Abstract
Background
There is currently no consensus on the optimal interval time between neoadjuvant therapy and surgery, and whether prolonged time interval from neoadjuvant therapy to surgery results in bad outcomes for locally advanced esophageal squamous cell carcinoma (ESCC). In this study, we aim to evaluate outcomes of time intervals ≤ 8 weeks and > 8 weeks in locally advanced ESCC.
Methods
This retrospective study consecutively included ESCC patients who received esophagectomy after neoadjuvant camrelizumab combined with chemotherapy at the Department of Thoracic Surgery, the First Affiliated Hospital, Zhejiang University School of Medicine. The primary endpoints were disease-free survival (DFS) and overall survival (OS), while the secondary endpoints were pathological response, surgical outcomes, and postoperative complications.
Results
From 2019 to 2021, a total of 80 patients were included in our study and were divided into two groups according to the time interval from neoadjuvant immunochemotherapy to surgery: ≤ 8 weeks group (n = 44) and > 8 weeks group (n = 36). The rate of MPR in the ≤ 8 weeks group was 25.0% and 27.8% in the > 8 weeks group (P = 0.779). The rate of pCR in the ≤ 8 weeks group was 11.4%, with 16.7% in the > 8 weeks group (P = 0.493). The incidence of postoperative complications in the ≤ 8 weeks group was 27.3% and 19.4% in the > 8 weeks group (P = 0.413). The median DFS in the two groups had not yet reached (hazard ratio [HR], 3.153; 95% confidence interval [CI] 1.383 to 6.851; P = 0.004). The median OS of ≤ 8 weeks group was not achieved (HR, 3.703; 95% CI 1.584 to 8.657; P = 0.0012), with the > 8 weeks group 31.6 months (95% CI 21.1 to 42.1). In multivariable analysis, inferior DFS and OS were observed in patients with interval time > 8 weeks (HR, 2.992; 95% CI 1.306 to 6.851; and HR, 3.478; 95% CI 1.481 to 8.170, respectively).
Conclusions
Locally advanced ESCC patients with time interval from neoadjuvant camrelizumab combined with chemotherapy to surgery > 8 weeks were associated with worse long-term survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal cancer (EC) is the seventh most prevalent tumor and the sixth most common cause of cancer-related death worldwide (Siegel et al. 2022). Among its two histological subtypes, esophageal squamous cell carcinoma (ESCC) is more common in Asia, accounting for approximately 90% of EC cases in China. (Arnold et al. 2015; Zhang 2013). And most patients are diagnosed with locally advanced EC (Yang et al. 2023). Nowadays, the standard treatment for patients with locally advanced EC is neoadjuvant therapy [chemotherapy (Ando et al. 2012), chemoradiotherapy (Hagen et al. 2012; Yang et al. 2018), immunochemotherapy (Yan et al. 2022; Liu et al. 2022), chemoradiotherapy plus immunotherapy (Li et al. 2021)] followed by surgical resection. Neoadjuvant treatment can reduce the tumor size, lower the tumor stage, and subsequent surgical resection can remove the tumor more thoroughly and result in better outcomes.
Although neoadjuvant therapy followed by surgery has been recommended as the standard treatment for locally advanced EC, there still has been no consensus on the optimal interval time between neoadjuvant therapy and surgery. Surgical procedure was usually suggested after an interval of 4 to 8 weeks after completion of neoadjuvant treatment in current clinical studies (Hagen et al. 2012; Yang et al. 2018; Mukherjee et al. 2017; Haisley et al. 2016). However, surgical resection may sometimes be performed beyond this time frame owing to adverse events of neoadjuvant therapy, personal or logistic reasons. Some studies showed that a prolonged interval between neoadjuvant therapy and esophagectomy resulted in similar outcomes (Kim et al. 2012; Kathiravetpillai et al. 2016; Nilsson et al. 2020). Several studies reported that a prolonged interval between neoadjuvant therapy and esophagectomy was associated with a higher pathological response rate and similar long-term survival (Shapiro et al. 2014; Lee et al. 2016; Klevebro et al. 2020). Several studies revealed increased pathological response with prolonged interval, but worse long-term survival (Levinsky et al. 2020; Franko et al. 2016; Ranney et al. 2017). Additionally, some studies found that a prolonged interval following neoadjuvant therapy before esophagectomy was associated with increased incidence of postoperative complications (Teman et al. 2013), increased mortality (Wang et al. 2015), and poorer long-term survival (Chidambaram et al. 2023). Therefore, we launched this retrospective study to explore whether time interval from neoadjuvant therapy to surgery affect outcomes for locally advanced ESCC. And the cut off of 8 weeks were usually reserved to distinguish between early surgery group and delayed surgery group (Tie et al. 2018; Qin et al. 2018; Shang et al. 2020; Karthyarth et al. 2023). Therefore, we set the interval time at 8 weeks in the current study.
Methods
Study design and patients
Our study was a retrospective study, which consecutively enrolled ESCC patients who received esophagectomy after neoadjuvant camrelizumab combined with chemotherapy at the Department of Thoracic Surgery, the First Affiliated Hospital, Zhejiang University School of Medicine. It had been permitted by the Clinical Research Ethics Committee of the First Affiliated Hospital of Zhejiang University School of Medicine (2021 IIT No. 742) and was in line with the Helsinki Declaration (revised in 2013) and Good Clinical Practice Guidelines.
Inclusion criteria included histopathologically diagnosed ESCC by gastroscopy, pre-treatment clinical stage II-IVA (according to the eighth edition of the AJCC TNM staging (Rice et al. 2017)), receipt of 2–4 cycles (3 weeks per cycle) of neoadjuvant camrelizumab (200 mg) combined with platinum-containing dualdrug chemotherapy (platinum + paclitaxel), age over 18 and under 80 years and Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Exclusion criteria were incomplete information at our hospital, previous anticancer treatment (such as radiotherapy, interventional therapy or drug treatment), autoimmune disease or infectious disease, ongoing systemic immunosuppressive treatment, other malignant tumors and distant metastases. These patients were split up into two groups according to time interval from neoadjuvant immunochemotherapy to surgery: ≤ 8 weeks group (n = 44) and > 8 weeks group (n = 36).
Treatment procedures and data collection
Immunotherapy regimen was camrelizumab 200 mg. Chemotherapy regimen included platinum (75 mg/m2 of cisplatin, or area under the curve (AUC) of the plasma concentration–time curve after a single dose = 5 of carboplatin, or 80 mg/m2 of nedaplatin) and paclitaxel (260 mg/m2 of albumin-bound paclitaxel). Before neoadjuvant treatment, systematic imaging evaluations were performed for all patients, including computed tomography (CT) of the esophagus, endoscopic ultrasound, positron emission tomography (PET)–CT, brain magnetic resonance imaging and abdominal ultrasound. During neoadjuvant therapy, CT of the esophagus was performed every 2 cycles until the patient underwent surgery or withdrew from treatment. Moreover, routine blood and biochemical blood examinations were conducted every week. And myocardial enzyme spectrum, thyroid function, and coagulation function examinations were done every 3 weeks. We evaluated patients’ gastrointestinal reactions and skin reactions by their complaints. The response evaluation criteria in solid tumor version 1.1 (RECIST 1.1) (Eisenhauer et al. 2009) was used to evaluate the tumor treatment response–complete response (CR): disappearance of all target lesions, partial remission (PR): ≥ 30% decline in the total diameter of target lesions, progressive disease (PD): ≥ 20% enlargement in the total diameter of target lesions or the appearance of new lesions, stable disease (SD): neither CR, PR nor PD. Objective response rate (ORR) included CR and PR. Adverse events (AEs) were graded on the basis of Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 (Common Terminology Criteria for Adverse Events (CTCAE), 2017).
Surgical approaches included open radical surgery, video-assisted thoracoscopic surgery (VATS), and robot-assisted thoracoscopic surgery (RATS). Surgical methods were comprised of Mc-Kewon and Ivor-Lewis. We considered Ivor-Lewis esophagectomy with at least a two-field lymph node dissection for inferior and medial ESCC, and McKeown esophagectomy with three-field lymph node dissection (neck, thoracic and abdominal lymph nodes) for superior ESCC. We adopted tumor regression grade (TRG) to express pathological response. TRG was divided into four categories according to the College of American Pathologists (CAP)/The National Comprehensive Cancer Network (NCCN) guidelines: TRG 0 (no remaining active tumor cells), TRG 1 (residual viable tumor cells ≤ 10%), TRG 2 (10% < residual viable tumor cells ≤ 50%) and TRG 3 (remaining active tumor cells > 50%). The pathological complete remission (pCR) rate and major pathological response (MPR) rate were considered as equal to TRG 0 and TRG 0–1 respectively. Postoperative complications were evaluated based on definitions proposed by the Esophagectomy Complications Consensus Group (ECCG) (Low et al. 2015).
After surgery, imaging assessments were conducted every 1–3 months. Patients continued to receive chemotherapy plus camrelizumab after surgery until the full 6 cycles, and then continued to receive camrelizumab alone for 1–2 years or until disease progression. And the follow-up date would not end until at least 1 year after surgery. The primary endpoints of this study were DFS and OS. DFS was defined as the time from surgery to disease progression according to the RECIST 1.1 or death, whichever occurred first. OS was defined as the time from surgery until death from any cause. Secondary endpoints of this study were pathological response (MPR and pCR), surgical outcomes and postoperative complications.
Statistical analysis
Categorical variables were expressed as frequencies (percentages), and continuous variables were shown as the median and interquartile range (IQR). Categorical variables were analyzed using the Chi-square test or Fisher’s exact test and continuous variables were compared with the t-test or Wilcoxon test. DFS and OS were estimated using the Kaplan–Meier method and compared with the stratified log-rank test. Median follow-up time was evaluated with the reverse Kaplan–Meier method. Stratified Cox proportional-hazards models were used to assess the correlation between each study variable and survival outcomes. Statistical analyses were performed using R software (version 4.1.2) and plotting was performed using GraphPad Prism version 9.0 (GraphPad Software, San Diego, CA, USA). A two-sided P value < 0.05 was considered to be statistically significant.
Results
Baseline characteristics
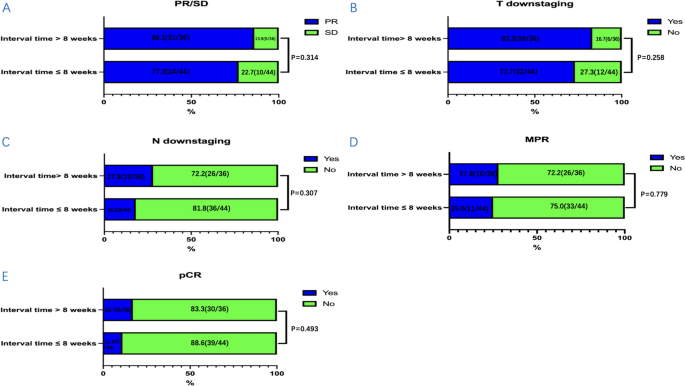
From 2019 to 2021, a total of 80 patients were included in our study and were divided into two groups according to the time interval from neoadjuvant immunochemotherapy to surgery: ≤ 8 weeks group (n = 44) and > 8 weeks group (n = 36). The median time to surgery was 51.0 days (IQR, 49.0–54.0 days) in the ≤ 8 weeks group and 96.0 days (IQR, 81.3–101.8 days) in the > 8 weeks group. Characteristics of these patients at baseline are shown in Table 1. There were no significant differences between the two groups in age, gender, ECOG performance status, smoking status, drinking status, comorbidities, pathological grade, tumor location, clinical stage, and treatment cycle. The ORR in the ≤ 8 weeks group was 77.3% and 86.1% in the > 8 weeks group (P = 0.314, Fig. 1A). The rate of T downstaging (assessed by CT before and after neoadjuvant immunochemotherapy) was 72.7% and 83.3% in the ≤ 8 weeks group and the > 8 weeks group, respectively (P = 0.258, Fig. 1B). The rate of N downstaging (assessed by CT before and after neoadjuvant immunochemotherapy) in the ≤ 8 weeks group was 18.2% and 27.8% in the > 8 weeks group (P = 0.307, Fig. 1C).
The distribution condition of clinical response, T downstaging, N downstaging and pathological response between the two groups: A PR/SD/PD, B T downstaging, C N downstaging, D MPR, and E pCR. Clinical response included partial remission (PR) and stable disease (SD). Pathological response included major pathological response (MPR) and pathological complete remission (pCR). T downstaging and N downstaging were assessed by CT before and after neoadjuvant immunochemotherapy. CT computed tomography
Adverse events
There were no previously unrecorded AEs in our study. Grade 3–4 AEs of neoadjuvant therapy were summarized in Table 2. The incidence of grade 3–4 AEs in the ≤ 8 weeks group was 27.3% and 36.1% in the > 8 weeks group. Grade 3–4 AEs were mainly distributed in hematological abnormalities (anemia). There were no significant differences in the occurrence of grade 3–4 AEs between the two groups. These AEs were quickly resolved after symptomatic treatment.
Surgical outcomes and pathological response
The outcomes of surgery and the pathological response were summarized in Table 3. There were no significant differences in the surgical approach, operation time, blood loss, length of hospital stays, TRG, and ypTNM stage between the two groups. The rate of R0 resection was 100.0% in the ≤ 8 weeks group and 97.2% in the > 8 weeks group. And more lymph nodes were removed during surgery in the ≤ 8 weeks group compared with the > 8 weeks group (P = 0.034). The rate of MPR in the ≤ 8 weeks group was 25.0% and 27.8% in the > 8 weeks group (P = 0.779, Fig. 1D). The rate of pCR in the ≤ 8 weeks group was 11.4%, with 16.7% in the > 8 weeks group (P = 0.493, Fig. 1E). Overall, the incidence of postoperative complications in the ≤ 8 weeks group was 27.3% and 19.4% in the > 8 weeks group (P = 0.413). There were no significant differences in the postoperative complications and no perioperative deaths occurred.
Survival
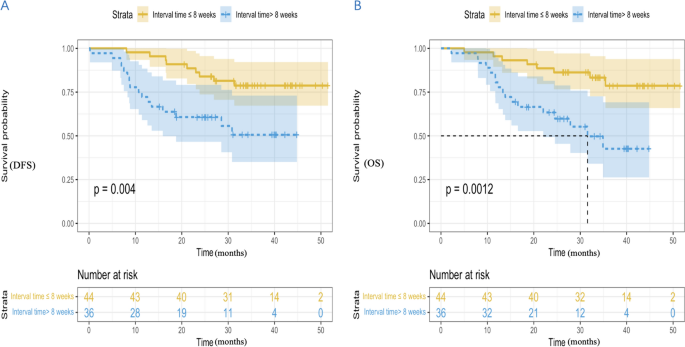
At the time of data cutoff (December 2023), the median follow-up time for the ≤ 8 weeks group was 35.7 months (95% confidence interval [CI] 32.5–39.0), while the median follow-up time for the > 8 weeks group was 31.0 months (95% CI 24.8–37.3). Among the ≤ 8 weeks group, 18.2% (8/44) patients experienced recurrence and metastasis, and 7 patients died due to recurrence and metastasis. Among the > 8 weeks group, 38.9% (14/36) patients experienced recurrence and metastasis, 1 patient died from COVID-19, and 14 patients died due to cancer recurrence and metastasis. The summary of recurrence and metastasis in the two groups is shown in Table 4.
The median DFS in the two groups had not yet reached (hazard ratio [HR], 3.153; 95% CI 1.383 to 6.851; P = 0.004) (Fig. 2A). The 1-year DFS rate, 2-year DFS rate, and 3-year DFS rate in the ≤ 8 weeks group were 97.7%, 84.1%, and 79.5%, with that in the > 8 weeks group 72.2%, 61.1% and 55.6%. The median OS of ≤ 8 weeks group was not achieved (HR, 3.703; 95% CI 1.584 to 8.657; P = 0.0012), with the > 8 weeks group 31.6 months (95% CI 21.1 to 42.1) (Fig. 2B). The 1-year OS rate, 2-year OS rate and 3-year OS rate in the ≤ 8 weeks group were 95.5%, 88.6% and 81.8%, with that in the > 8 weeks group 80.6%, 63.9% and 52.8%.
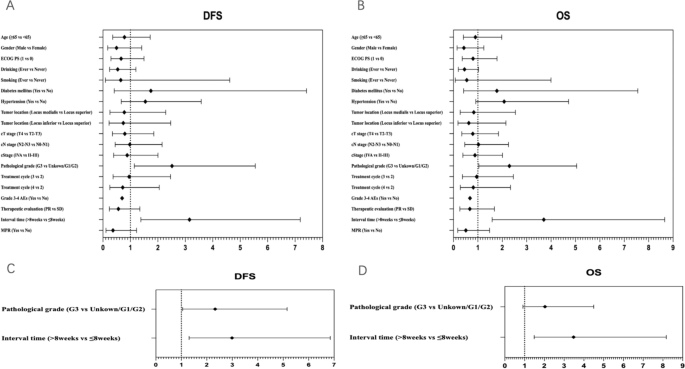
In univariable Cox regression analyses, there were no statistically significant correlations between these included factors and DFS (Fig. 3A) or OS (Fig. 3B), except for pathological grade and interval time. Patients with G3 had inferior DFS and OS (HR, 2.516; 95% CI 1.141 to 5.548; and HR, 2.292; 95% CI 1.040 to 5.051, respectively). Interval time > 8 weeks was associated with inferior DFS and OS (HR, 3.153; 95% CI 1.383 to 6.851; and HR, 3.703; 95% CI 1.584 to 8.657, respectively). Moreover, we performed multivariable Cox regression analyses on statistically significant factors identified through univariable analyses (Fig. 3C and D). Inferior DFS and OS were observed in patients with interval time > 8 weeks (HR, 2.992; 95% CI 1.306 to 6.851; and HR, 3.478; 95% CI 1.481 to 8.170, respectively). Patients with G3 were associated with inferior DFS (HR, 2.327; 95% CI 1.051 to 5.152), but not inferior OS (HR, 2.032; 95% CI 0.919 to 4.496). It can be seen that interval time ≤ 8 weeks independently predicted better survival.
Forest plot of hazard ratio of univariable and multivariable Cox regression analyses for DFS (A, C) and OS (B, D). DFS, disease-free survival, OS overall survival, ECOG PS eastern cooperative oncology group performance status, PR partial remission, SD stable disease, AEs adverse events, MPR major pathologic response
Discussion
Nowadays, there is still controversy over the outcomes of prolonged time intervals from neoadjuvant therapy to surgery for locally advanced esophageal squamous cell carcinoma (ESCC) (Kathiravetpillai et al. 2016; Shapiro et al. 2014; Franko et al. 2016; Teman et al. 2013). Therefore, we launched this retrospective study to explore whether the time interval from neoadjuvant therapy to surgery affects outcomes for locally advanced ESCC. Moreover, there is currently no consensus on the optimal interval time between neoadjuvant therapy and surgery. In clinical practice, the interval time has usually been set at 4 to 8 weeks (Yang et al. 2018; Haisley et al. 2016). In the current study, we set the interval time at 8 weeks. We found that the time interval from neoadjuvant camrelizumab combined with chemotherapy to surgery > 8 weeks was not associated with a difference in postoperative complications, postoperative morbidity, and pathological response. However, delaying surgery increases the risk of recurrence and metastasis for locally advanced ESCC patients. A longer interval between neoadjuvant therapy and surgery (> 8 weeks) was associated with worse long-term survival. Despite no significant differences in clinical oncologic factors (cStage) or surgical outcomes (R0 rate, complication) and tumor evaluation variables (pCR, TRG) between the two groups, the prognosis was poor in the surgery group after 8 weeks. In my opinion, the reasons for this result are as listed. Firstly, a longer waiting period may increase the risk of tumor repopulation, recurrence, and metastasis (Tessier et al. 2014; Chiu et al. 2013). Secondly, a longer waiting period was not a result of the patient’s preferences or opportunities, but rather because of their poor physical condition after neoadjuvant therapy, which may result in an inherent disadvantage in terms of survival. Finally, apart from clinical oncologic factors (cStage) or surgical outcomes (R0 rate, complication) and tumor evaluation variables (pCR, TRG), different factors have a significant impact on OS. Due to the various confounding factors of this issue, it may be necessary to conduct prospective randomized studies.
The findings of our study were different from the findings of other studies. Two studies and a meta-analysis showed there was no significant difference in the pathologic response and overall survival between timely esophagectomy and delayed esophagectomy (Kim et al. 2012; Tie et al. 2018; Tessier et al. 2014). A meta-analysis revealed a longer interval associated with unchanged pathological response and reduced overall survival (Lin et al. 2016). Three studies found a prolonged interval was associated with higher pathological response, without affecting survival (Haisley et al. 2016; Shapiro et al. 2014; Lee et al. 2016). Levinsky et al. and a meta-analysis showed that the delayed esophagectomy group (interval ≥ 90 days) had higher rates of pathological complete response and poorer overall survival (Levinsky et al. 2020; Qin et al. 2018). In our study, we found that there was no significant difference in the pathological response. The rates of MPR and pCR in the ≤ 8 weeks group and > 8 weeks group were similar (25.0% vs 27.8%, 11.4% vs 16.7%, P > 0.05). A longer interval (> 8 weeks) was associated with worse long-term survival. The median DFS in the two groups had not yet reached (P = 0.004). The median OS of the ≤ 8 weeks group was not achieved (P = 0.0012), with the > 8 weeks group at 31.6 months. The reasons for these differences may be different interval time, different neoadjuvant therapy regimens, and different treatment cycles.
In the study, we found that pathological grade (G3) and interval time > 8 weeks were associated with inferior DFS and OS in univariable Cox regression analyses. And after multivariable Cox regression analyses, inferior DFS and OS were observed in patients with interval time > 8 weeks. It can be seen that interval time ≤ 8 weeks independently predicted better survival. Therefore, it is not reasonable to delay esophagectomy beyond 8 weeks for patients who can tolerate surgery. However, patients with G3 were associated with inferior DFS (HR, 2.327; 95% CI 1.051 to 5.152), but not inferior OS (HR, 2.032; 95% CI 0.919 to 4.496). The reason for this may be the small sample size. Larger samples and randomized controlled trials are needed to confirm. Additionally, we found more lymph nodes were removed during surgery in the ≤ 8 weeks group compared with the > 8 weeks group (P = 0.034). The reason we speculated was that delaying surgery made surgical dissection more difficult. A longer waiting period may lead to tumor repopulation or increase fibrosis and adhesion. In our study, there were no significant differences in the postoperative complications and no perioperative deaths occurred. The incidence of postoperative complications varied in different clinical researches. Nilsson et al. and Tie et al. found there were no significant differences in postoperative complications and 90-day mortality (Nilsson et al. 2020; Tie et al. 2018). Chidambaram et al. and Karthyarth et al. revealed that delay in surgery was associated with higher mortality and complications rates (Chidambaram et al. 2023; Karthyarth et al. 2023).
There are some limitations in this study
Firstly, our study is a retrospective study. The patients may be allocated to the two groups in a non-randomized manner. This may result in potential bias. And our sample size was small. This may limit our statistical ability for research. Therefore, our findings require larger scale randomized controlled trials to validate. Secondly, there was heterogeneity in patients in our study and our findings were based on a post-hoc analysis, which may cause some impacts on the results. Moreover, delaying surgery after neoadjuvant therapy is inevitable owing to adverse events of neoadjuvant therapy, poor physical condition, personal or logistic reasons. This may result in impacts in terms of survival. And the cutoff point of the interval was different in different studies. In the current study, we set the interval time at 8 weeks. This may result in potential bias. Finally, the postoperative follow-up time of this study was relatively short. Therefore, further follow-up actions are needed to evaluate long-term outcomes.
In conclusion, prolonged time interval from neoadjuvant camrelizumab combined with chemotherapy to surgery may increase the risk of recurrence and metastasis for locally advanced ESCC patients. And a longer interval time (> 8 weeks) was associated with worse long-term survival, but similar pathological response rate. It is not reasonable to delay esophagectomy beyond 8 weeks for patients who can tolerate surgery.
Data availability
The data of the current study are available from the corresponding author on reasonable request.
References
Ando N, Kato H, Igaki H et al (2012) A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 19(1):68–74
Arnold M, Soerjomataram I, Ferlay J, Forman D (2015) Global incidence of oesophageal cancer by histological subtype in 2012. Gut 64(3):381–387
Chidambaram S, Owen R, Sgromo B et al (2023) Delayed surgical intervention after chemoradiotherapy in esophageal cancer: (DICE) study. Ann Surg 278(5):701–708
Chiu CH, Chao YK, Chang HK et al (2013) Interval between neoadjuvant chemoradiotherapy and surgery for esophageal squamous cell carcinoma: does delayed surgery impact outcome? Ann Surg Oncol 20:4245–4251
Common Terminology Criteria for Adverse Events (CTCAE) Version 5. Published: November 27. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
Franko J, Voynov G, Goldman CD (2016) Esophagectomy timing after neoadjuvant therapy for distal esophageal adenocarcinoma. Ann Thorac Surg 101(3):1123–1130
Haisley KR, Laird AE, Nabavizadeh N et al (2016) Association of intervals between neoadjuvant chemoradiation and surgical resection with pathologic complete response and survival in patients with esophageal cancer. JAMA Surg 151(11):e162743
Karthyarth MN, Mathew A, Ramachandra D et al (2023) Early versus delayed surgery following neoadjuvant chemoradiation for esophageal cancer: a systematic review and meta-analysis. Esophagus 20(3):390–401
Kathiravetpillai N, Koëter M, van der Sangen MJ et al (2016) Delaying surgery after neoadjuvant chemoradiotherapy does not significantly influence postoperative morbidity or oncological outcome in patients with oesophageal adenocarcinoma. Eur J Surg Oncol 42(8):1183–1190
Kim JY, Correa AM, Vaporciyan AA et al (2012) Does the timing of esophagectomy after chemoradiation affect outcome? Ann Thorac Surg 93(1):207–213
Klevebro F, Nilsson K, Lindblad M et al (2020) Association between time interval from neoadjuvant chemoradiotherapy to surgery and complete histological tumor response in esophageal and gastroesophageal junction cancer: a national cohort study. Dis Esophagus 33(5):1–8
Lee A, Wong AT, Schwartz D, Weiner JP, Osborn VW, Schreiber D (2016) Is there a benefit to prolonging the interval between neoadjuvant chemoradiation and esophagectomy in esophageal cancer? Ann Thorac Surg 102(2):433–438
Levinsky NC, Wima K, Morris MC et al (2020) Outcome of delayed versus timely esophagectomy after chemoradiation for esophageal adenocarcinoma. J Thorac Cardiovasc Surg 159(6):2555–2566
Li C, Zhao S, Zheng Y et al (2021) Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1). Eur J Cancer 144:232–241
Lin G, Han SY, Xu YP, Mao WM (2016) Increasing the interval between neoadjuvant chemoradiotherapy and surgery in esophageal cancer: a meta-analysis of published studies. Dis Esophagus 29(8):1107–1114
Liu J, Yang Y, Liu Z et al (2022) Multicenter, single-arm, phase II trial of camrelizumab and chemotherapy as neoadjuvant treatment for locally advanced esophageal squamous cell carcinoma. J Immunother Cancer 10(3):e004291
Low DE, Alderson D, Cecconello I et al (2015) International consensus on standardization of data collection for complications associated with esophagectomy: esophagectomy complications consensus group (ECCG). Ann Surg 262(2):286–294
Mukherjee S, Hurt CN, Gwynne S et al (2017) NEOSCOPE: A randomised phase II study of induction chemotherapy followed by oxaliplatin/capecitabine or carboplatin/paclitaxel based pre-operative chemoradiation for resectable oesophageal adenocarcinoma. Eur J Cancer 74:38–46
Nilsson K, Klevebro F, Rouvelas I et al (2020) Surgical morbidity and mortality from the multicenter randomized controlled neores II trial: standard versus prolonged time to surgery after neoadjuvant chemoradiotherapy for esophageal cancer. Ann Surg 272(5):684–689
Qin Q, Xu H, Liu J et al (2018) Does timing of esophagectomy following neoadjuvant chemoradiation affect outcomes? A Meta Anal Int J Surg 59:11–18
Ranney DN, Mulvihill MS, Yerokun BA et al (2017) Surgical resection after neoadjuvant chemoradiation for oesophageal adenocarcinoma: what is the optimal timing? Eur J Cardiothorac Surg 52(3):543–551
Rice TW, Ishwaran H, Ferguson MK, Blackstone EH, Goldstraw P (2017) Cancer of the esophagus and esophagogastric junction: an eighth edition staging primer. J Thorac Oncol 12(1):36–42
Shang QX, Yang YS, Gu YM et al (2020) Timing of surgery after neoadjuvant chemoradiotherapy affects oncologic outcomes in patients with esophageal cancer. World J Gastrointest Oncol 12(6):687–698
Shapiro J, van Hagen P, Lingsma HF et al (2014) Prolonged time to surgery after neoadjuvant chemoradiotherapy increases histopathological response without affecting survival in patients with esophageal or junctional cancer. Ann Surg 260(5):807–814
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics, 2022. CA Cancer J Clin 72(1):7–33
Teman NR, Silski L, Zhao L et al (2013) Delaying surgery for esophageal cancer increases postoperative complications. J Am Coll Surg 217(3):S35–S36
Tessier W, Gronnier C, Messager M et al (2014) Does timing of surgical procedure after neoadjuvant chemoradiation affect outcomes in esophageal cancer? Ann Thorac Surg 97(4):1181–1189
Tie H, He F, Shen J et al (2018) Prolonged interval between neoadjuvant chemoradiotherapy and esophagectomy does not benefit the outcome in esophageal cancer: a systematic review and meta-analysis. Dis Esophagus 31(1):1–9
van Hagen P, Hulshof MC, van Lanschot JJ et al (2012) Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 366(22):2074–2084
Wang BY, Chen HS, Hsu PK et al (2015) Clinical impact of the interval between chemoradiotherapy and esophagectomy in esophageal squamous cell carcinoma patients. Ann Thorac Surg 99(3):947–955
Yan X, Duan H, Ni Y et al (2022) Tislelizumab combined with chemotherapy as neoadjuvant therapy for surgically resectable esophageal cancer: a prospective, single-arm, phase II study (TD–NICE). Int J Surg 103:106680
Yang H, Liu H, Chen Y et al (2018) Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized Open-Label Clinical Trial. J Clin Oncol 36(27):2796–2803
Yang W, Niu Y, Sun Y (2023) Current neoadjuvant therapy for operable locally advanced esophageal cancer. Med Oncol 40(9):252
Zhang Y (2013) Epidemiology of esophageal cancer. World J Gastroenterol 19(34):5598–5606
Funding
This research was supported by the the National Key Research and Development Program of China (2022YFC2407303); Major Science and Technology Projects of Zhejiang Province (2020C03058); Research Center for Lung Tumor Diagnosis and Treatment of Zhejiang Province (JBZX-202007).
Author information
Authors and Affiliations
Contributions
Jiacong Liu (Conceptualization, Data curation, Formal Analysis, Investigation, Visualization, Methodology, Writing—original draft). Linhai Zhu (Conceptualization, Data curation, Formal Analysis, Investigation, Visualization, Methodology, Writing—review & editing). Xuhua Huang (Conceptualization, Data curation, Investigation, Visualization, Methodology, Writing—review & editing). Zhongjie Lu (Conceptualization, Investigation, Visualization, Methodology). Yanye Wang (Conceptualization, Investigation, Visualization, Methodology). Yuhong Yang (Conceptualization, Investigation, Visualization, Methodology). Jiayue Ye (Conceptualization, Investigation, Visualization, Methodology). Chen Gu (Conceptualization, Investigation, Visualization, Methodology). Wang Lv (Conceptualization, Investigation, Visualization, Methodology). Chong Zhang (Conceptualization, Investigation, Resources, Supervision, Validation). Jian Hu (Conceptualization, Funding acquisition, Investigation, Resources, Supervision, Validation). All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare.
Ethical statement
This trial was permitted by the Clinical Research Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine (2021 IIT No. 742), and was in line with the Declaration of Helsinki (as revised in 2013) and Good Clinical Practice Guidelines. What’s more, we gained written informed consent from enrolled patients so that we could get access to their electronic medical record information.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, J., Zhu, L., Huang, X. et al. Does the time interval from neoadjuvant camrelizumab combined with chemotherapy to surgery affect outcomes for locally advanced esophageal squamous cell carcinoma?. J Cancer Res Clin Oncol 150, 161 (2024). https://doi.org/10.1007/s00432-024-05696-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00432-024-05696-4