Abstract
Purpose
The present study aimed to develop a nomogram to predict the prognosis of patients with secondary bone tumors in the intensive care unit to facilitate risk stratification and treatment planning.
Methods
We used the MIMIC IV 2.0 (the Medical Information Mart for Intensive Care IV) to retrieve patients with secondary bone tumors as a study cohort. To evaluate the predictive ability of each characteristic on patient mortality, stepwise Cox regression was used to screen variables, and the selected variables were included in the final Cox proportional hazard model. Finally, the performance of the model was tested using the decision curve, calibration curve, and receiver operating characteristic (ROC) curve.
Results
A total of 1028 patients were enrolled after excluding cases with missing information. In the training cohort, albumin, APSIII (Acute Physiology Score III), chemotherapy, lactate, chloride, hepatic metastases, respiratory failure, SAPSII (Simplified Acute Physiology Score II), and total protein were identified as independent risk factors for patient death and then incorporated into the final model. The model showed good and robust prediction performance.
Conclusion
We developed a nomogram prognostic model for patients with secondary bone tumors in the intensive care unit, which provides effective survival prediction information.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone is one of the most common sites of metastasis for malignant tumors, affecting many patients with advanced cancer (Coleman et al. 2020a). Bone metastases often lead to skeletal morbidity called skeletal-related events (SREs) (Moos et al. 2019). In general, SREs reduce overall survival and are associated with loss of mobility and social function, decreased quality of life, and substantial increase in medical costs (Coleman et al. 2020b). In most cases, the treatment of bone metastases focuses on preventing disease progression and alleviating symptoms. And within the context of multidisciplinary supportive care, years of disease control and reduction of the impact of metastatic bone disease on physical function can be achieved (Coleman 2006). Cancer patients require ICU (intensive care unit) admission after cancer progression, surgery, radiotherapy-related complications, or complications from severe acute illness (Soares et al. 2010). Patients with bone metastases are more severely ill and more likely to have complications than cancer patients without bone metastases, and an increased need for medical care (Fornetti et al. 2018; Jimenez-Andrade et al. 2010). Therefore, it is important to identify high-risk patients with poor prognosis in the intensive care unit. It helps clinicians to improve treatment strategies in time to improve the prognosis of patients.
Currently, multiple studies have explored the prognostic factors and established models to predict the prognosis of patients with various types of malignant tumors (Baba et al. 2018; Vichapat et al. 2011; Fang et al. 2020; Gurney et al. 2013; Liu et al. 2016; Mao et al. 2018). Other studies have developed models to predict bone metastasis in patients with malignant tumors (Teng et al. 2020; Ellmann et al. 2019; Hou et al. 2021). Bone metastases are common in patients with malignant tumors, whereas few studies have been conducted with bone metastases as research subjects to explore the prognosis of patients (Guo et al. 2008; Abdelazeem et al. 2022).
The nomogram has been widely used as a predictive method for the prognosis of patients with various diseases (Park 2018; Lv et al. 2021; Hess 2021; Yuan and Wu 2021), and its visual interface allows accurate quantification of the risk of independent risk factors by score. Clinicians can calculate scores from the characteristics on the column line graphs to predict the probability of death or illness of a patient. In this study, a nomogram prognostic model based on Cox proportional hazard model was established by employing a large multicenter database MIMIC IV 2.0 as the data source, and patients with secondary bone tumors in the intensive care unit as the research subjects. The aim was to explore the independent risk factors affecting the prognosis of patients and to facilitate clinicians to identify high-risk patients for more accurate clinical decision-making.
Methods
Study cohort and data
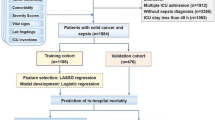
Data were extracted from the MIMIC IV 2.0 database on patients diagnosed with secondary bone tumors according to the International Classification of Diseases codes, Ninth Revision (198.5) and Tenth Revision (C7B.03, C79.5). To improve usability, we have collected routine, readily accessible clinical indicators. The collected data included patient demographics (age, gender, ethnicity), body mass index, comorbidities (cancers, acute kidney injury, hepatic metastases, pulmonary metastasis, brain metastases, acidosis, respiratory failure, heart failure, atrial fibrillation, hypertension), treatment information (chemotherapy, parenteral nutrition, radiotherapy, mechanical ventilation), laboratory results (hematology: atypical lymphocytes, metamyelocytes, mean corpuscular hemoglobin concentration, mean corpuscular volume, mean hemoglobin content; biochemical test: pO2, calculated total CO2, pCO2, pH, base excess, lactate, free calcium; biochemical test: glutamic-pyruvic transaminase, alkaline phosphatase, glutamic oxaloacetic transaminase, creatinine kinase MB, albumin, total protein, anion gap, bicarbonate, calcium, creatinine, chloride, potassium), and prognosis scores(APSIII, SOFA, SAPSII), with cases with missing data excluded. For patients with multiple ICU admissions, we selected data from the first ICU admission of the patient for analysis. In addition, we used data from patients within 24 h of admission to the ICU for the analysis. If the patient had multiple measurements within 24 h of admission to the ICU, the data from the first measurement were used.
Statistical analysis
Each variable was divided into training and validation data sets, with the categorical variables described by percentage (%), non-normally distributed continuous variables expressed using median and quartiles, and normally distributed continuous variables described using mean and standard error [mean (S.E.)]. The chi-square test was adopted to compare differences in categorical variables, and the t-test or Mann–Whitney U test was used to compare differences between two groups of continuous variables. The starting point for follow-up was defined as the time the patient was admitted to the ICU. The primary outcome indicator for this study was the long-term mortality of the patients. Date of death is extracted from two sources: the hospital information system and the Massachusetts State Registry of Vital Records and Statistics. For the training cohort, feature selection was performed using univariate Cox regression and stepwise Cox regression based on AIC (Akaike Information Criterion) with both selections. Variables with P < 0.05 in the univariate analysis were included in the stepwise Cox regression, while variables with P < 0.05 in the stepwise Cox regression were included in the final Cox proportional hazard model, and the corresponding nomogram was generated. The multicollinearity of the variables in the model was detected by calculating the variance inflation factor (VIF), and a VIF higher than 2 was considered to have multicollinearity among the variables. Overall survival at 1 month, 3 months, 1 year, and 3 years was estimated using the nomograms. The discrimination ability of the model was evaluated by the area under the time-dependent receiver operating characteristic curve (time-dependent AUC). The calibration graph was used to assess the agreement between the predicted and actual values of the model. The survival package (version 3.5-7) was used for univariate Cox regression and stepwise Cox regression, the rms package (version 6.7-0) was used for plotting nomogram and calibration curves, the survivalROC package (version 1.0.3.1) was used for plotting ROC curves, and the dcurves package (version 0.4.0.9) was used for plotting decision curves. All statistical analyses were performed using R 4.2.1., with a bilateral P-value < 0.05 considered statistically significant.
Results
Study cohort
A total of 1357 patients with bone metastases admitted to the ICU were identified from the database, and after excluding patients with missing information (N = 329), a total of 1028 patients were finally included in the study (median survival time: 642.50 days). Including 720 in the training cohort (median survival time: 624.00 days) and 308 in the validation cohort (median survival time: 695.50 days) (Table 1).
Feature selection and model building
Feature selection by univariate Cox regression and stepwise Cox regression showed that nine features, including albumin, APSIII, chemotherapy, lactate, chloride, hepatic metastases, respiratory failure, SAPSIII, total protein, were independent predictors of prognosis in patients with secondary bone tumors in the intensive care unit (Table 2). The VIF of the variables in the model was calculated and the results were all below 2 (albumin: 1.166, APSIII: 1.705, chemotherapy: 1.107, chloride: 1.080, hepatic metastases: 1.084, lactate: 1.129, respiratory failure: 1.079, SAPSII: 1.733, total protein: 1.181), showing no multicollinearity. The Cox proportional hazard model was established based on the above characteristics, and the nomogram was drawn as shown in Fig. 1. In the nomogram, the total score (Total Points) for each patient is calculated by adding the scores corresponding to each feature (Points), and the total score corresponds vertically to the scale on the predictor (1-month, 3-month, 1-year, and 3-year survival probability), i.e., the patient’s survival probability. If a patient’s ultimate total score (Total Points) is 300, then the patient’s probability of survival at 1 month, 3 months, 1 year, and 3 years is 90–95%, 80%, 60%, and 40%, respectively. In addition, for the categorical variables included in the model, we plotted Kaplan–Meier curves according to their grouping (Fig. 2).
Validation of the model
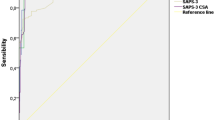
The ROC curve, calibration curve, and decision curve were plotted to validate the model. The results of the ROC curve analysis showed that the AUC of the nomogram model for predicting the mortality in the training cohort at 1 month, 3 months, 1 year, and 3 years was 0.862, 0.890, 0.826, and 0.831, respectively; the AUC of the for predicting model for predicting the mortality in the validation cohort at 1 month, 3 months, 1 year, 3 years was 0.854, 0.884, 0.872, and 0.839, respectively (Fig. 3). And the model exhibited good predictive accuracy. The calibration curve analysis revealed that the agreement between the predicted and the actual values was within an acceptable range (Fig. 4). In addition, we plotted decision curves (Fig. 5). The green horizontal line in the figure shows the benefit if none of the patients received the intervention, the red bias line shows the benefit if all the patients received the intervention, and the blue curve shows the benefit if they received the intervention as judged by the model. The figure shows that our model has a large net gain in both the training and validation cohorts.
Decision curve analysis of the nomogram: A 1‐month survival benefit in the training cohort. B 3‐month survival benefit in the training cohort. C 1‐year survival benefit in the training cohort. D 3‐year survival benefit in the training cohort. E 1‐month survival benefit in the training cohort. F 3‐month survival benefit in the training cohort. G 1‐year survival benefit in the training cohort. H 3‐year survival benefit in the training cohort
Discussion
In the present study, we studied patients with secondary bone tumors in the intensive care unit and developed a nomogram model to predict patient prognosis based on patient demographic information, laboratory test indicators, and comorbidities/surgical history. The model achieved an AUC of above 0.8 in both the training and validation cohorts, showing good predictive value.
Most current studies on secondary bone tumors have focused on bone metastases from specific tumors (Li et al. 2021; Lang et al. 2013; Huang et al. 2019; Sun et al. 2019), and few pan-cancer studies have been conducted on bone metastases in all cancer patients. However, there is a certain commonality in patients who develop secondary bone tumors, especially in patients with bone metastases admitted to the intensive care unit. An earlier similar study analyzed prognostic factors based on 216 patients with bone metastases (Teshima et al. 1990), but the study cohort was not limited to the intensive care unit. Independent predictors of prognosis in patients with bone metastases in the intensive care unit remain uncertain. Hence, we developed a predictive model that can predict the prognosis of patients with secondary bone tumors in the intensive care unit to provide supporting data for future studies.
Our model showed that nine characteristics, including low albumin, APSIII, chemotherapy, high lactate, low chloride, hepatic metastases, respiratory failure, SAPSIII, and low total protein, were independent predictors of prognosis in patients with secondary bone tumors in the intensive care unit. Among them, albumin, chemotherapy, chloride, and total protein were shown to be protective factors; while, APSIII, hepatic metastases, SAPSIII, SOFA, and lactate were promoting factors of mortality. Among the protective biomarkers, total protein and albumin are often used as indicators of nutritional status and hepatic synthetic function (Hülshoff et al. 2013), and exogenous albumin is frequently treated as a nutritional support drug in critically ill patients (Farrugia 2010). Bone metastasis means tumor progression. Hypoproteinemia is prevalent in cancer patients due to the damage inflicted on the body by the tumor and various treatment methods (Christina et al. 2023; Jiang et al. 2022; Sun et al. 2022); therefore, these patients require a higher protein intake to maintain body functions (Muscaritoli et al. 2021). Adequate plasma albumin has been demonstrated in many studies to be the basis for improved prognosis in patients with various medical conditions (Fanali et al. 2012; Yu et al. 1877; Amouzandeh et al. 2018; Arques 2018). Meanwhile, a prospective cohort study showed a significant negative correlation between serum albumin and the inflammatory marker C-reactive protein (Sheinenzon et al. 2021). Serum chloride ions are important electrolytes for maintaining body fluid homeostasis and are associated with the cardiac, renal and neurohormonal systems (Zandijk et al. 2021). Chloride was associated with acidosis and we included acidosis for analysis, but acidosis did not show a correlation with patient prognosis. The effect of serum chloride ions on patient prognosis is controversial to some extent. A study by Yaling Zhai et al. showed that elevated serum chloride levels were associated with poor prognostic outcomes in patients with IgA nephropathy, which contradicts our findings (Zhai et al. 2021). Nevertheless, some studies have shown that electrolyte disorders such as hypochlorhydria are significantly associated with poor prognosis in cancer patients (Li et al. 2020). In addition, a study on heart failure revealed a significant inverse association between serum chloride concentration and long-term mortality of patients (HR: 0.890; 95% CI: 0.863–0.918; P < 0.001), which is consistent with our study. Therefore, we believe that the effect of serum chloride ions on patient mortality is related to the disease characteristics of patients. However, no studies have directly illustrated the effect of serum chloride concentration on the prognosis of patients with bone metastases, and more research data are needed for validation. Among the biomarkers that manifest as mortality-promoting factors, lack of oxygen in the body affects the normal function of many organs (Fenves and Emmett 2021). In our model, the biomarkers incorporated into the model are mostly indicators reflecting acid–base and electrolyte balance and nutritional status in the patient’s body. Hence, for patients with secondary bone tumors in the intensive care unit, the administration of adequate nutrients and maintenance of acid-base balance are important measures to improve the prognosis of patients. In addition, hepatic metastases and the prognostic score were also major risk factors. This suggests that the severity of the cancer and the patient's physical condition are equally significant in predicting prognosis.
Our model can provide valid predictive information, but some limitations need to be mentioned: first, due to the limitation of the database, we could not include some important indicators, such as the primary tumor of the patient, the size of the primary tumor, and the site of metastasis. Second, we were unable to determine whether the patient’s combined tumor was the primary tumor. Moreover, some laboratory indicators may interact with each other, but we are unable to detect the interactions between covariates. Finally, our model only used data from a single center and needs to be validated using a large sample of data from multiple centers.
Conclusion
A prognostic model has been developed in this study for patients with secondary bone tumors in the intensive care unit. The prediction performance of the model is robust and it can provide valid forecasting information. The indicators included in the model suggest that nutritional support and maintenance of fluid balance are important therapeutic measures to improve the prognosis of patients with bone metastases in the intensive care unit.
Data availability
All data generated or analyzed during this study are included in this published article or are available from the corresponding author on reasonable request.
References
Abdelazeem B, Abbas KS, Rao DN, Tariq R, Wahab A (2022) Incidence and comparative prognosis of cancers with metastasis to noncommon sites: a population-based study. Medicine 101(29):e29743
Amouzandeh M, Nowak G, Januszkiewicz A, Wernerman J, Rooyackers O, Norberg Å (2018) Albumin mass balance and kinetics in liver transplantation. Crit Care (london, England) 22(1):152. https://doi.org/10.1186/s13054-018-2053-6
Arques S (2018) Human serum albumin in cardiovascular diseases. Eur J Intern Med 52:8–12. https://doi.org/10.1016/j.ejim.2018.04.014
Baba Y, Yoshida N, Kinoshita K, Iwatsuki M, Yamashita YI, Chikamoto A, Watanabe M, Baba H (2018) Clinical and prognostic features of patients with esophageal cancer and multiple primary cancers: a retrospective single-institution study. Ann Surg 267(3):478–483. https://doi.org/10.1097/sla.0000000000002118
Christina NM, Tjahyanto T, Lie JG, Santoso TA, Albertus H, Octavianus D, Putri D, Andrew J, Jatinugroho YD, Shiady C, Wijaya JH (2023) Hypoalbuminemia and colorectal cancer patients: any correlation? A systematic review and meta-analysis. Medicine (baltimore) 102(8):e32938. https://doi.org/10.1097/MD.0000000000032938
Coleman RE (2006) Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 12(20 Pt 2):6243s–6249s. https://doi.org/10.1158/1078-0432.Ccr-06-0931
Coleman RE, Croucher PI, Padhani AR, Clézardin P, Chow E, Fallon M, Guise T, Colangeli S, Capanna R, Costa L (2020a) Bone metastases. Nat Rev Dis Primers 6(1):83. https://doi.org/10.1038/s41572-020-00216-3
Coleman R, Hadji P, Body JJ, Santini D, Chow E, Terpos E, Oudard S, Bruland Ø, Flamen P, Kurth A, Van Poznak C, Aapro M, Jordan K (2020b) Bone health in cancer: ESMO Clinical Practice Guidelines. Ann Oncol 31(12):1650–1663. https://doi.org/10.1016/j.annonc.2020.07.019
Ellmann S, Seyler L, Evers J, Heinen H, Bozec A, Prante O, Kuwert T, Uder M, Bäuerle T (2019) Prediction of early metastatic disease in experimental breast cancer bone metastasis by combining PET/CT and MRI parameters to a Model-Averaged Neural Network. Bone 120:254–261. https://doi.org/10.1016/j.bone.2018.11.008
Fanali G, di Masi A, Trezza V, Marino M, Fasano M, Ascenzi P (2012) Human serum albumin: from bench to bedside. Mol Aspects Med 33(3):209–290. https://doi.org/10.1016/j.mam.2011.12.002
Fang SX, Chen C, Guo Q, Ke XX, Lu HL, Xu G (2020) High lncSNHG15 expression may predict poor cancer prognosis: a meta-analysis based on the PRISMA and the bio-informatics analysis. Biosci Rep. https://doi.org/10.1042/bsr20194468
Farrugia A (2010) Albumin usage in clinical medicine: tradition or therapeutic? Transfus Med Rev 24(1):53–63. https://doi.org/10.1016/j.tmrv.2009.09.005
Fenves AZ, Emmett M (2021) Approach to patients with high anion gap metabolic acidosis: core curriculum 2021. Am J Kidney Dis 78(4):590–600. https://doi.org/10.1053/j.ajkd.2021.02.341
Fornetti J, Welm AL, Stewart SA (2018) Understanding the bone in cancer metastasis. J Bone Miner Res 33(12):2099–2113
Guo W, Tang X, Yang Y, Ji T (2008) Surgical treatment and outcome of pelvic metastases. Zhonghua Wai Ke Za Zhi [chinese Journal of Surgery] 46(12):891–894
Gurney J, Sarfati D, Stanley J, Dennett E, Johnson C, Koea J, Simpson A, Studd R (2013) Unstaged cancer in a population-based registry: prevalence, predictors and patient prognosis. Cancer Epidemiol 37(4):498–504. https://doi.org/10.1016/j.canep.2013.03.005
Hess DR (2021) A nomogram for use of non-invasive respiratory strategies in COVID-19. Lancet Digital Health 3(3):e140–e141. https://doi.org/10.1016/s2589-7500(21)00006-6
Hou N, Yi J, Wang Z, Yang L, Wu Y, Huang M, Hou G, Ling R (2021) Development and validation of a risk stratification nomogram for predicting prognosis in bone metastatic breast cancer: a population-based study. Medicine 100(6):e24751. https://doi.org/10.1097/md.0000000000024751
Huang Z, Du Y, Zhang X, Liu H, Liu S, Xu T (2019) Clear cell renal cell carcinoma bone metastasis: What should be considered in prognostic evaluation. Eur J Surg Oncol 45(7):1246–1252. https://doi.org/10.1016/j.ejso.2019.01.221
Hülshoff A, Schricker T, Elgendy H, Hatzakorzian R, Lattermann R (2013) Albumin synthesis in surgical patients. Nutrition (burbank, Los Angeles County, Calif) 29(5):703–707. https://doi.org/10.1016/j.nut.2012.10.014
Jiang A, Shi X, Zheng H, Liu N, Chen S, Gao H, Ren M, Zheng X, Fu X, Liang X, Ruan Z, Tian T, Yao Y (2022) Establishment and validation of a nomogram to predict the in-hospital death risk of nosocomial infections in cancer patients. Antimicrob Resist Infect Control 11(1):29. https://doi.org/10.1186/s13756-022-01073-3
Jimenez-Andrade JM, Mantyh WG, Bloom AP, Ferng AS, Geffre CP, Mantyh PW (2010) Bone cancer pain. Ann N Y Acad Sci 1198(1):173–181
Lang BH, Wong KP, Cheung CY, Wan KY, Lo CY (2013) Evaluating the prognostic factors associated with cancer-specific survival of differentiated thyroid carcinoma presenting with distant metastasis. Ann Surg Oncol 20(4):1329–1335. https://doi.org/10.1245/s10434-012-2711-x
Li Y, Chen X, Shen Z, Wang Y, Hu J, Xu J, Shen B, Ding X (2020) Electrolyte and acid-base disorders in cancer patients and its impact on clinical outcomes: evidence from a real-world study in China. Ren Fail 42(1):234–243. https://doi.org/10.1080/0886022X.2020.1735417
Li P, Lin Z, Liu Q, Chen S, Gao X, Guo W, Gong F, Wei J, Lin H (2021) Enhancer RNA SLIT2 inhibits bone metastasis of breast cancer through regulating P38 MAPK/c-Fos signaling pathway. Front Oncol 11:743840. https://doi.org/10.3389/fonc.2021.743840
Liu FT, Qiu C, Luo HL, Zhang Y, Xia GF, Hao TF, Zhu PQ (2016) The association of HOTAIR expression with clinicopathological features and prognosis in gastric cancer patients. Panminerva Med 58(2):167–174
Lv J, Liu YY, Jia YT, He JL, Dai GY, Guo P, Zhao ZL, Zhang YN, Li ZX (2021) A nomogram model for predicting prognosis of obstructive colorectal cancer. World J Surg Oncol 19(1):337. https://doi.org/10.1186/s12957-021-02445-6
Mao Q, Xia W, Dong G, Chen S, Wang A, Jin G, Jiang F, Xu L (2018) A nomogram to predict the survival of stage IIIA-N2 non–small cell lung cancer after surgery. J Thoracic Cardiovasc Surg 155(4):1784–1792
Muscaritoli M, Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, Hutterer E, Isenring E, Kaasa S, Krznaric Z, Laird B, Larsson M, Laviano A, Muhlebach S, Oldervoll L, Ravasco P, Solheim TS, Strasser F, de van der Schueren M, Preiser JC, Bischoff SC (2021) ESPEN practical guideline: Clinical Nutrition in cancer. Clin Nutr 40(5):2898–2913. https://doi.org/10.1016/j.clnu.2021.02.005
Park SY (2018) Nomogram: An analogue tool to deliver digital knowledge. J Thorac Cardiovasc Surg 155(4):1793. https://doi.org/10.1016/j.jtcvs.2017.12.107
Sheinenzon A, Shehadeh M, Michelis R, Shaoul E, Ronen O (2021) Serum albumin levels and inflammation. Int J Biol Macromol 184:857–862
Soares M, Caruso P, Silva E, Teles JM, Lobo SM, Friedman G, Dal Pizzol F, Mello PV, Bozza FA, Silva UV (2010) Characteristics and outcomes of patients with cancer requiring admission to intensive care units: a prospective multicenter study. Crit Care Med 38(1):9–15
Sun XS, Lin C, Liang YJ, Chen QY, Tang LQ, Mai HQ (2019) Role of zoledronic acid in nasopharyngeal carcinoma patients with bone-only metastasis at diagnosis. Oral Oncol 97:31–36. https://doi.org/10.1016/j.oraloncology.2019.08.003
Sun W, Li G, Zhang J, Zhu J, Zhang Z (2022) The role of nutritional assessment for predicting radiotherapy-induced adverse events in patients with gastric cancer. Br J Radiol 95(1130):20201004. https://doi.org/10.1259/bjr.20201004
Teng X, Wei L, Han L, Min D, Du Y (2020) Establishment of a serological molecular model for the early diagnosis and progression monitoring of bone metastasis in lung cancer. BMC Cancer 20(1):562. https://doi.org/10.1186/s12885-020-07046-2
Teshima T, Chatani M, Inoue T, Hirokawa Y, Wadasaki K, Kasiwado K, Kagemoto M, Katsuta S, Honke Y, Koyama T et al (1990) Prognostic factors for patients with osseous metastasis: a multi-institutional prospective study. Strahlentherapie Und Onkologie: Organ Der Deutschen Rontgengesellschaft [et Al] 166(6):387–391
Vichapat V, Garmo H, Holmberg L, Fentiman IS, Tutt A, Gillett C, Lüchtenborg M (2011) Prognosis of metachronous contralateral breast cancer: importance of stage, age and interval time between the two diagnoses. Breast Cancer Res Treat 130(2):609–618. https://doi.org/10.1007/s10549-011-1618-8
von Moos R, Costa L, Gonzalez-Suarez E, Terpos E, Niepel D, Body JJ (2019) Management of bone health in solid tumours: from bisphosphonates to a monoclonal antibody. Cancer Treat Rev 76:57–67. https://doi.org/10.1016/j.ctrv.2019.05.003
Yu L, Hua Z, Luo X, Zhao T, Liu Y (2022) Systematic interaction of plasma albumin with the efficacy of chemotherapeutic drugs. Biochim Biophys Acta 1877(1):188655. https://doi.org/10.1016/j.bbcan.2021.188655
Yuan Q, Wu G (2021) ASO author reflections: clinical prediction nomogram for breast cancer-related lymphedema. Ann Surg Oncol 28(12):7329–7330. https://doi.org/10.1245/s10434-021-10013-1
Zandijk AJL, van Norel MR, Julius FEC, Sepehrvand N, Pannu N, McAlister FA, Voors AA, Ezekowitz JA (2021) Chloride in heart failure: the neglected electrolyte. JACC Heart Failure 9(12):904–915. https://doi.org/10.1016/j.jchf.2021.07.006
Zhai Y, Yao X, Qi Y, Gao J, Chen Y, Wang X, Wu F, Zhao Z (2021) Elevated serum chloride levels contribute to a poor prognosis in patients with IgA nephropathy. J Immunol Res 2021:1
Acknowledgements
Not applicable.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Study design: Weikang Li; Data collection: Weikang Li, Jinliang Li; Data analysis: Weikang Li, Jinliang Li; Manuscript writing and revisions for important intellectual content: Weikang Li, Jinkui Cai, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, W., Li, J. & Cai, J. Development of a nomogram to predict the prognosis of patients with secondary bone tumors in the intensive care unit: a retrospective analysis based on the MIMIC IV database. J Cancer Res Clin Oncol 150, 164 (2024). https://doi.org/10.1007/s00432-024-05667-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00432-024-05667-9