang: Data curation, Investigation, Formal Analysis. Shi Xu: Data curation, Investigation. Jiachen Li: Data curation, Investigation. Bokang Sun: Data curation, Investigation. Chunyue Gai: Data curation, Investigation. Zhao Liu: Data curation, Investigation. Mingbo Wang: Data curation, Investigation. Zhenhua Li: Data curation, Validation. Ziqiang Tian: Conceptualization, Methodology, Project administration, Supervision, Validation, Writing – review & editing.
The work reported in the paper has been performed by all authors. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki, and approved by the Ethics Committee of The Fourth Hospital of Hebei Medical University. Written informed consent were waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lv, H., Zhang, F., Huang, C. et al. Survival outcomes of neoadjuvant immunochemotherapy versus chemotherapy for locally advanced esophageal squamous cell carcinoma. J Cancer Res Clin Oncol 150, 260 (2024). https://doi.org/10.1007/s00432-024-05793-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00432-024-05793-4