Abstract
Copper is a necessary micronutrient for maintaining the well-being of the human body. The biological activity of organic ligands, especially their anticancer activity, is often enhanced when they coordinate with copper(I) and (II) ions. Copper and its compounds are capable of inducing tumor cell death through various mechanisms of action, including activation of apoptosis signaling pathways by reactive oxygen species (ROS), inhibition of angiogenesis, induction of cuproptosis, and paraptosis. Some of the copper complexes are currently being evaluated in clinical trials for their ability to map tumor hypoxia in various cancers, including locally advanced rectal cancer and bulky tumors. Several studies have shown that copper nanoparticles can be used as effective agents in chemodynamic therapy, phototherapy, hyperthermia, and immunotherapy. Despite the promising anticancer activity of copper-based compounds, their use in clinical trials is subject to certain limitations. Elevated copper concentrations may promote tumor growth, angiogenesis, and metastasis by affecting cellular processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metal-based compounds offer a promising opportunity to develop new cancer treatments (Aliabadi et al. 2021a, b; Abdolmaleki et al. 2021a, b). These compounds with their unique properties and mechanisms of action have shown many advantages in the fight against cancer compared to other chemical compounds (Abdolmaleki et al. 2023a,b, 2022b; Adibi et al. 2019). One of the advantages of these compounds is their ability to target specific cellular processes or proteins involved in cancer cell growth and survival (Khaksar et al. 2023a,b; Abdolmaleki et al. 2021a, b). The targeted approach can potentially minimize damage to healthy cells and reduce side effects compared to conventional chemotherapeutic agents (Abdolmaleki et al. 2020; Heydari et al. 2020a, b; Khaksar et al. 2023a, b). Metal ions commonly used in this method include platinum, ruthenium, gold, and copper (Abdolmaleki et al. 2023a, b).
Studies have shown that the complexation of copper ions with some organic ligands increases the biological activity of the resulting compound (Abdolmaleki et al. 2023a, b; Abdolmaleki et al. 2016; Abdolmaleki and Ghadermazi 2017). In 2023, our group reported significant cytotoxicity of copper(II) complexes with pyridine-2,6-dicarboxylate against BEL-7404 cells. The mechanistic study of the anticancer effect of this complex showed that it generates high ROS and stops the progression of the cell cycle in the G2/M phase. Treatment of BEL-7404 cells with copper(II) complex induced apoptosis through a caspase-dependent mitochondrial signaling pathway (Abdolmaleki et al. 2023a, b). In our other study, a strong cytotoxic effect was also observed for two mixed-ligand mono- and binuclear copper(II) complexes containing pyridine-2,6-dicarboxylate derivatives and 2-aminopyrimidine. In this study, the complexes were found to have stronger inhibitory effects than their ligands in all three cell lines tested, including βTC, MCF7, and HT29. The strongest antiproliferative properties of these complexes were observed in the MCF7 cell line (Abdolmaleki et al. 2016). This effect was also confirmed in our study on a polymeric copper(II) complex synthesized from pyridine dicarboxamide derivatives. Similar to a previous study, cancer cells showed the highest sensitivity to the copper(II) complex compared to the tested ligands and oxaliplatin (Abdolmaleki et al. 2017). In 2023, Climova et al. reported a remarkable inhibitory effect of three copper(II) complexes formed from hydrazonamide derivatives on cancer cell lines. They found that among the compounds tested, the complex containing N′-(benzylidene)-6-chloropyrazine-2-carbohydrazonamide was more effective and selective against LN229 cancer cells (Climova et al. 2023). In another study, copper(I) complexes with tridentate homoscorpionate tris(pyrazolyl)borate and monodentate phosphine auxiliary ligands were investigated due to their capacity to suppress the proliferation of cancer and normal cells. Some of them were found to be selective against tumors by inhibiting 26S proteasome activity associated with endoplasmic reticulum (ER) stress and activation of unfolded protein response (UPR). In this case, no signs of biochemical features related to apoptosis were observed, and morphological findings showed significant cytoplasmic vacuolization associated with a cell death process similar to paraptosis (Gandin et al. 2014).
Studies have shown that copper-based compounds act via a mechanism directed with DNA. The antitumor activities of these compounds rely on the interaction between copper and the ligand. The toxicity of copper arises from its ability to undergo cycles of oxidation and reduction, to remove ions from the binding sites of enzymes, bind strongly to DNA and cause DNA breaks (Marzano et al. 2009). Modification of copper-based compounds through the ligand scaffold increases its affinity, specificity, and stability toward DNA (Ceramella et al. 2020). These compounds can bind to DNA through noncovalent interactions, such as binding to major or minor grooves, intercalation, or electrostatic binding. Certain copper-based compounds produce ROS that overwhelm the body's antioxidant defenses and lead to oxidative damage in the cytoplasm, mitochondria, and DNA (Molinaro et al. 2020). A significant group of copper-based compounds acts as inhibitors of topoisomerases (Top) 1 and 2, leading to significant DNA damage, cell cycle arrest, and ultimately cell death (Shobha Devi et al. 2018). These compounds convert transient DNA enzyme complexes into lethal DNA breaks (Thomas and Pommier 2019).
Although copper is an essential trace element in cells, its high reactivity makes it toxic, which is why the body strictly regulates its levels. Copper metabolism is impaired in neoplastic diseases, and elevated blood copper levels are related to the progression and recurrence of various cancers (Geraki et al. 2002). The exact molecular mechanism behind the link between elevated copper and malignant cells is not yet fully understood, but it is suspected that copper has a role in the angiogenesis of early-stage tumors. According to studies, copper stimulates the growth and movement of endothelial cells, which are involved in the formation of blood vessels, and acts as a cofactor for angiogenic factors. The human copper transporter (hCTR1) also affects cell signaling pathways in embryogenic cells and may contribute to cancer development (Narayanan et al. 2013). The differential response of tumor cells and normal cells to copper suggests that copper-based compounds may be developed as anticancer agents (Wee et al. 2013; Khan et al. 2016). This depends on the choice of organic motifs, scaffolds, and the amount of donor atoms (Evans et al. 2015). In general, ligands have a prominent role in the cytotoxicity of metal compounds in cancer research (Abdolmaleki et al. 2018, 2017, 2019; Heydari et al. 2020a, b; Abdolmaleki et al. 2022a, b; Aliabadi et al. 2022; Zohrevandi et al. 2022). In a study, Hussain et al. evaluated the potential of copper complexes with a biocompatible Schiff base ligand as promising options for next-generation anticancer drugs and NSAIDs. These complexes showed strong binding ability to a model protein, indicating their potential for targeted therapy. In in vitro studies, the complex with 1,10-phenanthroline (phen) as a ligand was found to have remarkable efficacy against MCF-7 breast cancer cells compared to two other complexes. The mechanism by which these complexes exerted their cytotoxic effects was further investigated, and ROS generation was found to play an important role. The observed morphological changes in the cancer cells indicated that late apoptosis was induced by treatment with the complexes. Animal studies conducted in vivo showed that some of these complexes exhibited dose-dependent anti-inflammatory and analgesic activities, suggesting their potential as NSAIDs. Computational studies supported the notion that these complexes interact with a COX2 inhibitor, confirming their potential mechanism of action as NSAIDs (Hussain et al. 2019).
Researchers have also explored the utilization of copper-containing nanoparticles in the treatment of cancer, which is very promising. These innovative compounds have the potential to transform cancer therapy through innovation, enabling more effective and targeted treatments with fewer side effects. The results show that the copper ions released by the nanoparticles can trigger oxidative stress and induce programmed cell death in cancer cells. Copper nanoparticles also can selectively attack cancer cells and spare healthy cells. This selectivity is attributed to the EPR effect, which enables nanoparticles to accumulate in tumor tissue by exploiting leaky blood vessels (Mariani et al. 2021; Aishajiang et al. 2023).
While many copper complexes have shown significant cytotoxicity in in vitro studies, few have been tested in animal models (Khan et al. 2016). Further research is needed to understand their mechanisms of action, optimize their therapeutic potential, and evaluate safety and efficacy in clinical trials, as the history of copper complexes in cancer therapy is relatively limited compared to other metal complexes such as platinum. Therefore, in this review article, we focus on the anticancer effect investigation of copper-based compounds, their mechanisms of action, and their use in clinical trials. Nanoparticles containing copper are discussed as effective agents for the diagnosis and treatment of cancer. The limitations and challenges of using copper-based compounds are also explained. Although the use of copper-based compounds in the treatment of cancer is still at an early stage of development, studies have shown promising results concerning their antiproliferative and antiangiogenic effects.
Copper's chemical and biochemical properties
Copper is a vital nutrient for both plants and animals and serves as a cofactor in enzymes and as a component of pigments. In mammals, it is found mainly in the bloodstream and various organs (Elkanzi et al. 2023; Al-Fakeh et al. 2023). In humans, high copper concentrations can be harmful to cells, leading to the oxidation of DNA and structural changes in proteins and biomembranes. Copper ions, especially in blue copper proteins, play a role in redox reactions and electron transfer processes. Transition metal ions, including copper, exhibit selectivity in molecular recognition and can change their valence in redox reactions (Feng et al. 2023). One of the most important explanations for the cellular toxicity caused by copper is the ability of free copper ions to generate ROS.
Both copper(II) and (I) ions undergo oxidation and reduction reactions. When exposed to superoxide (O2−), ascorbic acid, or glutathione (GSH), copper(II) is reduced to copper(I). Copper(I) then can catalyze the production of hydroxyl radicals (.OH) from hydrogen peroxide (H2O2) through the Haber–Weiss reaction (Piroš et al. 2023). The highly reactive.OH interacts with biological molecules and causes damage by cleaving hydrogen from carbon atoms in amine-containing compounds and unsaturated fatty acids. This leads to the formation of protein and lipid radicals that contribute to oxidative damage in cells. Copper has been found to induce DNA damage and oxidation through the formation of ROS. GSH inhibits the formation of free radicals by copper ions in the presence of H2O2, ascorbate, and DNA. This defensive impact is due to the ability of GSH to stabilize copper(I) and thus prevent the creation of free radicals by redox cycling. Copper(II) also forms thioxyl radicals, copper cysteine, and copper methionine ions, possibly leading to the formation of metallothioneins (MT) and disulfides (RSSR), which can be harmful (Han 2023).
However, it is crucial to mention that the presence of free copper ions in cells is usually limited to less than one ion per cell. This suggests that the cells have a considerable capacity to chelate or bind copper, ensuring that it does not accumulate to toxic levels (Franco et al. 2023). Regulation of copper levels in the body is critical to maintaining health, as both excess and deficiency of this metal can be harmful. Metal-binding sequences, which are specialized domains rich in cysteine, methionine, or histidine, play a prominent role in maintaining the concentration of free copper in cells at an extremely low level of below 10–18 M (Bisaglia and Bubacco 2020).
ATP7A, also known as the Menkes protein, facilitates the absorption of copper from food in the stomach and small intestine. In normal human serum, most copper is bound to ceruloplasmin, an enzyme that contains 6 copper atoms in both the copper(II) and (I) states. However, this state of copper is not easily interchangeable. Copper in its interchangeable state is tightly attached to albumin and amino acids (Mhaske et al. 2020). The copper–histidine complex has been recognized as the primary copper–amino acid complex in human serum and human albumin has been discovered to create a ternary complex with copper-histidine (Ha et al. 2023; Song et al. 2022). During copper uptake, copper(II) is reduced to copper(I) and taken up by cells via transmembrane transporters. The major copper influx transporter in human cells is hCTR1, which is found predominantly in the plasma membrane and is expressed at higher levels in the liver, kidney, and heart. hCTR1 probably binds copper through its amino-terminal domain and transports it across the cell membrane (Magrì et al. 2022; Orlov et al. 2023).
Once in the cytoplasm, copper can form complexes with various ligands, with the majority being bound to GSH as copper(I) (Falcone et al. 2023). The Cu(I)-GS complex can then be transferred to intracellular proteins such as MT, which is important for metal detoxification (Ritacca et al. 2023). To prevent an accumulation of copper in mammalian cells, there are two closely related P-type ATPases known as ATP7A and ATP7B. These ATPases are responsible for facilitating the removal of copper from cells. ATP7A is found in various tissues other than the liver, while ATP7B is mainly found in the liver, kidney and to a lesser extent in the brain (Karpenko et al. 2023; Guan et al. 2023). Both proteins have a high affinity for copper and undergo regulated movement within cells when copper binds to them. Results have shown that copper transporters, including hCtr1, ATP7A, and ATP7B, play a role in importing, distributing, and exporting platinum-containing drugs such as cisplatin. This suggests that these copper transporters may influence the sensitivity of cancer cells to platinum-based medications. The mechanisms by which these transporters facilitate the transport of platinum drugs have not been fully elucidated (Zhao et al. 2023). However, their ability to discriminate between different metal ions suggests that changes in the expression of copper transporters may influence the cellular response to platinum drugs through secondary effects on other metabolic pathways, such as MT and GSH levels (Bisaglia and Bubacco 2020; Zhao et al. 2023).
Overview of copper-based compounds
Anticancer activity
The first discovery of the antitumor effect of copper-based compounds dates back to the 1960s (Graur et al. 2023; Jenkins et al. 2023). Since then, numerous papers have been devoted to investigating the chemical and biological properties of these complexes. Despite extensive research into the production of different types of copper-based compounds, there is little information on their physiological processing. Research has shown that the chelation of organic ligands with copper ions is important in enhancing their biological activity (Zhao et al. 2023).
Accordingly, copper(II) complexes of thiosemicarbazones (TSCs) (1a and b) were shown to have excellent inhibitory activity toward HeLa, HepG2, SGC-7901, and L02 cell lines compared with their corresponding ligands (Ma et al. 2014; Shao et al. 2014). In particular, these complexes showed promising results by inducing DNA fragmentation and increasing ROS production. Complex (1a) showed remarkable cytotoxicity against HeLa cells. This compound promoted apoptosis in the aforementioned cells via an oxidative DNA damage pathway, making it a potential anticancer agent. It was found that (1a) induces apoptosis in HeLa cells more strongly than (1b). The direct effects of (1a) on DNA replication, transcription, and protein synthesis are due to the DNA damage it causes. In general, copper complexes have shown promising anticancer activity in many reports, as they accumulate in tumors and selectively penetrate the membranes of cancer cells (Deegan et al. 2006; Deegan et al. 2007b, a). In particular, copper complexes containing N-donor ligands, such as TSCs, are effective against various cancer cell lines. In a study, the copper(II) complex containing 2-formylpyridine TSC (2) was reported to be able to inhibit ribonucleic acid (RNA)-dependent DNA polymerases as well as the transforming ability of Rous sarcoma virus (Kaska et al. 1978).
Ferrari et al. performed a study in which they modified derivatives of TSCs by replacing the 2-pyridine group with salicylaldehyde (Ferrari et al. 1999), pyridoxal (Ferrari et al. 2002), and 5-formyluracil (Ferrari et al. 1998). Among these derivatives, the salicylaldehyde TSC (H2 salt) showed selectivity for copper(II) and formed a dimeric metal complex (3). When in vitro evaluations were done with human leukemic U937 cells to study the effects of complex (3) on the suppression of cell proliferation and triggering of programmed cell death, the evaluations showed that this complex inhibited about 40% of cell proliferation, but no DNA fragmentation or apoptosis was confirmed. This behavior can be attributed to the specific arrangement of the copper atoms in a square-planar polyhedron of complex (3). In contrast, (4) formed with the pyridoxal-TSC ligand (Ferrari et al. 1994) and the compounds (5–7) formed with the 5-formyl-uracil-TSC ligand all exhibited a five-coordinated structure and induced process of breaking DNA molecules into smaller fragments that ultimately led to apoptosis [65]. Investigation of the cytotoxicity of the copper(II) complexes of isatin TSCs, particularly (8) and (9), on the U937 cell line revealed that both copper(II) complexes could strongly inhibit cell proliferation, with cytotoxicity of 70% at a dose of 20 μg mL−1 (Rodriguez-Arguelles et al. 1999). In another study, copper(II) complexes of 6-nitropiperonal TSC (10a-d) were evaluated for their interaction with DNA and HSA. These complexes exhibited antioxidant properties and strong binding to DNA, with a binding constant of 105 M−1. Binding to DNA was significantly stronger than binding to HSA. Here, the complexes were reported to have a similar core structure to oxolinic acid, a type of antibiotic known as quinolone that acts specifically on DNA gyrase and Top (IV). The strength of its interaction with DNA gyrase B varied between dissociation constants of 0.37 to 1.27 µM, while its binding to Top (IV) was even more potent with dissociation constants of 4.32 to 24.65 µM (Beckford and Webb 2017).
In 2023, Machado et al. demonstrated that copper(I) complexes (11a and b) containing a bioactive TSC ligand and a phosphane ligand (PP) exhibited dual anticancer and antiparasitic properties. Here, the in vitro anti-trypanosome and anticancer activities of the complexes against Trypanosoma cruzi and the cancer cells OVCAR3 and PC3 were investigated. To determine their selectivity against parasites and cancer cells, cytotoxicity against normal monkey kidney cells (VERO) and human skin fibroblasts (HDF) was also investigated. The synthesized heteroleptic complexes showed higher cytotoxicity against T. cruzi and chemoresistant prostate PC3 cells compared with the benchmark drugs nifurtimox and cisplatin. These complexes were observed to be effectively taken up by OVCAR3 cells, especially those containing the dppe-PP ligand, and activated the cell death mechanism mediated by apoptosis. However, there was no clear evidence of ROS production induced by these complexes (Machado et al. 2023). In another study, the affinity for binding of copper(II) complexes containing TSC (12a and b) with pET30a plasmid DNA was evaluated. The results proved that both complexes had nuclease activity, with more efficient DNA cleavage observed when the complexes were mixed with H2O2. Binding to DNA occured by partial intercalation binding and DNA degradation depended on the concentration of the complex. Complete DNA cleavage was observed at a concentration of 50 μM. (12a) showed stronger binding affinity to DNA compared to 12b, as evidenced by a greater increase in viscosity (Baldini et al. 2004). The structure of the above copper(II) complexes is represented in Fig. 1.
According to the literature, the biological activities of Schiff base ligands, such as anticancer, antibacterial, and antiviral activities, are enhanced when they coordinate with metal ions, especially copper(I) and (II) ions (Gomathi and Andy 2013). In a study, strong cytotoxicity and remarkable electrochemical activity were observed for a dinuclear copper(II) complex (13) prepared from a Schiff base ligand of N-(2-hydroxylbenzylidene)-benzo[d]imidazol-2-amine. In addition, (13) showed binding to DNA by groove binding and electrostatic interaction, resulting in conformational changes in the DNA structure. This complex could effectively cleave the supercoiled plasmid DNA into nicked and linear forms by an oxidative mechanism. In cell experiments, (13) was found to readily penetrate cancer cells, including the nucleus, induce cell apoptosis and exhibit cytotoxicity similar to cisplatin in tests with the cell lines HeLa and A549. In addition, (13) showed a higher inhibitory effect against MCF-7 cell lines compared to other cell lines (Zhao et al. 2022). Zhong et al. reported that a mononuclear distorted octahedral copper(II) complex (14) prepared from the Schiff base ligand (Z)-2-hydroxy-N'-(2-oxoindolin-3-ylidene)benzohydrazide (Zhong et al. 2007) exhibited pronounced cytotoxicity in four different human cancer cell lines (SPCA-1, Tb, MGC, and K562), and its potency was even higher compared to similar compounds reported previously (Bestwick et al. 2005). The authors suggested that the enhanced potential anticancer activity of the compounds could be attributed to the production of toxic copper(I) species through enzymatic reduction processes within the cells (Kostova et al. 2005). In another study, Schiff base compounds developed by attaching pharmacophores, such as amino group-bearing TSCs, to the central ketone function of ketoprofen (Saha et al. 2001; Padhye et al. 2005) were evaluated for their anticancer properties. These compounds showed remarkable antiproliferative activity against breast cancer cells when reacted with transition metals such as copper. In addition, the authors synthesized a selective COX-2 inhibitor derivative of ketoprofen, compound (15), which showed prominent effects on the inhibition of cell growth and promote programmed cell death in COX-2-positive cells (Ahmed et al. 2007). In addition, the authors synthesized a selective COX-2 inhibitor derivative of ketoprofen, compound (15), which showed outstanding effects on inhibiting cell growth and promoting programmed cell death in COX-2-positive cells (Zhao et al. 2023; Ahmed et al. 2007). Adsule et al. prepared Schiff base copper complexes of quinoline-2-carboxaldehyde ligands and investigated their ability to cause cytotoxicity and induce apoptosis in prostate cancer cell lines. The compounds induced apoptosis in prostate cancer cell lines without triggering oxidative stress, and the addition of thiocarbonyl side chains increased their anticancer activity. Here, compound (16) showed potent proteasome inhibitory activity (Adsule et al. 2006). Cerchiaro et al. prepared isatin-Schiff base copper(II) complexes that showed keto-enol equilibria and high stability (Cerchiaro et al. 2005). These complexes triggered the activation of the apoptotic program in human promonocytes and neuroblastoma cells. Two specific complexes, (17) and (18), induced apoptosis via the mitochondrial pathway. There was a correlation between the amount of apoptosis and the uptake of copper within the cells. It was hypothesized that these complexes transport copper into cells, produce ROS, and act specifically on mitochondria due to their delocalized lipophilic cations (Filomeni et al. 2007).
In 2017, Kathiresan et al. pointed out that a group of four copper(II) complexes with mixed ligands of the general formula [Cu(L)(diimine)](ClO4) (19), where the diimines are phen, 2,2’-bipyridine (bpy), 4,4’-dimethyl-2,2’-bipyridyl (dmbpy), or 2,2’-dipyridylamine (dpa) were able to effectively cause single-strand breakage in the pUC18 plasmid DNA by introducing ascorbic acid. Moreover, bovine serum albumin (BSA) showed static quenching when interacting with these complexes. Their ability as free radical scavengers and anti-inflammatory agents was evaluated using 2,2-diphenylpicrylhydrazyl (DPPH) and protein denaturation techniques. Moreover, in vitro inhibitory effect studies against AGS cancer cells showed strong anticancer effects (Kathiresan et al. 2017).
In another study, distorted square planar copper(II) complexes (20) synthesized as Schiff base derivatives of nimesulide were found to have a significant cytotoxic effect on both pancreatic tumor cell lines BxPC-3 (COX-2 positive) and MiaPaCa (COX-2 negative), with IC50 values of 3 to 26 μM for the COX-2-positive cell line and 5 to 9 μM for the COX-2-negative cell line, which was more remarkable compared with nimesulide with IC50 values of 35 μM for the COX-2-positive cell line and more than 100 μM for the COX-2-negative cell line. The observed biological activity of these compounds was attributed to the suppression of VEGF and COX-2 cells, as well as the down-regulation of the antiapoptotic proteins Bcl-2 and Bcl-XL (Ambike et al. 2007; Kroemer and Reed 2000). The structure of the aforementioned copper(II) complexes is illustrated in Fig. 2.
In a study, the copper(II) complex containing imidazole (21) was reported to have the strongest antitumor activity against the B16 mouse melanoma cell line (Tamura et al. 1987). In another study, a copper(II) complex (22) containing 1-methyl-4,5-diphenylimidazole with distorted octahedral geometry significantly delayed cell division, sister chromatid exchanges (SCEs), and mitotic indices (MIs) in cultured human lymphocytes (Raptopoulou et al. 1998). It also reduced proliferation rate indices (PRIs), indicating its cytostatic and cytotoxic effects. Low concentrations of compound (22) caused the unwinding of the plasmid pKS DNA and cleavages on both closed, supercoiled and open, relaxed forms of DNA, with guanosines being favored. In addition, strand cross-linking of DNA bases was shown in ds-DNA from calf thymus and plasmid DNA treated with the compound (22). According to studies, benzimidazole and its derivatives were recognized for their diverse biological properties, including antibacterial, antiviral, anticancer, and antifungal activities (Habib et al. 1997). For example, the trigonal bipyramidal copper(II) complex (23) five-coordinated with two chelating 2-(4-thiazolyl)benzimidazole or thiabendazole ligands and a chloride ion (Devereux et al. 2004) showed significant chemotherapeutic potential when tested against CAL-27 and SK-MEL-31 cells. In another study, Saczewski et al. synthesized and characterized a group of copper-based complexes with bidentate chelating ligands derived from benzimidazole (Saczewski et al. 2006). Among these compounds, complex (24) showed highly potent Cu,Zn-SOD activity in vitro (IC50 = 0.09 μM). This value was in line with Cu,Zn-SOD mimetics of ideal molecular weight reported in the literature, including heterodinuclear copper(II)–Zinc(II) complexes with imidazolate bridges. Moderate inhibitory effects on cell growth were observed in in vitro anticancer evaluations involving seven human tumor cell lines. Lung cancer cell line A427 showed the highest level of sensitivity to compound (24) (IC50 = 4.76 to 10.12 μM) (Szilagyi et al. 2005; Patel et al. 2002).
In 2022, Bello et al. concluded that copper(I) and (II) complexes (25a-d) containing pyrazole derivatives exhibited significant antitumor activity and were stronger than the standard drug cisplatin in a variety of human tumor cells. In addition, these complexes were able to overcome resistance to oxaliplatin and multidrug resistance. Notably, compared with cisplatin, the complexes showed superior efficacy when tested against 3D spheroids of PSN-1 pancreatic cancer cells. Among these complexes, the copper(I) complex (25d) containing triphenylphosphine (PPh3) showed the most hopeful results. Mechanistic evaluations showed that (25d) induced cancer cell death by an alternative form of apoptosis (Del Bello et al. 2022). Dallavalle et al. (Dallavalle et al. 2002) found that the copper(II) complex containing triazole (26) exhibited cytotoxic activity comparable to cisplatin in human fibrosarcoma HT1080 cells. Interestingly, normal human fibroblasts were not affected by (26). Further studies revealed that (26) triggered a particular model of programmed cell death characterized by the formation of large cytoplasmic vacuoles without nuclear fragmentation or caspase-3 activation. This suggests that (26) induces a non-apoptotic form of programmed cell death by inhibiting caspase-3-dependent apoptotic signaling pathways (Tardito et al. 2006). Similar results were observed in human ovarian adenocarcinoma cells treated with certain copper(I) phosphine complexes (Marzano et al. 2008). Tridentate copper(II) complexes with pyridine ring (27–29) were shown cytotoxic effects against HeLa, HepG2, and BEL-7402 cell lines. Their IC50 values (0.93 × 10–4–2.72 × 10–4 M) were comparable to cisplatin (0.14 × 10–4–0.26 × 10–4 M) and lower than 5-fluorouracil (8.76 × 10–4–24.31 × 10–4 M). These complexes were found to cause nuclear chromatin cleavage, as observed by AO/ EB staining assay and gel electrophoresis of pBR332 DNA cleavage in the presence of sodium ascorbate. They bind to calf thymus deoxyribonucleic acid (CT-DNA) by intercalation. (27) exhibited the highest interaction with CT-DNA, followed by (28) and (29). This binding to DNA led to the induction of apoptosis in BEL-7402 cells (Li et al. 2013). Apoptosis or programmed cell death can be induced by copper-based compounds containing isatin di-imine, (30) and (31). These complexes were tested on the cell lines SH-SY5Y, M14, and U937, and a mitochondria-dependent apoptosis pathway was detected. It was found that (30) with a lower penetration generated ROS and induced oxidative stress, while (31) with a higher penetration rapidly accumulated and damaged nuclear and mitochondrial components (Filomeni et al. 2007). The structure of the aforementioned copper(II) complexes is represented in Fig. 3.
In a study of copper(II) complexes formed from phen, stable bis-phen complexes were found to exhibit nuclease activity when exposed to reducing agents and molecular oxygen (Sigman et al. 1979). Here, the bis-phen complex (32) was identified as a compound capable of oxidatively degrading DNA and RNA by targeting the sugar groups (Sigman et al. 1996). Moreover, it has exhibited intriguing clinical effects, including antitumor, antifungal, antimycobacterial, and antimicrobial properties (Saha et al. 2004). In another study, Zhou et al. stated that (32) induced apoptosis specifically in the G1 phase of liver carcinoma cell line Bel-7402 (Zhou et al. 2002). Furthermore, Cai et al. confirmed that the apoptosis process in Bel-7402 cells upon treatment with this complex could be triggered by an excess of copper-induced through the lipophilic phen ligand (Cai et al. 2007). In the presence of intracellular reducing agents, this excess copper leads to an enhanced generation of ROS and a decrease in the amount of GSH compared to oxidized glutathione (GSSG). In addition, (32) showed a strong cytotoxic effect on human leukemic HL60 cells and human gastric cancer SGC-7901 cells (Zhang et al. 2004). However, there are limitations to the utilization of copper-phen compounds. Under physiological conditions, the formation of these complexes is harmful due to the low association constant of the second phen ligand. In addition, (32) shows low selectivity toward DNA sequences without specificity for nucleotides. To circumvent these limitations, Pitié et al. introduced a serinol bridge (clip) to connect two phen ligands and form the complexes (33) and (34) (Pitie et al. 1998a, b). These modifications offered several advantages. First, the linked phen ligands were coordinated to copper, and second, complexes (33) and (34) exhibited significantly higher DNA cleavage activity compared to (32), utilizing a pathway comparable to that of the bis-phen complex (Pitie et al. 2003; Pitie et al. 1998a, b). The increased DNA cleavage activity was attributed to structural features (Pitie et al. 2003). The incorporation of a serinol bridge was enabled the modification of copper–phenol compounds with antitumor pharmaceuticals that exhibited sequence specificity. This modification increased the selectivity of the compound toward specific DNA sequences, giving it potential anticancer properties (Zhao et al. 2023).
The development of the bifunctional complexes (35a) and (35b) was driven by an interest in improving the DNA cleavage specificity and double-strand break ability of complexes such as (34) and overcoming the drug resistance associated with cisplatin (Hoog et al. 2007). It has been suggested that protonation of the amino group in (34) facilitates binding to the polyanionic DNA structure, possibly through an interaction between hydrogen and phosphate oxygen, particularly in the minor groove (Pitie et al. 1998a, b). In vitro evaluations of the cytotoxic activities of the complexes against various human cancer cell lines revealed prominent cytotoxicity of (34), (35a), and (35b) in the L1210 cells. In particular, (35a) indicated greater efficacy than (35b) in most cases (Hoog et al. 2007; Ozalp-Yaman et al. 2008).
In a separate study, Devereux et al. investigated the structure–activity relationships (SARs) of copper complexes with two nitrogen atoms attached to the copper center. They synthesized a group of copper(II) carboxylate compounds, some of which contained chelating ligands such as phen or bipy. These complexes showed weak catalase mimetics in the presence of imidazole and were inactive without it. However, they showed excellent SOD mimetic activity. Compound (36) was demonstrated to be a potent inhibitory agent in vitro against cell lines HepG, A498, and A549, and its cytotoxicity was about sevenfold greater than that of cisplatin. Derivatives of this compound had a moderate level of solubility and exhibited inhibitory activity comparable to those of copper(II) complexes containing bis-phen. The lack of a relationship between SOD and cytotoxicity suggests that mechanisms other than mimicking SOD are involved in the cytotoxic activity of these complexes (Deegan et al. 2007a, b; Devereux et al. 2007). Rajendiran et al. synthesized and characterized a series of mixed ligand copper complexes with Htdp as the tetradentate ligand and N–N as phen, bpy, tmp, and dpq (Rajendiran et al. 2007). The complex (37) showed a six-coordinated geometry around the copper(II) center. The dpq and phen ligands partially intercalated into DNA base pairs in the minor groove, while the tmp complex interacted hydrophobically with DNA by its CH3 groups. The phen and dpq complexes showed higher efficiency in cleaving DNA when combined with ascorbic acid as a reducing agent. These results highlight the structural and functional properties of these copper complexes, especially their potential in DNA-related processes. In 2022, Alem et al. reported the significant inhibitory activity of two copper(II) complexes (38) and (39) containing phen against MCF-7 cells. The cytotoxicity of (38) (IC50 = 4.29 µM) and (39) (IC50 = 7.58 µM) proved to be stronger than cisplatin (IC50 = 18.62 µM) against the mentioned cells. Molecular docking analysis also agrees well with the experimental tests, with binding affinities of –7.35, –8.76, and –6.32 kcal/mol, respectively, for (38), (39), and cisplatin toward ERα (Alem et al. 2022). In 2023, Fernández et al. investigated the DNA binding of copper(II) complexes of dipeptide-bathophenanthroline (40) and demonstrated strong cytotoxicity for these compounds against the tumor cell lines MDA-MB-231, MCF-7 and A549 (Fernández et al. 2023). The structure of the aforementioned copper(II) complexes is shown in Fig. 4.
Mechanisms of action
Apoptosis
Copper is tightly regulated in the body and is not normally found as an independent ion as it is usually bound to proteins. Proteins that require copper ions for their function are important for copper transport and cancer treatment. The copper-containing SODs are a diverse group of enzymes that play a role in the degradation of ROS by converting O2•– into O2 and H2O2 through a process called disproportionation. ROS are harmful compounds that are formed during the body's oxidation reactions and contribute significantly to the progression of diseases, including cancer (Cheung and Vousden 2022). Cancer cells provide a balance between ROS and antioxidants, which affects certain signaling pathways involved in carcinogenesis. Factors, such as pressure, UV radiation, ischemia/reperfusion, and inflammation, can cause overproduction of ROS, leading to oxidative stress.
Ferredoxin-1 (FDX1) is a key component in cell death induced by copper exposure. It acts as a carrier that converts copper(II) to (I) and triggers cell death. It was identified as a regulator at an earlier stage and controls the modification of proteins with lipoic acid during cell death induced by copper and tricarboxylic acid (TCA) cyclin (Tsvetkov et al. 2022). In reduction-activated signaling, ROS combine with regulatory signals and initiate redox mechanisms that stimulate autophagy and apoptosis in cancer cells. Oxidative stress occurs when there is an imbalance between the generation and degradation of oxygen free radicals, which may indicate certain diseases, including cancer (Sies et al. 2022). While low levels of ROS are involved in signaling and regulate various cellular processes under normal conditions, high levels of ROS can lead to oxidative stress that damages lipids, proteins and DNA and triggers apoptosis (Nakamura and Takada 2021).
Excess copper in cells can generate a large amount of ROS, leading to cytotoxicity and activating multiple apoptotic pathways. Copper is involved in Fenton and Haber–Weiss reactions and catalyzes the creation of highly reactive.OH radicals and lipid peroxidation of membranes (Nakamura and Takada 2021). Exploiting copper's ability to trigger cancer cell death may offer potential strategies for the advancement of cancer treatments. Excess copper not only induces oxidative stress but also causes stress in the ER, leading to DNA damage and inhibition of cell proliferation (Oe et al. 2016). In tumor cells, various intrinsic and extrinsic cellular stress factors disrupt protein homeostasis in the ER, which can shift regulatory signals from adaptive to proapoptotic, leading to cell apoptosis. Copper increases ER permeability, inhibits ER corrective capacity, and increases ROS production, leading to proapoptotic signaling and the elimination of damaged cells (Wei and Fang 2021; Gul et al. 2020). In a previous study by our group on a pyridine-2,6-dicarboxylate copper(II) complex, apoptosis induction was confirmed after ROS generation and cell cycle arrest (in the G2–M phase) (Abdolmaleki et al. 2023a, b).
Regulation of the cell cycle is critical for maintaining cell proliferation and preventing tumor development. An interruption of the cell cycle can cause cell cycle arrest, which inhibits cell proliferation and induces apoptosis (Liu et al. 2022a, b). The cell cycle is a tightly regulated process, and abnormalities in cell cycle regulation can contribute to tumor cell proliferation. Gaining insights into the mechanisms of cell cycle regulation has important implications for the prevention and treatment of diseases associated with cell cycle dysregulation. Stages G1–S, S, and G2–M serve as important checkpoints in the cell cycle. Cell cycle arrest plays a critical role in the inhibitory potency of many cytotoxic compounds on tumor cell growth (Vakili-Samiani et al. 2022). NSC319726 has been introduced as a strong inhibitor of cancer cell cycle progression. Research has shown that dysregulation of copper can cause DNA damage resulting in cell cycle arrest (Shimada et al. 2018). The combination of NSC319726 with metal ions, especially copper ions, enhances its inhibitory effect on cancer cells. The binding of copper to NSC319726 triggers the production of ROS in cells and the degradation of deoxyribopurines, leading to cell cycle arrest in the G phase and apoptosis. These findings indicate that copper-mediated oxidative stress and DNA damage are major consequences of cell cycle arrest (Ji et al. 2023).
In 2021, it was shown that two tetragonal pyramidal copper(II) complexes containing benzimidazole derivatives have the potential to bind to DNA through insertion and groove binding. These compounds were able to cleave CT-DNA via an ascorbic acid-mediated pathway that relies on singlet oxygen. Both complexes showed significant inhibitory activity against A549, HeLa, and SGC-7901 cancer cells. The complex containing 5-chloro-2-(2′-pyridyl)benzimidazole showed higher cytotoxic activity compared with the complex with 2-(2′-pyridyl)benzimidazole as ligand, which correlated with its stronger binding and cleavage ability to DNA, suggesting that the inhibitory potency may be due to DNA binding. The mechanistic study performed on cells showed that the complexes arrested the cell cycle in the G2/M phase, enhanced intracellular ROS levels, and decreased mitochondrial membrane potential )MMP(. It was concluded that the complexes can induce apoptosis by causing DNA damage and disrupting mitochondrial function through the generation of ROS. Moreover, an in vivo study showed that the complex with 5-chloro-sunstitution suppressed the growth of tumors by 50.44%, outperforming the efficacy of cisplatin (40.94%) (Cai et al. 2021). In another study, Xu et al. concluded that a copper complex containing disulfiram (DSF), a drug known to make cancer cells dependent on copper and known to have deleterious effects on their viability, induces apoptosis and cell cycle arrest in multiple myeloma (MM) cells. They also observed disruption of mitochondrial membrane integrity and activation of apoptotic signaling pathways. In animal models, DSF/Cu demonstrated the ability to reduce tumor volume and improve overall survival. These results underline the promising potential of DSF/Cu as a suitable therapeutic compound for MM (Xu et al. 2020).
Angiogenesis
Angiogenesis is regulated by a complex interplay of various stimulating and inhibitory factors. The most important signaling system involved in the proliferation and migration of endothelial cells, which form the basis of blood vessels, is the vascular endothelial growth factor (VEGF) and its receptors. This system is crucial for the development of the embryonic vascular system and is also activated during neoangiogenesis in tumor growth. The biological effects of the VEGF system on cells depend on the presence of various factors and receptors in the tissue, in particular the ratio of the different isoforms of the VEGF. Other signaling systems, such as notch ligands Delta-like 4 (Dll4/Notch), play a role in the selection of endothelial cells for angiogenic expansion. Vascular stabilization and maturation involve the formation of the vessel wall, which is regulated by the PDGFB/PDGFRbeta signaling system and the angiopoietins (Ang1, Ang2) and their receptor tyrosine kinase with Tie2, which recruit mural cells, such as pericytes and smooth muscle cells (Shi et al. 2023).
Angiogenesis is a crucial process for tumor progression and metastatic spread driven by copper-dependent angiogenic factors and enzymes (Hariprabu et al. 2021). Tumors can upregulate copper-related signaling pathways to promote angiogenesis. Although tumor immunotherapy shows promise, it is only effective in certain patients and may only have a transient effect (Yang 2015). The copper deficiency can inhibit angiogenesis by blocking the expression of VEGF and impairing tumor blood vessel formation, effectively starving the tumor. In 2024, Wang et al. synthesized a series of new copper complexes, including mono-, bi-, and tri-nuclear complexes with thiophene-2-formaldehyde TSC and a tetra-nuclear complex derived from 1,2,4-triazole, to develop more effective metal agents to inhibit tumor growth. They investigated the SARs of these complexes. The tri-nuclear copper complex showed the highest inhibitory activity against T24 cells compared to the other copper complexes. In vivo studies showed that this complex had a better antitumor effect than cisplatin and fewer side effects. Further studies on the mechanism of action showed that the copper complexes induced apoptosis in cancer cells and inhibited tumor angiogenesis by preventing migration and invasion of vascular endothelial cells, arresting the cell cycle in the G1 phase, and inducing autophagy (Wang et al. 2024). In another study, a modified phen–copper(II) complex, CPT8, containing a triphenylphosphonium group attached to an alkyl chain, showed potent antiproliferative activity against various cancer cells, including TNBC and MDA-MB-231 cells. CPT8 induced mitophagy by activating the PINK1/Parkin and BNIP3 signaling pathways in cancer cells, which was primarily due to mitochondrial damage. Specifically, CPT8 reduced the ability of human umbilical vein endothelial cells (HUVEC) to form tubes by downregulating Nrf2. The antiangiogenic potential of CPT8 was confirmed by the decreased expression of VEGF and CD34 in HUVEC. In addition, CPT8 inhibited the formation of vasculogenic mimicry by suppressing the expression of vascular endothelial cadherin and the matrix metalloproteinases MMP2 and MMP9. CPT8 also attenuated the metastatic potential of MDA-MB-231 cells. In vivo studies showed that CPT8 suppressed tumor proliferation and vascularization, as indicated by the downregulation of Ki67 and CD34 expression. This makes CPT8 a promising metallic drug candidate for the treatment of TNBC (Zheng et al. 2023). Unver et al. reported that a copper(II) complex containing ImCF3 and bipy as ligands exhibited significantly potent antiangiogenic potential. The complex showed higher antiangiogenic activity (mean = 1.06 ± 0.01) compared to the standard antiangiogenic drug ( ±)-thalidomide (mean = 0.8 ± 0.2). In addition, it showed no irritation or embryotoxicity. However, both ligands showed weak antiangiogenic effects. In particular, one of the ligands, ImCF3, showed a significant irritation potential (86 ± 20%) at a concentration of 50 µg/pellet compared to the standard irritant SDS (84 ± 10%) on the CAM assay (Ünver et al. 2022). In another study, the antiangiogenic properties of copper(II) complexes with 1-adamantoylhydrazone with pyridine rings were investigated. The results confirmed that all three copper(II) complexes can inhibit angiogenesis in vascular endothelial cells (Rodić et al. 2016). In general, studies confirm that copper-based compounds involved in the regulation of angiogenesis could lead to the development of drugs that can inhibit or stimulate angiogenesis in various pathological conditions (Wang et al. 2024; Zheng et al. 2023; Ünver et al. 2022).
Cuproptosis
Cuproptosis is a recently discovered type of cell death caused by the accumulation of copper. Tsvetkov et al. made this discovery in 2022 while investigating the anticancer effects of the copper ionophore elesclomol (ES). They observed that copper interacts with a mitochondrial enzyme called FDX1, leading to an increase in ROS, which ultimately leads to cell death (Tsvetkov et al. 2022). Cuproptosis is characterized by the aggregation of lipoylated mitochondrial enzymes and a loss of iron-sulfur proteins. The excess copper is transported into the mitochondria via ionophores, and the reduction from copper(II) to (I) leads to the aggregation of lipoylated proteins and destabilization of iron-sulfur cluster proteins, which ultimately triggers cuproptosis (Wang et al. 2022; Xie et al. 2023). It is worth noting that cuproptosis differs from other known forms of cell death, such as apoptosis, necroptosis, pyroptosis, and ferroptosis. Unlike these forms, cuproptosis is not dependent on ROS and cannot be inhibited by antioxidants. In addition, cuproptosis is linked to mitochondrial function and energy metabolism. Cancer cells that rely on mitochondrial respiration are more susceptible to cuproptosis than cells that rely on glycolysis. Overall, cuproptosis sheds new light on the mechanisms behind copper-induced toxicity and cell death. The discovery of cuproptosis has prompted researchers to investigate the role of copper in cancer development and its potential as a therapeutic target. By harnessing the toxicity of copper, it may be possible to kill cancer cells. Copper-based compounds, both new and repurposed, can be used in combination with existing anticancer drugs or as a starting point for further optimization of treatment (Tsvetkov et al. 2022; Wang et al. 2022). Cuproptosis has attracted considerable interest in cancer research due to its potential to inhibit tumor cell proliferation, reverse drug resistance, and provide novel therapeutic approaches similar to ferroptosis. Promising compounds have been identified that can promote cuproptosis in preclinical models. In colorectal cancer, downregulation of the FDX1, DLAT, SDHB, and DLST genes in primary tumor tissues suggests a role of cuproptosis in cancer progression. Patients with higher expression of these genes in tumor tissue have a better prognosis (Yang et al. 2023a, b, c, d; Yang et al. 2023a, b, c, d; Yang et al. 2023a, b, c, d; Yang et al. 2023a, b, c, d). Treatment with ES-Cu significantly reduces the viability of colorectal cancer cells. The copper chelator tetrathiomolybdate (TTM) inhibits cuproptosis, indicating its role as a cuproptosis inhibitor. In addition, 2-deoxy-D-glucose, an inhibitor of glucose metabolism, sensitizes cancer cells to cuproptosis. Galactose and octyl itaconate (4-OI) also promote cuproptosis, with 4-OI inhibiting GAPDH-mediated aerobic glycolysis and silencing of FDX1 reversing the cuproptosis-promoting effects. In vivo experiments show that ES-Cu enhances the antitumor effect of 4-OI (Yang et al. 2023a, b, c, d; Yang et al. 2023a, b, c, d; Yang et al. 2023a, b, c, d; Yang et al. 2023a, b, c, d). Anisomycin, similar to ES and buthionine sulfoximine (BSO), inhibits the proliferation of ovarian cancer stem cells (OCSCs) by possibly promoting cuproptosis. Curcumin affects ferroptosis and cuproptosis in various HCC cells in a cell-specific manner, and analysis of single-cell transcriptome data suggests its potential as a cuproptosis inducer (Liu et al. 2023a, b). The cuproptosis-related gene CDKN2A is associated with the malignant behavior of head and neck squamous cell carcinoma (HNSCC), and plicamycin inhibits the progression of HNSCC, indicating its potential as a cuproptosis inducer (Fan et al. 2022). Sorafenib, a multi-tyrosine kinase inhibitor used in HCC treatment, and erastin, a ferroptosis inducer, enhance copper ionophore ES and ES-Cu-induced cuproptosis in HCC cells by promoting copper-dependent lipoylated protein aggregation, inhibiting FDX1 protein degradation, and decreasing intracellular GSH synthesis. These results suggest a link between cuproptosis and ferroptosis, and the combination of copper ionophores and ferroptosis inducers could be a promising therapeutic strategy for HCC (Wang et al. 2023a, b).
It can be concluded from the studies that copper complexes have the potential to induce cuproptosis depending on their composition and properties. However, it should be noted that the specific mechanisms and effects of copper complexes may vary depending on the specific complex and cell type. Further research is needed to fully understand the mechanisms and potential of copper complexes in inducing cuproptosis (Xie et al. 2023).
Paraptosis
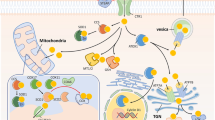
Paraptosis is a form of programmed cell death that differs from apoptosis and is characterized by specific morphological changes in the cell. It was first identified by Sperandio et al. in 2000 and is known as type III programmed cell death (PCD). Paraptosis is characterized by increased cytoplasmic density, vacuolization, swelling of the mitochondria, ER, and the formation of multimembrane vesicles (Sperandio et al. 2000; Tardito and Marchiò, 2009). Finally, macrophages phagocytize the cells without causing inflammation in the surrounding tissues. Various assays, including DNA labeling and enzymatic analysis, are commonly used to study paraptosis (Ji et al. 2023). Recent research has shown that certain copper complexes can induce paraptosis in tumor cells by suppressing the activity of proteasomes and promoting the accumulation of misfolded proteins, thereby triggering ER homeostasis. This effect is comparable to the well-known chemotherapeutic agent cisplatin (Ji et al. 2023; Gandin et al. 2012). In a study, it was observed that hinokitiol–copper complex (HK–Cu) caused a significant accumulation of ubiquitinated proteins in A549 and K562 cells. Moreover, HK–Cu showed strong inhibition of 19S proteasomal deubiquitinases (DUBs) compared to its effect on the chymotrypsin-like activity of the 20S proteasome. HK–Cu effectively induced caspase-independent and paraptosis-like cell death in A549 and K562 cells. Finally, the researchers discovered that HK–Cu-induced cell death was dependent on ATF4-associated ER stress, but was not related to the generation of ROS. Overall, these results suggest that HK–Cu has the potential to inhibit the activity of 19S proteasomal DUBs and induce paraptosis-like cell death, making it a promising candidate for cancer treatment (Chen et al. 2017). Paraptosis can be triggered by different mechanisms (Hanson et al. 2023). Translation and transcription are essential for triggering this process. The ER plays an important role in paraptosis by accumulating misfolded proteins, leading to ER stress and the UPR. Inhibition of the proteasome can lead to the assembly of polyubiquitinated proteins and ER stress. The ER is connected to the mitochondria by MAMs, which help to maintain calcium balance. ER stress triggers the UPR and activates proteins like BiP and CHOP. Paraptosis occurs when the apoptosis machinery is not functioning, and one of its distinct features is the dilation of the ER (Zhao et al. 2023). In cells, the ER serves as a storage site for calcium, and IP3 receptors (IP3R) are located in the mitochondria-associated ER membranes. Disturbances in calcium levels can lead to cell death. Calcium leaves the ER via IP3R and enters the mitochondria via the MCU. The ER is responsible for protein synthesis, folding and processing, and calcium plays a role in supporting chaperone function. When calcium is deficient, chaperones are impaired, leading to an accumulation of misfolded proteins and swelling of the ER. Mitochondrial calcium overload leads to oxidative stress. There may be a control loop between ROS and calcium, whereby calcium is upstream of ROS formation and ROS influences the release of calcium. ROS also increases ER stress and thus damages both the ER and the mitochondria. Mitochondrial calcium overload disrupts the distribution of ions, reduces the potential at the membrane and leads to organelle collapse. ROS stimulates the release of carbon monoxide, activates ion channels and affects potassium and sodium levels, leading to ATP depletion and cell death. Paraptosis involves the impairment of both the ER and the mitochondria. The increased presence of mitochondrial proteins, such as ATP synthase and prohibitin, indicates the involvement of the mitochondria. Prohibitin acts as a mediator and agonist in the paraptotic death pathway and influences various cellular processes (Yokoi et al. 2022; Shubin et al. 2016). The types of copper-induced cell death mechanisms are shown in Fig. 5
Clinical studies and translational potential
[64Cu]Cu-ATSM, is a copper complex currently being evaluated in clinical trials for its ability to represent oxygen deprivation in advanced-stage tumors in a localized area of rectal cancer (NCT03951337) and bulky tumors (NCT04875871) (Savi et al. 2017). Liu et al. reported the automated cyclotron generation and preparation of [64Cu]Cu-ATSM using a synthesis module that is readily available for commercial use (Liu et al. 2022a, b). However, it is important to note that multiple administrations of [64Cu]Cu-ATSM can lead to liver toxicity (Yoshii et al. 2018). To mitigate this risk, the administration of penicillamine, a heavy metal chelator, has been suggested to minimize the amount of radiation absorbed by vital organs, such as liver and small intestine (Yoshii et al. 2014).
Researchers have successfully developed and confirmed the efficacy of [64Cu]Cu-ATSM for diagnostic and theranostic applications (Matsumoto et al. 2019). The potential risk of chemical contamination from the degradation of [64Cu]Cu-ATSM is considered insignificant, even at therapeutic doses. Positron emission tomography (PET) with [64Cu]Cu-ATSM has been shown to provide relevant and useful information about tumor oxygenation in a clinical context to predict response to therapy and survival of patients with solid tumors (Liu et al. 2020; Xie and Wei 2022). [64Cu]Cu-ATSM as a theranostic agent has been exploited in Japan. Further exploration of its mechanisms of action may contribute to the understanding of its therapeutic effects (Xie and Wei 2022). The use of 64Cu-labeled agents offers advantages for multicenter clinical trials compared with 60Cu- or 62Cu-labeled agents because they have a longer half-life, which facilitates shipment to multiple centers. The β-decay and Auger electron emission of 64Cu also allows potential therapeutic applications with [64Cu]Cu-ATSM under appropriate conditions (McMillan et al. 2015). In tumor masses, [64Cu]Cu-ATSM tends to accumulate mainly in the outer periphery, i.e., in regions of the tumor where there is a lack of oxygen but active cancer cells are still present (Obata et al. 2003). [64Cu]Cu-ATSM has been granted Investigational New Drug status by the US Food and Drug Administration. The comparison of [60Cu]Cu-ATSM and [64Cu]Cu-ATSM in patients with cervical cancer confirmed a prominent correlation in tracer uptake, with [64Cu]Cu-ATSM PET images showing a better tumor to-background ratio than [60Cu]Cu-ATSM [142]. [64Cu]Cu-ATSM is better absorbed in hypoxic tissues and offers several significant advantages over other radiotracers. It may have better penetration through the blood–brain barrier (BBB) than [18F]FAZA tracer, which is excreted through the urinary tract (Savi et al. 2017). In addition, [64Cu]Cu-ATSM shows more uptake in hypoxic tissues and faster washout in normoxic cells compared to [18F]FMISO, which requires at least a two-hour equilibration time before scanning (Xie and Wei 2022; Takasawa et al. 2008).
The Cu(II)-GTSM (39) (Xie and Peng 2017), can transport exogenously bound copper directly into cells, activating mechanisms in which copper is a critical cofactor. On the other hand, Cu(II)-ATSM (40) is a biosimilar that releases exogenously bound copper only under conditions of low oxygen levels (Fig. 6a) (Xie and Wei 2022). Both Cu-ATSM and ATSM are rapidly eliminated from the circulation with half-lives (T1/2) of approximately 21.5 and 22.4 min, respectively (Xie and Wei 2022; Nam et al. 2022). The BBB is a highly selective gateway that regulates the movement of molecules between the systemic circulation and the brain and in the reverse direction. Copper(II)-ATSM exhibits an increased ability to penetrate and cross the BBB (Nam et al. 2022). The penetration of this compound in cells is influenced by the properties and nature of copper. The exact mechanisms of Cu-ATSM uptake have not been fully elucidated, but several possibilities have been proposed. A mechanism states that Cu(II)-ATSM can enter mitochondria and be reduced from copper(II) to copper(I), resulting in long-term storage of the metal or radiometal in hypoxic cells. Cu(II)-ATSM can easily penetrate cells due to its small size, excellent ability to penetrate membranes, and low redox potential. In cells that are too reduced, such as under hypoxic conditions, the copper(II) in Cu(II)-ATSM is reduced to copper(I). This reduction results in copper(I) being released from the ATSM and subsequently trapped in the cells (Fig. 6b) (Nam et al. 2022).
Recent research has also shown that Cu-ATSM uptake is not only indicative of hypoxia but also reflects intracellular states characterized by mitochondrial damage and over-reduction. In normoxic cells, Cu(I)-ATSM is rapidly reoxidized to Cu(II)-ATSM by molecular oxygen and subsequently eliminated from the cells (Maurer et al. 2002). Another possible pathway is the dissociation of copper(II) from copper(II) complexes and the reduction of it to copper(I) by reductases present in the blood circulation or the tumor surroundings. Tumor cells suffering from oxygen deficiency often have an increased concentration of CTR-1, which facilitates the transport of copper(I) into the cells (White et al. 2009).
Cu-ATSM labeled with copper isotopes, such as 60Cu, 62Cu, and 64Cu, has been used to visualize tumor hypoxia and blood perfusion. It shows significant absorption in the brain and heart with enhanced permeation under hypoxic conditions. Translational studies have demonstrated its safety profile, rapid clearance from the bloodstream, and accumulation in tumors within minutes. The penetration of Cu-ATSM depends on the oxygen pressure in the tissue, making it a valuable imaging tool for hypoxia. In different cancer types, the uptake patterns of Cu-ATSM differ from those of 18F-FDG, indicating different characteristics in squamous cell carcinoma (SCC) and adenocarcinoma. Cu-ATSM was also recognized to visualize regional oxidative stress in patients with MELAS, a mitochondrial disorder. Cu-ATSM has been correlated with 15O tracers to assess cerebral blood flow and oxygen extraction fraction in patients with cerebrovascular disease (Xie et al. 2022). Moreover, Cu-ATSM uptake is noticeably greater in grade IV gliomas compared with grade III gliomas and correlates with HIF-1α expression, a marker of hypoxia (Tateishi et al. 2013). In addition, the tumor-to-muscle activity ratio of Cu-ATSM has shown promise for predicting treatment outcomes in non-small cell lung cancer (NSCLC) and cervical cancer. To tumor hypoxia imaging, some clinical trials are investigating the safety data and initial efficacy of Cu(II)ATSM in patients with Parkinson's disease (PD) or amyotrophic lateral sclerosis/motor neuron disease (Savi et al. 2017; Xie and Wei 2022).
Capasso et al. investigated the role of 64Cu-PET in assessing the stage of individuals with prostate cancer (PCa). The study included seven PCa patients, three of whom were undergoing hormone therapy. The lack of urinary 64CuCl2 excretion helped identify pelvic prostate cancer lesions. The penetration of 64Cu-PET showed higher values in the original tumors of patients without hormone therapy than in treated patients. Uptake in nodes varied, with a focal dose in normal-sized nodes and no noticeable absorption in suspicious lymph nodes. These initial findings suggest that 64Cu-PET has the potential for the diagnosis of PCa (Capasso et al. 2015). A subsequent study by Piccardo et al. examined the distribution, radiation dose, and behavior of lesions in a group of 50 patients with PCa who experienced biochemical recurrence after surgery or radiotherapy. The accuracy of the diagnosis of 64Cu-PET/CT, 18F-choline PET/CT, and multiparametric MRI (mpMRI) was compared. The research confirmed the efficacy of 64Cu-PET/CT in the detection of local recurrence as well as bone and lymph node metastases (Piccardo et al. 2018). The better performance of 64Cu-PET/CT can be attributed to its better biodistribution compared to 18F-choline PET/CT. Unlike choline, copper ions are not excreted by the kidneys and do not accumulate in the urinary system, allowing a more accurate assessment of the pelvic region and prostate bed with early visualization of lesions in the pelvic area. Dosimetry studies have also shown that the amount of radiation absorbed by recurrent PCa and its spread to other areas is minimal, without considering the therapeutic outcome. Righi et al. have shown that the therapeutic effect of 64Cu in PCa may be due to the emission of Auger electrons with high linear energy transfer rather than beta radiation. Although its efficacy may be limited in large PCa lesions, it may be effective against residual disease or micrometastases because of the damaging effects of Auger electrons released by 64Cu and absorbed into the cells (Fig. 7a–d) (Righi et al. 2018). In addition, 64Cu-PET/MRI was shown to have a higher overall detection rate than 18F-choline PET/MRI, 64Cu-PET/CT, 18F-choline PET/CT, and mpMRI alone when assessing the presence of PCa recurrence in the same area (Paparo et al. 2020). Although there are promising results regarding the use of 64CuCl2-PET/CT for the evaluation of PCa, Cantiello et al. pointed out that the technique has its limitations and is not always superior to imaging modalities currently used in PCa (Cantiello et al. 2021). There are no significant differences between mpMRI and 64CuCl2-PET/CT in the detection of metastatic lymph nodes in the initial diagnosis of primary PCa. However, in re-staging, a significantly higher detection rate is observed in lesion-based analysis than in 18F-choline PET/CT, both in local and lymph node-based staging, but not in patient-based analysis. In addition, 64CuCl2 is mainly degraded by the liver, which may fail to detect liver metastases. Mascia et al. reported a prospective study to assay the safety and efficacy of 64CuCl2 as a PET radiopharmaceutical for imaging other urologic malignancies (Mascia et al. 2021). The research included a total of 23 patients with renal, bladder, and penile cancers. Uptake of 64Cu was highest in PCa lesions (SUVmax 11.5), relatively high in bladder cancer (SUVmax 6.2), and lower in penile cancer (SUVmax 3.9) and renal cancer (SUVmax 5.0). This suggests that 64Cu-PET/CT may be beneficial to assess primary and locally recurrent bladder cancer lesions because of a significant contrast between the desired area and the surrounding background. However, the potent background uptake of 64Cu in the kidneys (SUVmax 10.4) may reduce its utilization in the assessment of primary renal cancer tumors (Mascia et al. 2021). In another study, Panichelli et al. investigated the feasibility of 64CuCl2 PET/CT for imaging in patients with glioblastoma (GBM). In this study, all patients with GBM had high tumor uptake of 64Cu, whereas patients with low-grade astrocytoma had low tumor uptake of 64Cu. Importantly, neoplastic tissue was rapidly and unequivocally detected and radioactivity remained stable over time (Fig. 7e). This research explained further reasons for the use of 64CuCl2 as a radiopharmaceutical for PET imaging (Panichelli et al. 2016). Fiz et al. performed a study of the use of 64Cu-PET/CT in pediatric patients with diffuse high-grade glioma and found desirable dosimetry and the ability to detect tumor recurrence in cases with unclear MRI results (Fiz et al. 2022). Another study by García-Pérez et al. investigated 64Cu uptake in individuals diagnosed with non-small cell lung carcinoma, finding high uptake in peripheral primary lung cancer lesions and nodal metastases. Patients with high 64Cu uptake had a partial response to chemotherapy, whereas patients with low uptake had disease progression. Expression of the CTR1 is associated with 64Cu uptake, indicating the potential utility of 64Cu PET/CT in identifying individuals who may benefit from platinum-based treatment (García-Pérez et al. 2020).
64CuCl2 PET/CT imaging: a Uptake of the tracer in the prostate apex, near the midline, 1 h after injection, b Marked reduction of tracer uptake 4 h after injection, c 24 h after injection, d Maximum intensity of tracer uptake and e Presence of cerebral glioblastoma in the brain, imaged 1 h after injection
64CuCl2 is a potential radiopharmaceutical for the diagnosis and therapy of various cancers, such as PCa and GBM. PCa cells have been found to have increased uptake of 64CuCl2, leading to DNA damage and genomic instability (Guerreiro et al. 2018). In preclinical models, 64CuCl2 has shown therapeutic effects by significantly reducing the growth and viability of PCa cells. In GBM, 64CuCl2 has shown an increased affinity for tumor cells and potential as a diagnostic PET probe. In addition, it has shown therapeutic potential for GBM, with synergistic effects observed when used in combination with other treatments. Regarding copper toxicity, calculations show that the amount of copper administered during PET imaging or therapy with 64CuCl2 is not harmful to patients. The copper concentration required for cytotoxic effects is much higher than the amount administered in PET studies or therapy. The dose of 64Cu administered in PET imaging is below the cytotoxic threshold, and even in therapy, the dose remains below the toxicity threshold (Capriotti et al. 2022). In addition, 64Cu shows the possibility of radionuclide therapy in other copper-metabolizing tumors, including HCC, GBM, and MM. The results demonstrate the promising capabilities of 64CuCl2 as a valuable tool for both diagnosis and therapy in various types of cancer (Righi et al. 2018).
Copper nanoparticles in cancer therapy
Metal-based nanoparticles have proven to be useful in various therapeutic areas due to their unique properties and synergistic effects. Copper nanoparticles have gained special attention in this regard as ideal candidates for cancer therapy. These nanoparticles have remarkable properties, including a large surface area to volume ratio, excellent compatibility with living organisms, and the ability to generate ROS when exposed to an acidic tumor microenvironment (Kang et al. 2023; Amatya et al. 2023; Liu et al. 2021a, b, c).
One of the main advantages of copper nanoparticles in cancer therapy is their capacity to specifically target cancer cells while preserving healthy cells. The targeting approach can be achieved by functionalizing the surface of the nanoparticles with specific ligands or antibodies that can recognize and bind to cancer cells. The targeting mechanism allows therapeutic agents to be delivered directly to the tumor, minimizing off-target effects (Liu et al. 2023a, b; Shen et al. 2022). Several studies have shown that copper nanoparticles can be used as effective agents in chemodynamic therapy (CDT) (Liu et al. 2021a, b, c; Liu et al. 2023a, b; Shen et al. 2022). This is a relatively new method in cancer therapy that utilizes ROS generated by the catalytic action of transition metal-based nanomaterials. Unlike traditional chemotherapy, which relies on cytotoxic drugs, CDT uses the intrinsic properties of the nanomaterials to induce tumor cell death. CDT is a promising treatment strategy for cancer that utilizes the in situ Fenton reaction, which is activated by endogenous substances, such as GSH and H2O2 without the need for external energy input (Yu et al. 2021). CDT overcomes the limitations of light penetration into tissues and has gained attention in recent years. Copper-based substrates have been developed that generate H2O2 internally and function effectively in weakly acidic tumor microenvironments (TME) (Liu et al. 2021a, b, c). In a study, copper peroxide nanodots were used to improve CDT. These nanoparticles self-supply H2O2 and release copper ions in an acidic tumor environment. The released copper ions react with H2O2 to produce.OH, which can induce cell death. The nanoparticles also accumulate in tumors and successfully inhibit tumor proliferation with minimal side effects. This study introduces a new method to prepare metal peroxide nanomaterials and offers a promising strategy to improve CDT efficacy (Lin et al. 2019).
In 2022, copper–iron peroxide nanoparticles (CFp NPs) were developed as a new CDT system for synergistic therapy in TME. These CFp NPs release H2O2, copper ions, and iron ions under the slightly acidic conditions of the TME. Copper and iron ions, especially at lower oxidation states, work together to efficiently generate.OH. This unique synergism, aided by the copper(I)-assisted conversion of Fe3+ to Fe2+, distinguishes this CDT system from previous systems. Remarkably, this approach achieves almost complete tumor ablation with a minimal treatment dose, without the need for additional therapies. In addition, CFp NPs can generate O2 during the catalysis process and show TME-dependent T1 contrast enhancement in magnetic resonance imaging. These properties are beneficial for alleviating hypoxia or monitoring tumors in vivo (Koo et al. 2022). In 2023, researchers developed another copper-based CDT agent called nanoparticle Cu@cLAs, which effectively addresses the issues of high copper consumption and potential toxicity associated with previous copper-based CDT agents. Cu@cLAs consist of copper anchored on cross-linked lipoic acid nanoparticles. When Cu@cLAs are taken up by tumor cells, they release copper (I) and (II) ions upon dissociation into lipoic acid and dihydrolipoic acid. These copper ions undergo a self-recycling process within the cells that leads to efficiently killing cancer cells by delaying the loss of copper metabolism and enhancing ROS levels (Fig. 8a). The efficacy of this self-recycling process was confirmed by sustained high copper/dihydrolipoic acid (DHLA) content and sustained ROS generation. To investigate the therapeutic efficacy of Cu@cLAs, an animal model of MCF-7/R in nude mice was used. Cu@cLAs were injected intravenously at a dose of 5 mg/kg, which corresponds to 10% of the safe dose (Fig. 8b). During the 21-day treatment process, the control groups treated with saline and the chemotherapeutic agent doxorubicin (DOX) showed rapid tumor growth, while the tumor size increased only slightly in the Cu@cLAs group (Fig. 8c and d). Further validation of the superiority of Cu@cLAs in tumor therapy was provided by the examination of removed tumors (Fig. 8e). The results showed that Cu@cLAs had higher efficacy in tumor suppression compared to DOX. Importantly, the copper content of Cu@cLAs at 5 mg/kg was significantly lower than the previously reported optimal counterparts. The therapeutic efficacy of Cu@cLAs was also confirmed by histological staining of tumor sections, which showed greater apoptosis and necrosis compared to DOX-treated mice (Fig. 8h). Consistent with the therapy results, the survival experiment showed a higher survival rate in mice injected with Cu@cLAs compared to saline- and DOX-treated mice (Fig. 8f). The Cu@cLAs-treated group exhibited a favorable level of biosafety throughout the treatment, as evidenced by the absence of significant weight changes (Fig. 4g). In general, Cu@cLAs showed an antitumor effect of up to 77.9% at a copper dose lower (0.05 mg/kg) than the normal serum copper level (0.83 ± 0.21 mg/kg). This research not only represents a promising clinical strategy to reduce excessive copper consumption in copper-mediated CDT but also provides insights for other metal-mediated therapies with high metal consumption (Cui et al. 2023).
a Graphical representation of the self-cycling system based on Cu@cLAs for copper-mediated chemotherapeutic drug delivery. b Schematic of the process of MCF-7/R tumor xenograft preparation, drug delivery, and survival tracking. c Images showing the different sizes of tumors in mice subjected to different treatments. d Graphical representation of the change in tumor volume during the treatment period. e Digital photographs of the tumors of mice bearing MCF-7/R tumors after 21 days of therapy, along with the percentage of tumor inhibition for each treatment group. f Percentage of survival of mice after different treatments. g Changes in body weight of the mice during therapy and h Histological analysis of tumor sections from mice with MCF-7/R tumors, involving the use of H&E staining and TUNEL staining
Copper nanoparticles can also be used in phototherapy. Phototherapy includes two main approaches: photothermal therapy (PTT) and photodynamic therapy (PDT). PDT is a noninvasive treatment method that uses photosensitizers (PS) to generate cytotoxic ROS in premalignant and neoplastic conditions. In contrast, PTT focuses on inducing cell death by hyperthermia using photoabsorbing agents (Zhuo et al. 2023). PDT uses light, O2, and PS to produce cytotoxic ROS that trigger apoptosis in cancer cells by type I and/or type II photochemical reactions (Zhuo et al. 2023; Cheng et al. 2023). In type I reactions, the excited triplet state of PS interacts directly with cancer cell biomolecules, resulting in the formation of radical cations or anions, which then react with O2 to generate cytotoxic ROS (O2−, −OH, H2O2, etc.). In type II reactions, the excited triplet state of PS sensitizes O2 and produces singlet oxygen (1O2) within cancer cells (Cheng et al. 2023). Although PDT shows promise in the treatment of superficial tumors, there are challenges in achieving high tumor selectivity, efficient ROS production, and monitoring treatment success. Copper-based nanomaterials offer potential solutions to these limitations. For example, copper-doped carbon dots (Cu-CDs) developed by Wang et al. exhibit high fluorescence quantum yield, low cytotoxicity, and efficient 1O2 production, effectively inhibiting the growth of multicellular spheroids (Wang et al. 2019a, b). Guo et al. designed tumor-selective nanoparticles (Cu(ii)Chl-HA NPs) that replace magnesium(II) in chlorophyll to enhance 1O2 production and achieve receptor-mediated targeting in CD44-overexpressing cancer cells (Guo et al. 2019).
Researchers have developed copper-based complexes that combine type I and type II PDT with gene silencing to overcome limitations in 1O2 yield and selectivity for cancer. For example, Liu et al. developed an ultrathin 2D copper(I)-1,2,4-triazolate coordination polymer nanosheet (Ce6-DNAzyme/[Cu(tz)]) that responds to GSH and light. This nanosheet carrier released a tumor-targeted DNAzyme that generated 1O2 through type II PDT and triggered a type I response for effective antitumor activity in hypoxic TMS (Fig. 9a and b). To evaluate the efficacy of the treatment, the nanosheet was administered intravenously to mice with MCF-7 tumors. The body weight of the mice and the relative tumor volume were determined every three days. A gradual increase in body weight of all mice was observed as time progressed, indicating minimal side effects of Ce6-DNAzyme/[Cu(tz)] in vivo. The relative tumor volumes of mice in the phosphate-buffered saline (PBS)-treated group with 660 nm and 808 nm laser irradiation (group i) and the Ce6-cDNA/[Cu(tz)] group in the dark (group ii) increased around 14-fold over the 21-day treatment, indicating negligible effects of laser irradiation alone or Ce6-cDNA/[Cu(tz)] injection. In contrast, gene silencing was the only method used (group iii), type II PDT alone (group iv) and type I PDT alone (group v) only partially inhibited tumor proliferation. However, in group vi, in which the combination of gene silencing, type II PDT, and type I PDT was administered, tumors regressed markedly. These findings are in agreement with the images and weights of the removed tumors from the mice that were euthanized after 21 days of treatment (Fig. 9c, d). In comparison to the tumor weights in group i, at day 21, tumors in groups iii, iv, and v treated with single therapies had decreased by 44.7%, 48.7%, and 52.7%, respectively. In contrast, the combination therapy in group vi inhibited tumor proliferation by 88.0%, demonstrating the enhanced efficacy of the combination of gene therapy, type II PDT and type I PDT (Liu et al. 2021a, b, c). Xu et al. developed glucose oxidase (GOx)-loaded copper nanoparticles (GOx@[Cu(tz)]), which triggered cuproptosis-mediated synergistic photodynamic therapy in bladder cancer. GSH stimulation initiated GOx activity, which degraded glucose and GSH and sensitized tumor cells to GOx@[Cu(tz)]-associated cuproptosis through elevated intracellular H2O2 (Xu et al. 2022). Chen et al. introduced copper-cysteamine (Cu-Cy) nanoparticles as a novel generation of PDT sensitizers activated by X-rays. These nanoparticles inhibited the proliferation and migration of cancer cells without apparent toxicities in vivo, as demonstrated by the downregulation of proliferating cell nuclear antigen (PCNA) and E-cadherin expression (Chen et al. 2022). In addition, He et al. evaluated a PDT treatment with copper-doped calcium phosphate nanoparticles (CCPCA NPs) that reduced cancer hypoxia and continuously delivered oxygen generated by catalase. This approach improved the concentration of protoporphyrin IX (PpIX) and metabolic half-life by reducing PpIX efflux and increasing PpIX formation (He et al. 2022).
a Process for the preparation of 2D [Cu(tz)] nanosheets and the Ce6 DNAzyme/[Cu(tz)] therapeutic platform. b Schematic representation of the proposed combination therapy with DNAzyme-based gene silencing, Ce6-based PDT type II and [Cu(tz)] nanosheet-based PDT type I. c Photographs and d weights taken and recorded for analysis
Photothermal therapy (PTT) is an effective treatment method for inhibiting tumor growth using a near-infrared (NIR) laser to generate hyperthermia and selectively destroy cancer cells. Copper sulfide nanoparticles (CuS NPs) have been developed for nucleus-targeted PTT, which allows direct targeting of cancer cells and induces apoptosis through NIR irradiation (Li et al. 2018). In another study, a NIR-activated CuS nano-platform (CuS-RNP/DOX@PEI) was presented to deliver Cas9 RNP and DOX for synergistic antitumor therapy and enhance the PTT effect by accelerating the release of therapeutic agents (Chen et al. 2021). Copper-based PTT has also been shown to enhance radiotherapy (RT). A CuS-based nano-platform significantly delayed tumor growth and improved survival when combined with RT (Aishajiang et al. 2023). Furthermore, PTT can be combined with immunotherapy, as shown by a strategy using CuS-RNP@PEI, which triggers PTT and promotes factors of the immune response (Yan et al. 2021).
Copper nanoparticles have also shown success in destroying cancer tissue by hyperthermia. This method is a local anticancer treatment in which cells are exposed to high temperatures. It has shown promising results when used in combination with radiotherapy or chemotherapy for various tumor types. Researchers are continually working to improve hyperthermia by studying its effects on DNA repair pathways, immune response, and its ability to target specific tumor areas. Recent studies have found that hyperthermia can inhibit DNA repair pathways and increase tumor cell killing when combined with ionizing radiation. It has also been demonstrated that hyperthermia at 42 °C can induce an immune response and inhibit tumor growth and metastasis. Comparisons of heating methods have shown that microwave heating is more effective at killing cancer cells and increasing the release of HSP70, a heat shock protein. These results contribute to the advancement of knowledge about the effect of hyperthermia on tumor cells and provide valuable insights for further research and clinical application (Crezee et al. 2021).
Hyperthermia is a promising clinical treatment that, when used in combination with other therapies, has shown significant benefits in improving tumor control and disease-free survival without increasing side effects. Researchers have focused on determining the dose–response relationship of hyperthermia and have found that tumors with a pathologic response to preoperative radio(chemo)therapy and locoregional hyperthermia have higher tumor temperatures (Datta et al. 2015; Unsoeld et al. 2020). In 2022, Gajare et al. presented two nanomagnetic copper complexes, [Nano-magnetite-Lys@Cu(PPh3)I] and [Nano-magnetite-Arg@Cu(PPh3)I], with sizes of approximately 12 nm and 15 nm, respectively. These compounds showed notable anticancer effects toward MCF-7 cells with IC50 values of 104.38 μg/mL and 95.81 μg/mL, respectively, compared with the reference drug cisplatin (IC50 = 88.91 μg/mL). Hyperthermia analyses showed that both complexes had impressive SAR values, ranging from 36.83 − 85.81 W/g and 97.67 − 125.58 W/g at therapeutic temperatures of 48 °C and 43 °C, respectively. These results suggest that both complexes may be effective in the treatment of breast cancer using a dual approach, combining the effects of hyperthermia and chemotherapy (Gajare et al. 2022). In another study, CuO NPs (25, 50, and 100 μg/ml), hyperthermia (41 °C for 1 h), and irradiation (200 cGy) were able to inhibit the proliferation of MCF7 cells and induce cell apoptosis via MMP collapse (Ghaleh et al. 2019).
In 2023, Huang et al. reported Cu-doped polypyrrole for cancer treatment via hyperthermia-triggered nitric oxide (NO) release. According to studies, NO as a gaseous medium has a significant role in tumor progression. Thus, high concentrations of NO can lead to mitochondrial dysfunction and DNA damage. The delivery and release of NO in gas therapy to eradicate malignant tumors at non-toxic levels is challenging and unpredictable. To overcome these challenges, a multifunctional nanocatalyst called Cu-doped polypyrrole (CuP) was developed as a smart nanoplatform (CuP-B@P). This nano-platform is designed to target the NO precursor BNN6 to tumors and release NO. In the abnormal metabolic conditions of the tumor, CuP-B@P catalyzes the conversion of the antioxidant GSH to GSSG and excess H2O2 to −OH through the copper(I)/copper(II) cycle. This process leads to oxidative damage to tumor cells and the release of BNN6. Moreover, when irradiated with laser light, the nanocatalyst CuP can absorb photons and convert them into hyperthermia, which further enhances the catalytic efficiency and pyrolyzes BNN6 into NO. Through the synergistic effects of hyperthermia, oxidative damage, and NO release, the nanocatalyst achieves nearly complete eradication of tumors within the body while causing minimal harm or toxicity. In this study, the distribution of CuP-B@P was investigated in vivo after intravenous injection. The fluorescence of CuP-B/Cy5@P at tumor sites elevated from 2 to 4 h and remained high from 4 to 24 h (Fig. 10a and c). On the other hand, the fluorescence of free Cy5 initially maintained a robust presence throughout the entire body but rapidly decreased, indicating that the tumor was not targeted. After the experiment, the major organs and tumors were examined using an in vivo imaging system. The analysis showed that the fluorescence intensity in the distant tumor tissue was significantly stronger in the CuP-B/Cy5@P group than in the Cy5 group, confirming the specific accumulation and storage of CuP-B/Cy5@P in the tumor (Fig. 10b and d). Next, in vivo photothermal imaging of CuP-B@P was performed using a thermal imager. When the tumor tissue in the CuP-B@P group was exposed to laser irradiation of 808 nm (1 W/cm2, 5 min), the temperature gradually increased and reached 57 °C after 5 min. This temperature was significantly higher than the temperature (40 °C) observed in the PBS group (Fig. 10e and f) (Huang et al. 2023).
a In vivo fluorescence imaging of mice bearing MCF-7 tumor over time after intravenous injection of free Cy5 or CuP-B/Cy5@P. c Corresponding quantitative analysis of fluorescence intensity in the tumor region. b Ex vivo fluorescence imaging of tumors and major organs, together with d Corresponding quantitative analysis. e and f Infrared thermal imaging of mice bearing MCF-7 tumors and the temperature at the tumor sites after intravenous injection of PBS or CuP-B@P during 808 nm laser irradiation (1 W/cm.2)
According to various studies, copper-based compounds can enhance the effectiveness of immunotherapy in the treatment of cancer (Guo et al. 2022; Zhao et al. 2018; Kong and Sun 2023). Unlike traditional cancer treatments, such as chemotherapy and radiotherapy, which target both cancer and healthy cells, immunotherapy focuses on strengthening the body's immune system to specifically recognize and eliminate cancer cells. One possible way in which copper can improve immunotherapy is by releasing DAMPs from dying cancer cells. DAMPs are molecules that can trigger the immune system to initiate a response against tumors. Treatment with copper can also increase the activity of immune cells, such as T cells and natural killer cells, which have a main effect in the recognition and elimination of cancer cells (Hu et al. 2020). Zhang et al. conducted a study where they explored the application of nanoparticles containing copper and cysteamine to deliver radiation therapy, oxidative therapy, and immunotherapy simultaneously to treat melanoma (Fig. 11a) (Zhang et al. 2020). This research highlights the potential of metal complexes, particularly copper-based compounds, to enhance the outcomes of immunotherapy in cancer treatment. The distribution of Cu-Cy nanoparticles (NPs) within cells was investigated by confocal fluorescence microscopy. The results showed that the uptake of Cu-Cy NPs into the nucleus increased significantly after 6 h of incubation compared with 2 and 4 h. The cytotoxicity of Cu-Cy on B16 cells was evaluated using the CCK8 viability assay. After cells were incubated with various concentrations of Cu-Cy for 24 h, they were irradiated with either 0 or 2.5 Gy of X-rays. The results shown in Fig. 11b and c confirm that the viability of the cells in the control group (Cu-Cy only) did not decrease noticeably. However, in the 2.5 Gy group, there was a dose-dependent decrease in cell viability, indicating that the Cu-Cy NPs have weak toxicity to cells but can be activated by X-rays to cause significant cytotoxic activity. In Fig. 11d, the Cu-Cy + X-ray group showed higher green fluorescence of dichlorofluorescein (DCF) compared with the PBS, Cu-Cy, and PBS + X-ray groups, demonstrating the production of ROS. In the apoptosis assay, the Cu-Cy group exhibited a low apoptosis rate of 24.1%, whereas the combined treatment with Cu-Cy and X-ray showed a significant killing effect with an apoptosis rate of about 84.7% (Fig. 11e and f). In the in vivo study, the PBS and Cu-Cy groups had minimal effects on tumor proliferation, whereas the PBS + X-ray and Cu-Cy + X-ray groups illustrated a strong antiproliferative effect from day 12. The Cu-Cy + X-ray group had the most significant inhibitory effect (Fig. 11g and h). No noticeable changes in body weight or pathological damage to the spleen were observed in any of the groups. These results indicate that the cytotoxicity of Cu-Cy nanoparticles can be enhanced by X-ray irradiation. Cu-Cy-based PDT generates ROS that leads to tumor cell destruction and induction of an immune response. The immune response in the tumor and spleen was examined and it was concluded that only Cu-Cy + x-ray treatment increased CD4 + T and CD8 + T cell levels in the spleen (Fig. 11i). However, no significant changes were observed in other immune cells in the spleen. To understand the inhibitory potency of Cu-Cy-based PDT, the infiltration of immune cells into the TME was investigated. Tumor tissues treated with Cu-Cy + X beams had a higher proportion of dendritic cells (DCs), cluster of differentiation 8 with T cells (CD8 + T cells), and Natural Killer (NK) cells (Fig. 11j and k). Mature DCs play a critical role in initiating an effective adaptive immune response, and activated NK cells can support mature DCs and kill tumor cells directly. Cu-Cy-mediated PDT also reduced the number of M2 macrophages in the TME, whereas the proportion of M1 and MDSC macrophages did not change significantly. In summary, Cu-Cy-based PDT elicits an immune response by increasing CD4 + T and CD8 + T cell numbers in the spleen and promoting infiltration of DCs, CD8 + T, and NK cells into the TME. This immune response, together with the reduction of M2 macrophages, contributes to the cytotoxicity of Cu-Cy-based PDT (Zhang et al. 2020).
a Schematic representation of the use of Cu-Cy NPs for simultaneous radiotherapy, oxidative therapy, and immunotherapy in the treatment of melanoma. b) Morphological images of B16 cells treated with Cu-Cy NPs (~ 40 nm) under X-ray irradiation (0 or 2.5 Gy) c Cell viability of B16 cells treated with different concentrations of Cu-Cy NPs under 2.5 Gy X-ray irradiation compared to other groups. d Increased intracellular ROS levels in B16 cells treated with Cu-Cy NPs and X-rays compared to other groups. e, f The highest rates of cell apoptosis and/or necrosis in B16 cells treated with Cu-Cy NPs and X-rays compared to other groups. g Tumor volumes in different groups at the end of treatment. h Tumor growth curves in different groups. i–k Tumor tissue using flow cytometry to detect changes in infiltrating immune cells. i Percentage of CD8 + T cells in the spleen of mice treated with Cu-Cy NPs and X-rays compared to other groups. P value versus the PBS group (n = 6). j, k Percentage of CD8 + T cells and NK cells in tumor tissue of mice treated with Cu-Cy NPs and X-rays compared to other groups. P value versus the PBS group
In general, copper nanoparticles are promising in cancer immunotherapy because they enhance immune-based therapies (Xu et al. 2023). They can be used as adjuvants in cancer vaccines to enhance immune response and attack cancer cells. Copper nanoparticles can also be combined with immune checkpoint inhibitors to target them to the tumor, increasing the therapeutic efficacy of immunosuppressive factors that promote a more favorable immune response against cancer cells. Although research is still at an early stage, copper nanoparticles can enhance the effectiveness of immunotherapeutic approaches, which requires further studies to optimize (Wang et al. 2019a, b; Li et al. 2023). Generally, the reports on copper-based nanoparticles are also limited to in vitro, in vivo, or ex vivo phases (Kang et al. 2023). The clinical implementation of these nanoparticles remains a challenge, and more efforts should be directed to in vivo and ex vivo studies [196]. Compared to other metallic nanoparticles (such as silver, gold, or platinum) copper nanoparticles have not been extensively studied in biomedical applications. Although the number of scientific reports on copper nanomaterials is increasing, more research is needed before they can be used in clinical trials (Gu et al. 2023; Ilbasmis-Tamer et al. 2023; Abu-Serie and Abdelfattah 2023; Zhuang et al. 2022; Wózniak-Budych et al. 2023). Some examples of copper-based compounds and nanoparticles with different applications and mechanisms of action are listed in Table 1.
Limitations and challenges in the use of copper-based compounds
Although copper-based compounds are promising for cancer therapy, there are some problems with their use. Copper is a necessary trace mineral for the human body, but high concentrations of copper can be toxic (Tang et al. 2023). When using copper compounds in cancer therapy, a balance must be struck between therapeutic efficacy and toxicity to healthy cells and tissues (Wang et al. 2023a, b).
According to studies, increased values of copper have been detected in various types of tumors, which can be attributed to several factors. Tumors with high metabolic demands, especially fast-growing tumors, require copper as a cofactor for enzymes involved in cellular energy metabolism and antioxidant defense. This increased need for copper in cancer cells contributes to the higher copper content (Gao and Zhang 2023; Fiadjoe et al. 2023; Su et al. 2022). In addition, hypoxia, which is common in tumors, leads to upregulation of CTR1. Hypoxia-inducible factor 1-alpha indirectly activates genes involved in copper metabolism, including those that control CTR1, further increasing copper levels in tumor cells. Up-regulation of CTR1 has been observed in a variety of tumors (Lopez et al. 2019; Wooton-Kee et al. 2020). Tumors often exhibit an imbalance in copper levels that affects mitochondrial respiration, glycolysis, insulin resistance, and lipid metabolism. Liver cancer occurs more frequently in patients with Wilson's disease and animal models. There is evidence that the abnormal accumulation of copper may contribute to the transformation of liver cells, although the exact mechanisms are not yet fully understood. Extensive research has shown that cancer cells require an increased copper content to support their rapid growth compared to normal cells (Wooton-Kee et al. 2020). Some studies proved that copper metabolism is closely associated with tumor development. Cancer cells have a higher requirement for copper compared to normal cells, as it is essential for their rapid proliferation and energy needs (Atakul et al. 2020). Copper acts as a cofactor for cytochrome c oxidase, an enzyme involved in ATP synthesis and electron transport in the mitochondrial respiratory chain (Fang et al. 2019). Abnormal copper levels have been detected in the blood and tissue of various cancer patients. Impaired copper homeostasis, as evidenced by notable alterations in copper-binding proteins, is closely associated with cancer occurrence, development, and metastasis (Tsang et al. 2020). To gain more copper, cancer cells often show increased expression of the CTR1. Despite the increased glycolysis observed in cancer cells, the administration of copper chelators can greatly reduce ATP generation. This suggests that tumor cells rely heavily on mitochondrial respiration and oxidative phosphorylation to meet their energy needs. Here, copper may act as a limiting factor in tumorigenesis and cancer progression as it regulates ATP production via oxidative phosphorylation, which is necessary to meet the high energy demands of rapidly proliferating cancer cells (Atakul et al.2020; Fang et al. 2019; Tsang et al. 2020).
Copper is an important cofactor for the autophagy kinases ULK1 and ULK2 and can increase the activity of these kinases, leading to higher autophagy flux. This promotes the proliferation and viability of neoplastic lung cells. In Wilson's disease, copper accumulation in the liver is associated with autophagy activation. In colorectal cancer with KRAS gene mutations, tumor cells take up more copper through macro-pinocytosis, which promotes tumor growth. On the other hand, lower availability of copper suppresses the growth of KRAS-mutated cancer cells (Tsang et al. 2020; Aubert et al. 2020). Research suggests that enhanced copper levels can stimulate tumor progression by boosting mitochondrial energy metabolism, leading to the mis-folding of tumor-associated proteins, triggering the activation of signaling pathways related to tumor development, and influencing the activity of autophagy kinases (Tsang et al. 2020; Aubert et al. 2020). Copper has a significant impact on the promotion of angiogenesis, as demonstrated in the 1979 study by McAuslan et al. Subsequent research has shown that copper stimulates endothelial cell migration, a crucial step in the formation of blood vessels. Excessive copper levels are observed during the process of neovascularization, and the addition of copper promotes the growth of blood vessels. In animal models, inhibition of copper leads to a decrease in blood vessel formation. Copper promotes angiogenesis by several mechanisms, including direct binding to growth factors involved in angiogenesis, increasing nitric oxide production, regulating angiogenic factors, such as FGF and IL-1α, and modulating the activity of the NF-κB cells. Copper also interacts with HIF-1α, an important regulator of angiogenesis, influencing its transcriptional activity and promoting tumor growth (Karginova et al. 2019). Clinical trials have shown a remarkable relationship between serum copper levels and HIF-1α levels in liver cancer patients, demonstrating that copper has a considerable role in HIF-1α activation and liver cancer progression. Inhibition of copper chaperone proteins can disrupt copper balance, inhibit tumor angiogenesis, and induce apoptosis in breast cancer cells. The results also suggest that copper has a significant effect on tumor angiogenesis (Wu et al. 2019). Copper has a remarkable role in advancing the growth of blood vessels in tumors by influencing the activity of HIF-1α and regulating the release of various factors that promote blood vessel formation. By reducing the copper content in tumor tissue or preventing copper transport in cancer cells, it may be possible to effectively block the growth of blood vessels in tumors. This could be a valuable therapeutic strategy for the treatment of cancer (Karginova et al. 2019; Wu et al. 2019).
Copper plays a decisive role in tumor cells, influencing their growth, invasion, and spread. It influences these processes through the activity of LOX enzymes, which contribute to the strengthening of the extracellular matrix. LOX and LOXL proteins depend on copper for their function, and the copper exporter ATP7A protein transports copper for them. In breast cancer, inhibition of ATP7A disrupts LOX activity and impedes metastasis. High levels of LOXL2 are associated with aggressive breast cancer by reducing cell adhesion and promoting EMT. Targeted inhibition of LOX and LOXL by copper can successfully inhibit the invasion and spread of cancer cells. The MEMO1, another copper-dependent protein, is involved in cell movement and metastasis of breast cancer (Shanbhag et al. 2019). Treatment with a copper chelator called TTM lowers copper levels, inhibits MEMO1, delays blood vessel formation, and prevents breast cancer metastasis. Lowering copper levels by chelation or targeting copper transporters not only suppresses the growth of cancer cells but also downregulates the expression of genes associated with EMT. Copper also plays a role in the activation of genes associated with EMT, as it is involved in the binding of HIF-1α to specific gene sequences (Shanbhag et al. 2019). Copper can affect the activity of LOX and LOXL enzymes, the expression of MEMO1, and the binding of HIF-1α to target genes with HRE (Hypoxia Response Elements) sequences. This regulation of gene expression leads to the modulation of EMT-related genes and the promotion of tumor cell metastasis [216]. Consequently, the use of copper in therapy can interrupt this signaling mechanism and effectively prevent the spread of tumor cells (Shanbhag et al. 2019). Tumor cells undergo a unique metabolic process known as aerobic glycolysis. This allows them to adapt to different oxygen levels, promote cell proliferation, and prevent cell death. Under aerobic conditions, tumor cells increase their glycolysis, glucose uptake, and lactate production while weakening oxidative phosphorylation. This metabolic reprogramming provides tumor cells with the energy and nutrients they need to survive and proliferate, giving them an advantage over normal cells. Hypoxia, or low oxygen levels, has a vital role in stimulating the overexpression of genes related to glycolysis in tumor cells (Wu et al. 2019; Shanbhag et al. 2019; Feng et al. 2009). The HIF-1α protein is an imperative regulator of this process and is involved in cancer development. Copper also affects the regulation of glycolysis and the activity of HIF-1α. It can prevent the degradation of HIF-1α and thus improve its function by interfering with the activation of certain proteins. However, copper is also crucial for the functioning of respiratory complex IV in mitochondria, which affects overall cellular activity. Copper chelators can decrease mitochondrial respiration and activate glycolysis (Wu et al. 2019). Therefore, the involvement of copper in metabolic reprogramming of tumors is not a simple correlation, but rather a complex interplay of different pathways. To develop better treatment approaches, more in-depth investigation is needed to fully understand the underlying mechanisms of copper's role in the metabolic reprogramming of tumors (Wu et al. 2019; Feng et al. 2009; Jensen et al. 2019).
Conclusions
Copper complexes synthesized from various organic ligands, such as thiosemicarbazone, Schiff base, imidazole, benzimidazole, pyrazole, triazole, pyridine, and isatin diamine, as well as 1,10-phenanthroline derivatives, have demonstrated their potential as anticancer agents. They can induce cell death, and inhibit angiogenesis, tumor growth and metastasis via various mechanisms. However, side effects must be considered, such as potential toxicity, lack of selectivity, and the development of drug resistance. Clinical studies with copper complexes, particularly [64Cu]Cu-ATSM, have shown promise for imaging tumor hypoxia and predicting patient response to therapy. Ongoing research and clinical studies are investigating the use of copper complexes in various medical fields. Copper nanoparticles hold promise for cancer therapy due to their unique properties, as they can selectively target cancer cells and deliver drugs. Copper nanoparticles have shown remarkable efficacy in CDT due to their properties that can generate ROS and induce tumor cell death. They can be used as effective photosensitizers to selectively target and destroy cancer cells by light activation in phototherapy. The use of copper nanoparticles in hyperthermia promises to improve the effectiveness of cancer treatment. Copper nanoparticles can be used to generate heat when exposed to certain wavelengths of light or alternating magnetic fields. This ability to generate heat makes them suitable for targeted heating of tumor cells. By targeting copper nanoparticles in the tumor and activating them with external stimuli, it is possible to raise the temperature of the cancer tissue while causing as little damage as possible to the healthy surrounding cells. Copper nanoparticles can be used for cancer immunotherapy because they support immune-based treatments. When used as an adjuvant in cancer vaccines, they can improve the immune response and attack cancer cells. In combination with immune checkpoint inhibitors, copper nanoparticles can also be targeted to tumors to improve the effectiveness of therapy. While copper is an essential mineral needed for various bodily functions, excessive amounts can be harmful, such that high levels of copper in the body may be associated with an enhanced risk of certain cancers. Copper is involved in the formation of free radicals, unstable molecules that can damage DNA and other cellular components. This oxidative damage can lead to mutations and the development of cancer cells. In addition, copper is also involved in angiogenesis, the process by which new blood vessels are formed. Tumor cells rely on angiogenesis to grow and spread, and elevated copper levels can promote this process. Lowering copper concentrations or inhibiting copper transport in tumor cells may be promising strategies to inhibit angiogenesis and metastasis of tumors. However, it should be noted that the correlation between copper and cancer is complex, and further study is needed to fully understand the mechanisms involved.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- AO/EB:
-
Acridine orange/ethidium bromide
- ATP7A:
-
ATPase copper transporting alpha
- ATSM:
-
Diacetyl-bis(N4-methylthiosemicarbazone
- BNN6:
-
N,N′-di-sec-butyl-N,N′-dinitroso-1,4-phenylenediamine
- CAM:
-
Chick embryo chorioallantoic membrane
- COX2:
-
Cyclooxygenase-2
- Cu,Zn-SOD:
-
Copper, zinc superoxide dismutases
- DAMPs:
-
Damage-associated molecular patterns
- DLAT:
-
Dihydrolipoamide S-acetyltransferase
- DLST:
-
Dihydrolipoamide S-succinyltransferase
- Dpq:
-
Dipyrido-[3,2-d:2’,3’-f]-quinoxaline
- EPR:
-
Enhanced permeability and retention
- EMT:
-
Epithelial-to-mesenchymal transition
- FAZA:
-
Fluoroazomycin arabinoside
- FDG:
-
Fluorodeoxyglucose
- FMISO:
-
Fluoromisonidazole
- FGF:
-
Fibroblast growth factor
- GTSM:
-
Glyoxal-bis(4-methylthiosemicarbazonato)
- HIF-1α:
-
Hypoxia-inducible factor 1-alpha
- HSA:
-
Human serum albumin
- Htdp:
-
2-[(2-(2-Hydroxyethylamino)ethylimino)methyl]phenol
- IL-1α:
-
Interleukin-1 alpha
- KRAS:
-
Ki-ras2 Kirsten rat sarcoma viral oncogene homolog
- LOX:
-
Lysyl oxidase
- LOXL:
-
Lysyl oxidase-like
- MAMs:
-
Mitochondria-associated membranes
- MCU:
-
Mitochondrial uniporter
- MEMO1:
-
Mediator of ErbB2-driven cell motility
- MELAS:
-
Mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes
- NF-κB:
-
Nuclear factor kappa B
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- PDGFB:
-
Platelet-derived growth factor B
- PDGFRbeta:
-
Platelet-derived growth factor receptor beta
- RNA:
-
Ribonucleic acid
- SDHB:
-
Succinate dehydrogenase complex iron sulfur subunit B
- Tie2:
-
Immunoglobulin-like loops and epidermal growth factor homology domains-2
- Tmp:
-
3,4,7,8-Tetramethyl-1,10-phenanthroline
References
Abdolmaleki S, Ghadermazi M (2017) Novel pyridinedicarboxamide derivatives and a polymeric copper (II) complex: synthesis, structural characterization, electrochemical behavior, catalytic and cytotoxic studies. Inorg Chim Acta 461:221
Abdolmaleki S, Ghadermazi M, Fattahi A, Sheshmani S (2016) Synthesis, characterization, spectral studies and cytotoxic effects of mixed-ligand mono and binuclear copper (II) complexes and their amide ligands. Inorg Chim Acta 443:284
Abdolmaleki S, Ghadermazi M, Fattahi A, Shokraii S, Alimoradi M, Shahbazi B, Judy Azar AR (2017) Synthesis, crystallographic and spectroscopic studies, evaluation as antimicrobial and cytotoxic agents of a novel mixed-ligand nickel (II) complex. J Coord Chem 70:1406
Abdolmaleki S, Ghadermazi M, Ashengroph M, Saffari A, Sabzkohi SM (2018) Cobalt (II), zirconium (IV), calcium (II) complexes with dipicolinic acid and imidazole derivatives: X-ray studies, thermal analyses, evaluation as in vitro antibacterial and cytotoxic agents. Inorg Chim Acta 480:70
Abdolmaleki S, Yarmohammad N, Adibi H, Ghadermazi M, Ashengroph M, Amiri Rudbari H, Brunoe G (2019) X-ray studies, electrochemical properties, evaluation as in vitro cytotoxic and antibacterial agents of two antimony (III) complexes with dipicolinic acid. Polyhedron 159:239
Abdolmaleki S, Ghadermazi M, Bagheri F, Rudbari HA, Bruno G (2020) Evaluation of two novel macrocycles containing pyridine-2, 6-dicarboxamide unit as cationic fluorescent sensor. Polyhedron 176:114292
Abdolmaleki S, Ghadermazia M, Aliabadi A (2021a) Study on electrochemical behavior and in vitro anticancer effect of Co (II) and Zn (II) complexes containing pyridine-2, 6-dicarboxylate. Inorg Chim Acta 527:120549
Abdolmaleki S, Ghadermazi M, Aliabadi A (2021b) Novel Tl (III) complexes containing pyridine-2, 6-dicarboxylate derivatives with selective anticancer activity through inducing mitochondria-mediated apoptosis in A375 cells. Sci Rep 11:1
Abdolmaleki S, Aliabadi A, Ghadermazi M (2022a) Two La (III) complexes containing pyridine-2, 6-dicarboxylate as in vitro potent cytotoxic agents toward human lymphocyte cells. Inorg Chim Acta 542:121152
Abdolmaleki S, Aslani A, Aliabadi A, Khazayel S, Amininasab SM, Izadi Z, Ghadermazi M, Motieiyan E, Marabello D, Hugo Nunes Rodrigues V (2022b) Study on a Ru (III) complex containing picolinate with potent inhibition effect against melanoma cell line. J Coord Chem 75:147
Abdolmaleki S, Panjehpour A, Khaksar S, Ghadermazi M, Rostamnia S (2023a) Evaluation of central-metal effect on anticancer activity and mechanism of action of isostructural Cu (II) and Ni (II) complexes containing pyridine-2, 6-dicarboxylate. Eur J Med Chem 245:114897
Abdolmaleki S, Khaksar S, Aliabadi A, Panjehpour A, Motieiyan E, Marabello D, Faraji MH, Beihaghi M (2023b) Cytotoxicity and mechanism of action of metal complexes: an overview. Toxicology 492:153516
Abu-Serie MM, Abdelfattah EZ (2023) A comparative study of smart nanoformulations of diethyldithiocarbamate with Cu4O3 nanoparticles or zinc oxide nanoparticles for efficient eradication of metastatic breast cancer. Sci Rep 13:3529
Adibi H, Abdolmaleki S, Shahabadi N, Golabi A, Mahdavi M, Zendehcheshm S, Ghadermazi M, Ansari M, Amiri Rudbari H, Bruno G, Nemati A (2019) Investigation of crystallographic structure, in vitro cytotoxicity and DNA interaction of two La (III) and Ce (IV) complexes containing dipicolinic acid and 4-dimethylaminopyridine. Polyhedron 163:20
Adsule S, Barve V, Chen D, Ahmed F, Dou QP, Padhye S, Sarkar FH (2006) Novel schiff base copper complexes of quinoline-2 carboxaldehyde as proteasome inhibitors in human prostate cancer cells. J Med Chem 49:7242
Ahmed F, Adsule S, Ali AS, Banerjee S, Ali S, Kulkarni S, Padhye S, Sarkar FH (2007) A novel copper complex of 3-benzoylalpha methyl benzene acetic acid with antitumor activity mediated via cyclooxygenase pathway. Int J Cancer 120:734
Aishajiang R, Liu Z, Wang T, Zhou L, Yu D (2023a) Recent advances in cancer therapeutic copper-based nanomaterials for antitumor therapy. Molecules 28:2303
Alem MB, Damena T, Desalegn T, Koobotse M, Eswaramoorthy R, Ngwira KJ, Ombito JO, Zachariah M, Demissie TB (2022) Cytotoxic mixed-ligand complexes of Cu (II): a combined experimental and computational study. Front Chem 10:1028957
Al-Fakeh MS, Messaoudi S, Alresheedi FI, Albadri AE, El-Sayed WA, Saleh EE (2023) Preparation, characterization, DFT calculations, antibacterial and molecular docking study of Co (II), Cu (II), and Zn (II) mixed ligand complexes. Crystals 13:118
Aliabadi A, Motieiyan E, Hosseinabadi F, Ghadermazi M, Abdolmaleki S (2021a) One-pot synthesis, crystallographic characterization, evaluation as in vitro antibacterial and cytotoxic agents of two mercury (II) complexes containing pyridine dicarboxylic acid derivatives. J Mol Struct 1226:129405
Aliabadi A, Hakimi M, Hosseinabadi F, Motieiyan E, Hugo Nunes Rodrigues V, Ghadermazid M, Marabello D, Abdolmaleki S (2021b) Investigation of X-ray crystal structure and in vitro cytotoxicity of two Ga (III) complexes containing pyridine dicarboxylic acid derivatives and 2-aminobenzimidazole. J Mol Struct 1223:129005
Aliabadi A, Zangeneh M, Izadi Z, Badzohre M, Ghadermazi M, Marabello D, Bagheri F, Farokhi A, Motieiyan E, Abdolmaleki S (2022) Green synthesis, X-ray crystal structure, evaluation as in vitro cytotoxic and antibacterial agents of a new Zn (II) complex containing dipicolinic acid. J Mol Struct 1247:131327
Amatya R, Lee D, Sultana M, Min KA, Shin MC (2023) Albumin-coated copper nanoparticles for photothermal cancer therapy: synthesis and in vitro characterization. Heliyon 9:e17732
Ambike V, Adsule S, Ahmed F, Wang Z, Afrasiabi Z, Sinn E, Sarkar F, Padhye S (2007) Copper conjugates of nimesulide Schiff bases targeting VEGF, COX and Bcl-2 in pancreatic cancer cells. J Inorg Biochem 101:1517
Atakul T, Altinkaya SO, Abas BI, Yenisey C (2020) Serum copper and zinc levels in patients with endometrial cancer. Biol Trace Elem Res 195:46
Aubert L, Nandagopal N, Steinhart Z, Lavoie G, Nourreddine S, Berman J et al (2020) Copper bioavailability is a KRAS-specific vulnerability in colorectal cancer. Nat Commun 11:3701
Baldini M, Belicchi-Ferrari M, Bisceglie F, Dall’Aglio PP, Pelosi G, Pinelli S, Tarasconi P (2004) Copper(II) complexes with substituted thiosemicarbazones of alpha-Ketoglutaric acid: synthesis, X-ray structures, DNA binding studies, and nuclease and biological activity. Inorg Chem 43:7170
Beckford FA, Webb KR (2017) Copper complexes containing thiosemicarbazones derived from 6-nitropiperonal: antimicrobial and biophysical properties. Spectrochim Acta Part A Mol Biomol Spect 183:158
Bestwick CS, Milne L, Pirie L, Duthie SJ (2005) The effect of short-term kaempferol exposure on reactive oxygen levels and integrity of human (HL-60) leukaemic cells. Biochim Biophys Acta Mol Basis Dis 1740:340
Bisaglia M, Bubacco L (2020) Copper Ions and Parkinson’s disease: why is homeostasis so relevant? Biomolecules 10:195
Cai X, Pan N, Zou G (2007) Copper-1,10-phenanthroline-induced apoptosis in liver carcinoma Bel-7402 cells associates with copper overload, reactive oxygen species production, glutathione depletion and oxidative DNA damage. Biometals 20:1
Cai DH, Zhang CL, Liu QY, He L, Liu YJ, Xiong YH, Le XY (2021) Synthesis, DNA binding, antibacterial and anticancer properties of two novel water-soluble copper (II) complexes containing gluconate. Eur J Med Chem 213:113182
Cantiello F, Crocerossa F, Cascini GL, Russo GI, Ferro M, Cimino S, Lucarelli F, Damiano R (2021) 64CuCl2 PET/CT as a potential new imaging method in prostate cancer: illusion or reality? Minerva Urol, Nephrol 73:668
Capasso G, Durzu A, Piras S, Zandieh S, Knoll P, Haug A (2015) Role of 64CuCl2 PET/CT in staging of prostate cancer. Ann Nucl Med 6:482
Capriotti G, Piccardo A, Giovannelli E, Signore A (2022) Targeting copper in cancer imaging and therapy: a new theragnostic agent. J Clin Med 12:223
Ceramella J, Mariconda A, Iacopetta D, Saturnino C, Barbarossa A, Caruso A, Rosano C, Sinicropi MS, Longo P (2020) From coins to cancer therapy: Gold, silver and copper complexes targeting human topoisomerases. Bioorg Med Chem Lett 30:126905
Cerchiaro G, Aquilano K, Filomeni G, Rotilio G, Ciriolo MR, Ferreira AMDC (2005) Isatin-Schiff base copper(II) complexes and their influence on cellular viability. J Inorg Biochem 99:1433
Chen X, Zhang X, Chen J, Yang Q, Yang L, Xu D, Zhang P, Wang X, Liu J (2017) Hinokitiol copper complex inhibits proteasomal deubiquitination and induces paraptosis-like cell death in human cancer cells. Eur J Pharmacol 815:147–155
Chen C, Ma Y, Du S, Wu Y, Shen P, Yan T, Li X, Song Y, Zha Z, Han X (2021) Controlled CRISPR-Cas9 ribonucleoprotein delivery for sensitized photothermal therapy. Small 17:e2101155
Chen X, Liu J, Li Y, Pandey NK, Chen T, Wang L, Amador EH, Chen W, Liu F, Xiao E et al (2022) Study of copper-cysteamine based X-ray induced photodynamic therapy and its effects on cancer cell proliferation and migration in a clinical mimic setting. Bioact Mater 7:504
Cheng Q, Li Y, Huang W, Li K, Lan M, Wang B, Wang J, Song X (2023) Copper coordination-based conjugated polymer nanoparticles for synergistic photodynamic and chemodynamic therapy. Chem Commun 59:5886
Cheung EC, Vousden KH (2022) The role of ROS in tumor development and progression. Nat Rev Cancer 22:280
Climova A, Pivovarova E, Szczesio M, Gobis K, Ziembicka D, Korga-Plewko A, Kubik J, Iwan M, Antos-Bielska M, Krzyżowska M, Czylkowska A (2023) Anticancer and antimicrobial activity of new copper (II) complexes. J Inorg Biochem 240:112108
Crezee J, Franken NAP, Oei AL (2021) Hyperthermia-based anti-cancer treatments. Cancers 13:1240
Cui R, Li B, Liao C, Zhang S (2023) Copper-mediated chemodynamic therapy with ultra-low copper consumption by doping cupric ion on cross-linked (R)-(+)-lipoic acid nanoparticles. Regen. Biomater. 10:021
Dallavalle F, Gaccioli F, Franchi-Gazzola R, Lanfranchi M, Marchio L, Pellinghelli MA, Tegoni M (2002) Synthesis, molecular structure, solution equilibrium, and antiproliferative activity of thioxotriazoline and thioxotriazole complexes of copper(II) and palladium(II). J Inorg Biochem 92:95
Datta NR, Ordonez SG, Gaipl US, Paulides MM, Crezee H, Gellermann J, Marder D, Puric E, Bodis S (2015) Local hyperthermia combined with radiotherapy and/or chemotherapy: Recent advances and promises for the future. Cancer Treat Rev 41:742
de Hoog P, Boldron C, Gamez P, Sliedregt-Bol K, Roland I, Pitie M, Kiss R, Meunier B, Reedijk J (2007) New approach for the preparation of efficient DNA cleaving agents: ditopic copper-platinum complexes based on 3-clip-phen and cisplatin. J Med Chem 50:3148
Deegan C, Coyle B, McCann M, Devereux M, Egan DA (2006) In vitro anti-tumor effect of 1,10-phenanthroline-5,6-dione (phendione), [Cu(phendione)3](ClO4)2·4H2O and [Ag(phendione)2]ClO4 using human epithelial cell lines. Chemico-Biol Interact 164:115
Deegan C, McCann M, Devereux M, Coyle B, Egan D (2007a) In vitro cancer chemotherapeutic activity of 1,10-phenanthroline(phen), [Ag2(phen)3(mal)].2H2O, [Cu(phen)2(mal)].2H2O and [Mn(phen)2(mal)].2H2O (malH2 = malonic acid) using human cancer cells. Cancer Lett 247:224
Deegan C, McCann M, Devereux M, Coyle B, Egan DA (2007b) In vitro cancer chemotherapeutic activity of 1,10-phenanthroline (phen), [Ag2(phen)3(mal)].2H2O, [Cu(phen)2(mal)].2H2O and [Mn(phen)2(mal)].2H2O (malH2 = malonic acid) using human cancer cells. Cancer Lett 247:224
Del Bello F, Pellei M, Bagnarelli L, Santini C, Giorgioni G, Piergentili A, Quaglia W, Battocchio C, Iucci G, Schiesaro I, Meneghini C (2022) Cu (I) and Cu (II) complexes based on lonidamine-conjugated ligands designed to promote synergistic antitumor effects. Inorg Chem 61:4919
Devereux M, McCann M, Shea DO, Kelly R, Egan D, Deegan C, Kavanagh K, McKee V, Finn G (2004) Synthesis, antimicrobial activity and chemotherapeutic potential of inorganic derivatives of 2-(4’-thiazolyl)benzimidazole{thiabendazole}: X-ray crystal structures of [Cu(TBZH)2Cl]Cl.H2O.EtOH and TBZH2NO3 (TBZH = thiabendazole). J Inorg Biochem 98:1023
Devereux M, O’Shea D, O’Connor M, Grehan H, Connor G, McCann M, Rosair G, Lyng F, Kellett A, Walsh M, Egan D, Thati B (2007) Synthesis, catalase, superoxide dismutase and antitumour activities of copper(II) carboxylate complexes incorporating benzimidazole, 1,10-phenanthroline and bipyridine ligands: X-ray crystal structures of [Cu(BZA)2(bipy)(H2O)], [Cu(SalH)2(BZDH)2], and [Cu(CH3COO)2(5,6-DMBZDH)2] (SalH2 = salicylic acid; BZAH = benzoic acid; BZDH = benzimidazole and 5,6-DMBZDH = 5,6-dimethylbenzimidazole). Polyhedron 26:4073
Elkanzi NA, Hrichi H, Salah H, Albqmi M, Ali AM, Abdou A (2023) Synthesis, physicochemical properties, biological, molecular docking and DFT investigation of Fe (III), Co (II), Ni (II), Cu (II) and Zn (II) complexes of the 4-[(5-oxo-4, 5-dihydro-1, 3-thiazol-2-yl) hydrazono] methyl} phenyl 4-methylbenzenesulfonate Schiff-base ligand. Polyhedron 230:116219
Evans CW, Atkins C, Pathak A, Gilbert BE, Noah JW (2015) Benzimidazole analogs inhibit respiratory syncytial virus G protein function. Antivir Res 121:31
Falcone E, Stellato F, Vileno B, Bouraguba M, Lebrun V, Ilbert M, Morante S, Faller P (2023) Revisiting the pro-oxidant activity of copper: interplay of ascorbate, cysteine and glutathione. Metallomics 15:mfad040
Fan K, Dong Y, Li T, Li Y (2022) Cuproptosis-associated CDKN2A is targeted by plicamycin to regulate the microenvironment in patients with head and neck squamous cell carcinoma. Front Genet 13:1036408
Fang A-P, Chen P-Y, Wang X-Y, Liu Z-Y, Zhang D-M, Luo Y et al (2019) Serum copper and zinc levels at diagnosis and hepatocellular carcinoma survival in the guangdong liver cancer cohort. Int J Cancer 144:2823
Feng W, Ye F, Xue W, Zhou Z, Kang YJ (2009) Copper regulation of hypoxia-inducible factor-1 activity. Mol Pharmacol 75:174
Feng LC, Zhang J, Dou L, Dong WK (2023) Synthesis, structure, theoretical studies, and fluorescence properties of two multinuclear Cu (II) bis (salamo)-like complexes. J Coord Chem 76:648
Fernández CY, Alvarez N, Rocha A, Ellena J, Costa-Filho AJ, Batista AA, Facchin G (2023) New Copper (II)-L-dipeptide-bathophenanthroline complexes as potential anticancer agents—synthesis, characterization and cytotoxicity studies—and comparative DNA-binding study of related phen complexes. Molecules 28:896
Ferrari MB, Gasparri Fava F, Tarasconi P, Albertini R, Pinelli S, Starcich R (1994) Synthesis, spectroscopic and structural characterization, and biological activity of aquachloro(pyridoxal thiosemicarbazone)copper(II) chloride. J Inorg Biochem 53:13
Ferrari MB, Fava GG, Leporati E, Pelosi G, Rossi R, Tarasconi P, Albertini R, Bonati A, Lunghi P, Pinelli S (1998) Synthesis, characterization and biological activity of three copper(II) complexes with a modified nitrogenous base: 5-formyluracil thiosemicarbazone. J Inorg Biochem 70:145
Ferrari MB, Capacchi S, Pelosi G, Reffo G, Tarasconi P, Albertini R, Pinelli S, Lunghi P (1999) Synthesis, structural characterization and biological activity of helicin thiosemicarbazone monohydrate and a copper(II) complex of salicylaldehyde thiosemicarbazone. Inorg Chim Acta 286:134
Ferrari MB, Bisceglie F, Leporati E, Pelosi G, Tarasconi P (2002) Synthesis, solution chemistry, x-ray structure and biological activity of novel pyridoxal thiosemicarbazone derivatives. Bull Chem Soc Jpn 75:781
Fiadjoe HK, Lambring C, Sankpal UT, Alajroush D, Holder AA, Basha R (2023) Anti-proliferative effect of two copper complexes against medulloblastoma cells. Cancer Res 83:1538
Filomeni G, Cerchiaro G, Da Costa Ferreira AM, De Martino A, Pedersen JZ, Rotilio G, Ciriolo MR (2007a) Pro-apoptotic activity of novel isatin-schiff base copper(II) complexes depends on oxidative stress induction and organelle-selective damage. J Biol Chem 282:12010
Fiz F, Bottoni G, Ugolini M, Righi S, Cirone A, Garganese MC, Verrico A, Rossi A (2022) Diagnostic and dosimetry features of [64Cu]CuCl2 in high-grade paediatric infiltrative gliomas. Mol Imaging Biol 25:391
Franco A, Buoso S, Zanin L, Pinton R, Tomasi N (2023) Copper toxicity in maize: the severity of the stress is reduced depending on the applied fe-chelating agent. J Plant Growth Regul 42(3):1567–1581. https://doi.org/10.1007/s00344-022-10641-1
Gajare SP, Bansode PA, Patil PV, Aalhusaini TN, Chavan SS, Pore DM, Chhowala TN, Khot VM, Rashinkar GS (2022) Nano-magnetic copper complexes as double-edged sword against MCF-7 breast cancer cells. Chem Select 7:e202103818
Gandin V, Pellei M, Tisato F, Porchia M, Santini C, Marzano C (2012) A novel copper complex induces paraptosis in colon cancer cells via the activation of ER stress signaling. J Cell Mol Med 16:142
Gandin V, Tisato F, Dolmella A, Pellei M, Santini C, Giorgetti M, Marzano C, Porchia M (2014) In vitro and in vivo anticancer activity of copper (I) complexes with homoscorpionate tridentate tris (pyrazolyl) borate and auxiliary monodentate phosphine ligands. J Med Chem 57:2014
Gao L, Zhang A (2023) Copper-instigated modulatory cell mortality mechanisms and progress in oncological treatment investigations. Front Immun. https://doi.org/10.3389/fimmu.2023.1236063
García-Pérez FO, Medina-Ornelas SS, Barron-Barron F, Arrieta-Rodriguez O (2020) Evaluation of non-small cell lung cancer by PET/CT with 64CuCl2: initial experience in humans. Am J Nucl Med Mol Imaging 10:143
Geraki K, Farquharson MJ, Bradley DA (2002) Concentrations of Fe, Cu and Zn in breast tissue: a synchrotron XRF study. Phys Med Biol 47:2327
Ghaleh HEG, Zarei L, Mansori Motlagh B, Jabbari N (2019) Using CuO nanoparticles and hyperthermia in radiotherapy of MCF-7 cell line: synergistic effect in cancer therapy. Artif Cells Nanomed Biotechnol. 47:1396
Gomathi R, Andy R (2013) Synthesis, Characterization of novel Cu(II) complexes of isatin derivatives as potential cytotoxicity DNA binding, cleavage and antibacterial agents. Intl J Innov Res Sci Eng Techn 2:4852
Graur V, Usataia I, Graur I, Garbuz O, Bourosh P, Kravtsov V, Lozan-Tirsu C, Balan G, Fala V, Gulea A (2023) Novel Copper (II) complexes with N 4, S-diallylisothiosemicarbazones as potential antibacterial/anticancer drugs. Inorganics 11:195
Gu J, Chen F, Zheng Z, Bi L, Morovvati H, Goorani S (2023) Novel green formulation of copper nanoparticles by Foeniculum vulgare: chemical characterization and determination of cytotoxicity, anti-human lung cancer and antioxidant effects. Inorg Chem Commun 150:110442
Guan D, Zhao L, Shi X, Ma X, Chen Z (2023) Copper in cancer: from pathogenesis to therapy. Biomed Pharmacother 63:114791
Guerreiro JF, Alves V, Abrunhosa AJ, Paulo A, Gil OM, Mendes F (2018) Radiobiological characterization of 64CuCl2 as a simple tool for prostate cancer theranostics. Molecules 23:2944
Gul NS, Khan TM, Chen M, Huang KB, Hou C, Choudhary MI, Liang H, Chen ZF (2020) New copper complexes inducing bimodal death through apoptosis and autophagy in A549 cancer cells. J Inorg Biochem 213:111260
Guo Z, Zhou X, Hou C, Ding Z, Wen C, Zhang L-J, Jiang B-P, Shen X-C (2019) A chloroplast-inspired nanoplatform for targeting cancer and synergistic photodynamic/photothermal therapy. Biomater Sci 7:3886
Guo Y, Fan Y, Wang Z, Li G, Zhan M, Gong J, Majoral JP, Shi X, Shen M (2022) Chemotherapy mediated by biomimetic polymeric nanoparticles potentiates enhanced tumor immunotherapy via amplification of endoplasmic reticulum stress and mitochondrial dysfunction. Adv Mater 34:2206861
Ha JW, Choi JY, Boo YC (2023) Differential effects of histidine and histidinamide versus cysteine and cysteinamide on copper ion-induced oxidative stress and cytotoxicity in HaCaT keratinocytes. Antioxidants 12:801
Habib NS, Rida SM, Badawey EA, Fahmy HT, Ghozlan HA (1997) Synthesis and biological investigations of some novel thiazolylbenzimidazoles, and benzimidazolyl-thiazolo[4,5-d]pyrimidines. Pharmazie 52:346
Han J (2023) Copper trafficking system in cells: insights into coordination chemistry and toxicity. Dalton Trans 52:15277
Hanson S, Dharan A, Pv J, Pal S, Nair BG, Kar R, Mishra N (2023) Paraptosis: a unique cell death mode for targeting cancer. Front Pharmacol 14:1159409
Hariprabu KNG, Sathya M, Vimalraj S (2021) CRISPR/Cas9 in cancer therapy: a review with a special focus on tumor angiogenesis. Int J Biol Macromol 192:913
He G, Li Y, Younis MR, Fu L-H, He T, Lei S, Lin J, Huang P (2022) Synthetic biology-instructed transdermal microneedle patch for traceable photodynamic therapy. Nat Commun 13:6238
Heydari R, Motieiyan E, Aliabadi A, Abdolmaleki S, Ghadermazi M, Yarmohammadi N (2020a) Synthesis, crystallographic studies, electrochemical and in vitro cytotoxicity properties of two Mn (II) and U (IV) complexes containing dipicolinic acid and 4-dimethylaminopyridine. Polyhedron 181:114477
Heydari R, Motieiyan E, Abdolmaleki S, Aliabadi A, Ghadermazi M, Bagheri F, Amiri Rudbari H (2020b) X-ray crystal structure, thermal behavior and evaluation as an in vitro cytotoxic agent of a tin (IV) complex containing dipicolinic acid. J Coord Chem 73:2347
Hu C, Cai L, Liu S, Liu Y, Zhou Y, Pang M (2020) Copper-doped nanoscale covalent organic polymer for augmented photo/chemodynamic synergistic therapy and immunotherapy. Bioconjug Chem 31:1661
Huang C, Tang J, Liu Y, Chen T, Qi J, Sun S, Hao H, Zeng W, Zhao J, Wu M (2023) Hyperthermia-triggered NO release based on Cu-doped polypyrrole for synergistic catalytic/gas cancer therapy. Acta Biomater 167:463
Hussain A, AlAjmi MF, Rehman MT, Amir S, Husain FM, Alsalme A, Siddiqui MA, AlKhedhairy AA, Khan RA (2019) Copper (II) complexes as potential anticancer and nonsteroidal anti-inflammatory agents: in vitro and in vivo studies. Sci Rep 9:5237
Ilbasmis-Tamer S, Turk M, Evran Ş, Boyaci IH, Ciftci H, Tamer U (2023) Cytotoxic, apoptotic and necrotic effects of starch coated copper nanoparticles on Capan 1 pancreatic cancer cells. J Drug Deliv Sci Technol 79:104077
Jenkins H, MacLean L, McClean S, Cooke G, Devereux M, Howe O, Pereira MD, May NV, Enyedy ÉA, Creaven BS (2023) Structural and solution speciation studies on selected [Cu (NN)(OO)] complexes and an investigation of their biomimetic activity, ROS generation and their cytotoxicity in normoxic, hypoxic and anoxic environments in MCF-7 breast cancer-derived cells. J Inorg Biochem 249:112383
Jensen EL, Gonzalez-Ibanez AM, Mendoza P, Ruiz LM, Riedel CA, Simon F et al (2019) Copper deficiency-induced anemia is caused by a mitochondrial metabolic reprograming in erythropoietic cells. Metallomics 11:282
Ji P, Wang P, Chen H, Xu Y, Ge J, Tian Z, Yan Z (2023) Potential of copper and copper compounds for anticancer applications. Pharmaceuticals 16:234
Kang X, Wang J, Huang CH, Wibowo FS, Amin R, Chen P, Li F (2023) Diethyldithiocarbamate copper nanoparticle overcomes resistance in cancer therapy without inhibiting P-glycoprotein. Nanomed Nanotech Biol Med 47:102620
Karginova O, Weekley CM, Raoul A, Alsayed A, Wu T, Lee SS-Y et al (2019) Inhibition of copper transport induces apoptosis in triple-negative breast cancer cells and suppresses tumor angiogenesis. Mol Cancer Ther 18:873
Karpenko MN, Muruzheva ZM, Ilyechova EY, Babich PS, Puchkova LV (2023) Abnormalities in copper status associated with an elevated risk of Parkinson’s phenotype development. Antioxidants 12:1654
Kaska WC, Carrans C, Michalowski J, Jackson J, Levinson W (1978) Inhibition of the RNA dependent DNA polymerase and the malignant transforming ability of Rous sarcoma virus by thiosemicarbazone- transition metal complexes. Bioinorg Chem 8:245
Kathiresan S, Mugesh S, Annaraj J, Murugan M (2017) Mixed-ligand copper (II) Schiff base complexes: the vital role of co-ligands in DNA/protein interactions and cytotoxicity. New J Chem 41:1267
Khaksar S, Panjehpour A, Ghadermazi E, Motieiyan E, Aliabadi A, Rostamnia S, Marabello D, Abdolmaleki S (2023a) Study on crystallographic structure and antiproliferative effect of mixed-ligand strontium (II) complex and N, Nˊ–bis (2-hydroxy-5-methylphenyl) pyridine-2, 6-dicarboxamide ligand. J Mol Struct 1274:134432
Khaksar S, Aliabadi A, Panjehpour A, Abdolmaleki S (2023b) Effect of the extra-nuclear cation on the cytotoxicity and mechanism of action of pyridine-2, 6-dicarboxylate Ga (III) complexes. Toxicology 495:153609
Khan RA et al (2016) Transition-metal norharmane compounds as possible cytotoxic agents: new insights based on a coordination chemistry perspective. J Inorg Biochem 165:128
Kong R, Sun G (2023) Targeting copper metabolism: a promising strategy for cancer treatment. Front Pharmacol. https://doi.org/10.3389/fphar.2023.1203447
Koo S, Park OK, Kim J, Han SI, Yoo TY, Lee N, Kim YG, Kim H, Lim C, Bae JS, Yoo J (2022) Enhanced chemodynamic therapy by Cu–Fe peroxide nanoparticles: tumor microenvironment-mediated synergistic Fenton reaction. ACS Nano 16:2535
Kostova I, Momekov G, Zaharieva M, Karaivanova M (2005) Cytotoxic activity of new lanthanum(III) complexes of bis-coumarins. Eur J Med Chem 40:542
Kroemer G, Reed JC (2000) Mitochondrial control of cell death. Nat Med 6:513
Li GY, Du KJ, Wang JQ, Liang JW, Kou JF, Hou XJ, Ji LN, Chao H (2013) Synthesis, crystal structure, DNA interaction and anticancer activity of tridentate copper (II) complexes. J Inorg Biochem 119:43
Li N, Sun Q, Yu Z, Gao X, Pan W, Wan X, Tang B (2018) Nuclear-targeted photothermal therapy prevents cancer recurrence with near-infrared triggered copper sulfide nanoparticles. ACS Nano 12:5197
Li Y, Dong Y, Zhou X, Fan K (2023) Nanotechnology connecting copper metabolism and tumor therapy. MedComm–Biomater Appl. https://doi.org/10.1002/mba2.36
Lin LS, Huang T, Song J, Ou XY, Wang Z, Deng H, Tian R, Liu Y, Wang JF, Liu Y, Yu G (2019) Synthesis of copper peroxide nanodots for H2O2 self-supplying chemodynamic therapy. J Am Chem Soc 141:9937
Liu T, Karlsen M, Karlberg AM, Redalen KR (2020) Hypoxia imaging and theranostic potential of [(64)Cu][Cu(ATSM)] and ionic Cu(II) salts: a review of current evidence and discussion of the retention mechanisms. Eur J Nucl Med Mol Imaging Res 10:33
Liu B, Bian Y, Liang S, Yuan M, Dong S, He F, Gai S, Yang P, Cheng Z, Lin J (2021a) One-step integration of tumor microenvironment-responsive calcium and copper peroxides nanocomposite for enhanced chemodynamic/ion-interference therapy. ACS Nano 16:617
Liu M, Wu H, Wang S, Hu J, Sun B (2021b) Glutathione-triggered nanoplatform for chemodynamic/metal-ion therapy. J Mater Chem B 9:9413
Liu S-Y, Xu Y, Yang H, Liu L, Zhao M, Yin W, Xu Y-T, Huang Y, Tan C, Dai Z et al (2021c) Ultrathin 2D Copper(I) 1,2,4-triazolate coordination polymer nanosheets for efficient and selective gene silencing and photodynamic therapy. Adv Mater 33:e2100849
Liu J, Peng Y, Wei W (2022a) Cell cycle on the crossroad of tumorigenesis and cancer therapy. Trends Cell Biol 32:30
Liu T, Redalen KR, Karlsen M (2022b) Development of an automated production process of [(64)Cu][Cu (ATSM)] for positron emission tomography imaging and theranostic applications. J Labelled Comp Radiopharm 65:191
Liu Z, Ma H, Lai Z (2023a) The role of ferroptosis and cuproptosis in curcumin against hepatocellular carcinoma. Molecules 28:1623
Liu L, Zhang H, Peng L, Wang D, Zhang Y, Yan B, Xie J, Xing S, Peng F, Liu X (2023b) A copper-metal organic framework enhances the photothermal and chemodynamic properties of polydopamine for melanoma therapy. Acta Biomater 158:660
Lopez J, Ramchandani D, Vahdat L (2019) Copper depletion as a therapeutic strategy in cancer. Met Ions Life Sci 19:303
Ma Z, Shao J, Bao W, Qiang Z, Xu J (2014) A thiosemicarbazone copper(II) complex as a potential anticancer agent. J Coord Chem 68:277
Machado JF, Marques F, Pinheiro T, de Brito MJ, Scalese G, Pérez-Díaz L, Otero L, António JP, Gambino D, Morais TS (2023) Copper (I)-thiosemicarbazone complexes with dual anticancer and antiparasitic activity. ChemMedChem 18:e202300074
Magrì A, Tabbì G, Naletova I, Attanasio F, Arena G, Rizzarelli E (2022) A deeper insight in metal binding to the hCtr1 N-terminus fragment: affinity, speciation and binding mode of binuclear Cu2+ and mononuclear ag+ complex species. Int J Mol Sci 23:2929
Mariani D, Ghasemishahrestani Z, Freitas W, Pezzuto P, Costa-da-Silva AC, Tanuri A, Kanashiro MM, Fernandes C, Horn A Jr, Pereira MD (2021) Antitumoral synergism between a copper (II) complex and cisplatin improves in vitro and in vivo anticancer activity against melanoma, lung and breast cancer cells. Biochim Biophys Acta-Gen Subj 1865:129963
Marzano C, Gandin V, Pellei M, Colavito D, Papini G, Lobbia GG, Del Giudice E, Porchia M, Tisato F, Santini C (2008) In vitro antitumor activity of the water soluble copper(I) complexes bearing the Tris(hydroxymethyl)phosphine ligand. J Med Chem 51:798
Marzano C, Pellei M, Tisato F, Santini C (2009) Copper complexes as anticancer agents. Anticancer Agents Med Chem 9:185
Mascia M, Villano C, De Francesco V, Schips L, Marchioni M, Cindolo L (2021) Efficacy and safety of the 64Cu(II)Cl2 PET/CT for urological malignancies: phase IIa clinical study. Clin Nuclear Med 46:443
Matsumoto H, Yoshii Y, Baden A, Kaneko E, Hashimoto H, Suzuki H et al (2019) Preclinical pharmacokinetic and safety studies of copper-diacetyl-bis(N(4)-methylthiosemicarbazone) (Cu-ATSM): translational studies for internal radiotherapy. Transl Oncol 12:1206
Maurer RI, Blower PJ, Dilworth JR, Reynolds CA, Zheng Y, Mullen GE (2002) Studies on the mechanism of hypoxic selectivity in copper bis(thiosemicarbazone) radiopharmaceuticals. J Med Chem 45:1420
McMillan DD, Maeda J, Bell JJ, Genet MD, Phoonswadi G, Mann KA et al (2015) Validation of 64Cu-ATSM damaging DNA via high-LET Auger electron emission. J Radiat Res 56:784
Mhaske A, Dileep KV, Kumar M, Poojary M, Pandhare K, Zhang KY, Scaria V, Binukumar BK (2020) ATP7A Clinical Genetics Resource—a comprehensive clinically annotated database and resource for genetic variants in ATP7A gene. Comput Struct Biotechnol J 18:2347
Molinaro C, Martoriati A, Pelinski L, Cailliau K (2020) Copper complexes as anticancer agents targeting topoisomerases I and II. Cancers 12:2863
Nakamura H, Takada K (2021) Reactive oxygen species in cancer: current findings and future directions. Cancer Sci 112:3945
Nam B, Lee W, Sarkar S, Kim JH, Bhise A, Park H et al (2022) In vivo detection of hydrogen sulfide in the brain of live mouse: application in neuroinflammation models. Eur J Nucl Med Mol Imaging 49:4073
Narayanan G, Bharathidevi SR, Vuyyuru H, Muthuvel B, Konerirajapuram Natrajan SC (2013) CTR1 silencing inhibits angiogenesis by limiting copper entry into endothelial cells. PLoS ONE 8:e71982
Obata A, Yoshimoto M, Kasamatsu S, Naiki H, Takamatsu S, Kashikura K et al (2003) Intra-tumoral distribution of (64)Cu-ATSM: a comparison study with FDG. Nucl Med Biol 30:529
Oe S, Miyagawa K, Honma Y, Harada M (2016) Copper induces hepatocyte injury due to endoplasmic reticulum stress in cultured cells and patients with Wilson disease. Exp Cell Res 347:192
Orlov IA, Sankova TP, Skvortsov AN, Klotchenko SA, Sakhenberg EI, Mekhova AA, Kiseleva IV, Ilyechova EY, Puchkova LV (2023) Properties of recombinant extracellular N-terminal domain of human high-affinity copper transporter 1 (hNdCTR1) and its interactions with Cu (ii) and Ag (i) ions. Dalton Trans 52:3403
Ozalp-Yaman S, de Hoog P, Amadei G, Pitie M, Gamez P, Dewelle J, Mijatovic T, Meunier B, Kiss R, Reedijk J (2008) Platinated copper(3-Clip-Phen) complexes as effective DNA-cleaving and cytotoxic agents. Chem Eur J 14:3418
Padhye S, Afrasiabi Z, Sinn E, Fok J, Mehta K, Rath N (2005) Antitumor metallothiosemicarbazonates: structure and antitumor activity of palladium complex of phenanthrenequinone thiosemicarbazone. Inorg Chem 44:1154
Panichelli P, Villano C, Cistaro A, Bruno A, Barbato F, Piccardo A (2016) Imaging of brain tumors with copper-64 chloride: early experience and results. Cancer Biother Radiopharm 31:159
Paparo F, Peirano A, Matos J, Bacigalupo L, Rossi U, Mussetto I (2020) Diagnostic value of retrospectively fused (64)CuCl(2) PET/MRI in biochemical relapse of prostate cancer: comparison with fused (18)F-Choline PET/MRI, (64)CuCl2 PET/CT, (18)F-Choline PET/CT, and mpMRI. Abdom Radiol 45:3896
Patel RN, Kumar S, Pandeya KB (2002) E.s.r., visible and SOD studies of imidazolate bridged Cu2II, II, CuIIZnII and CuIINiII complexes with pentamethyldiethylenetriamine as capping ligand: a plausible model for superoxide dismutase. J Inorg Biochem 89:61
Piccardo A, Paparo F, Puntoni M, Righi S, Bottoni G, Bacigalupo L (2018) 64CuCl2 PET/CT in prostate cancer relapse. J Nucl Med 59:444
Piroš M, Schoeller M, Koňariková K, Valentová J, Švorc Ľ, Moncoľ J, Valko M, Švorec J (2023) Structural and biological properties of heteroligand copper complexes with diethylnicotinamide and various fenamates: preparation, structure, spectral properties and hirshfeld surface analysis. Inorganics 11:108
Pitie M, Donnadieu B, Meunier B (1998a) Preparation of the new bis(phenanthroline) ligand “Clip-Phen” and evaluation of the nuclease activity of the corresponding copper complex. Inorg Chem 37:3486
Pitie M, Sudres B, Meunier B (1998b) Dramatic increase of the DNA cleavage activity of Cu(Clip-phen) by fixing the bridging linker on the C3 position of the phenanthroline units. Chem Commun 23:2597
Pitie M, Boldron C, Gornitzka H, Hemmert C, Donnadieu B, Meunier B (2003) DNA cleavage by copper complexes of 2- and 3-clip-phen derivatives. Eur J Inorg Chem 3:528
Rajendiran V, Karthik R, Palaniandavar M, Stoeckli-Evans H, Periasamy VS, Akbarsha MA, Srinag BS, Krishnamurthy H (2007) Mixed-ligand Copper(II)-phenolate complexes: effect of coligand on enhanced DNA and protein binding, DNA cleavage, and anticancer activity. Inorg Chem 46:8208
Raptopoulou CP, Paschalidou S, Pantazaki AA, Terzis A, Perlepes SP, Lialiaris T, Bakalbassis EG, Mrozinski J, Kyriakidis DA (1998) Bis(acetato)bis(1-methyl-4,5-diphenylimidazole)copper(II): preparation, characterization, crystal structure, DNA strand breakage and cytogenetic effect. J Inorg Biochem 71:15
Righi S, Ugolini M, Bottoni G, Puntoni M, Iacozzi M, Paparo F (2018) Biokinetic and dosimetric aspects of 64CuCl2 in human prostate cancer: possible theranostic implications. Eur J Nucl Med Mol Imaging Res 8:18
Ritacca AG, Falcone E, Doumi I, Vileno B, Faller P, Sicilia E (2023) Dual role of glutathione as a reducing agent and Cu-ligand governs the ROS production by anticancer Cu-thiosemicarbazone complexes. Inorg Chem 62:3957
Rodić MV, Leovac VM, Jovanović LS, Spasojević V, Joksović MD, Stanojković T, Matić IZ, Vojinović-Ješić LS, Marković V (2016) Synthesis, characterization, cytotoxicity and antiangiogenic activity of copper (II) complexes with 1-adamantoyl hydrazone bearing pyridine rings. Eur J Med Chem 115:75–81
Rodriguez-Arguelles MC, Sanchez A, Ferrari MB, Fava GG, Pelizzi C, Pelosi G, Albertini R, Lunghi P, Pinelli S (1999) Transition-metal complexes of isatin-β-thiosemicarbazone X-ray crystal structure of two nickel complexes. J Inorg Biochem 73:7
Saczewski F, Dziemidowicz-Borys E, Bednarski PJ, Gruenert R, Gdaniec M, Tabin P (2006) Synthesis, crystal structure and biological activities of copper(II) complexes with chelating bidentate 2-substituted benzimidazole ligands. J Inorg Biochem 100:1389
Saha DK, Padhye S, Padhye S (2001) Targeting estrogen receptor sites in human breast cancer cell line T47D with copper conjugates of nonsteroidal antiinflammatory drug derivatives: antiproliferative activity of ketoprofen derivative and its copper complex. Met-Based Drugs 8:73
Saha DK, Sandbhor U, Shirisha K, Padhye S, Deobagkar D, Anson CE, Powell AK (2004) A novel mixed-ligand antimycobacterial dimeric copper complex of ciprofloxacin and phenanthroline. Bioorg Med Chem Lett 14:3027
Savi A, Incerti E, Fallanca F, Bettinardi V, Rossetti F, Monterisi C et al (2017) First evaluation of PET-based human biodistribution and dosimetry of (18)F-FAZA, a tracer for imaging tumor hypoxia. J Nucl Med 58:1224
Shanbhag V, Jasmer-McDonald K, Zhu S, Martin AL, Gudekar N, Khan A et al (2019) ATP7A delivers copper to the lysyl oxidase family of enzymes and promotes tumorigenesis and metastasis. Proc Natl Acad Sci USA 116:6836
Shao J, Ma Z-Y, Li A, Liu Y-H, Xie C-Z, Qiang Z-Y, Xu J-Y (2014) Thiosemicarbazone Cu(II) and Zn(II) complexes as potential anticancer agents: syntheses, crystal structure, DNA cleavage, cytotoxicity and apoptosis induction activity. J Inorg Biochem 136:13
Shen W-Y, Jia C-P, Liao L-Y, Chen L-L, Hou C, Liu Y-H, Liang H, Chen Z-F (2022) Copper(II) complexes of halogenated quinoline Schiff base derivatives enabled cancer therapy through glutathione-assisted chemodynamic therapy and inhibition of autophagy flux. J Med Chem 65:5134
Shi Z, Yao C, Shui Y, Li S, Yan H (2023) Research progress on the mechanism of angiogenesis in wound repair and regeneration. Front Phys 14:1284981
Shimada K, Reznik E, Stokes ME, Krishnamoorthy L, Bos PH, Song Y, Quartararo CE, Pagano NC, Carpizo DR, deCarvalho AC et al (2018) Copper-binding small molecule induces oxidative stress and cell-cycle arrest in glioblastoma-patient-derived cells. Cell Chem Biol 25:585
Shobha Devi C, Thulasiram B, Aerva RR, Nagababu P (2018) Recent advances in copper intercalators as anticancer agents. J Fluoresc 28:1195
Shubin AV, Demidyuk IV, Komissarov AA, Rafieva LM, Kostrov SV (2016) Cytoplasmic vacuolization in cell death and survival. Oncotarget 7:55863–55889
Sies H, Belousov VV, Chandel NS, Davies MJ, Jones DP, Mann GE, Murphy MP, Yamamoto M, Winterbourn C (2022) Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat Rev Mol Cell Biol 23:499
Sigman DS, Graham DR, D’Aurora V, Stern AM (1979) Oxygen-dependent cleavage of DNA by the 1,10-phenanthroline-cuprous complex Inhibition of E coli DNA polymerase I. J Biol Chem 254:12269
Sigman DS, Landgraf R, Perrin DM, Pearson L (1996) Nucleic acid chemistry of the cuprous complexes of 1,10-phenanthroline and derivatives. Met Ions Biol Syst 33:485
Song Y, Zhan J, Li M, Zhao H, Shi G, Wu M, Fang H (2022) Enhancement of the water affinity of histidine by zinc and copper ions. Int J Mol Sci 23:3957
Sperandio S, de Belle I, Bredesen DE (2000) An alternative, nonapoptotic form of programmed cell death. Proc Natl Acad Sci USA 97:14376
Su Y, Zhang X, Li S, Xie W, Guo J (2022) Emerging roles of the copper-CTR1 axis in tumorigenesis. Mol Cancer Res 20:1339
Szilagyi I, Labadi I, Hernadi K, Palinko I, Nagy NV, Korecz L, Rockenbauer A, Kele Z, Kiss T (2005) Speciation study of an imidazolate-bridged copper(II)-zinc(II) complex in aqueous solution. J Inorg Biochem 99:1619
Takasawa M, Moustafa RR, Baron JC (2008) Applications of nitroimidazole in vivo hypoxia imaging in ischemic stroke. Stroke 39:1629
Tamura H, Imai H, Kuwahara J, Sugiura Y (1987) A new antitumor complex: bis(acetato)bis(imidazole)copper(II). J Am Chem Soc 109:6870
Tang X, Yan Z, Miao Y, Ha W, Li Z, Yang L, Mi D (2023) Copper in cancer: from limiting nutrient to therapeutic target. Front Oncol 13:1209156
Tardito S, Marchiò L (2009) Copper compounds in anticancer strategies. Curr Med Chem 16:1325
Tardito S, Bussolati O, Gaccioli F, Gatti R, Guizzardi S, Uggeri J, Marchio L, Lanfranchi M, Franchi-Gazzola R (2006) Nonapoptotic programmed cell death induced by a copper(II) complex in human fibrosarcoma cells. Histochem Cell Biol 126:473
Tateishi K, Tateishi U, Sato M, Yamanaka S, Kanno H, Murata H et al (2013) Application of 62Cu-diacetyl-bis(N4-methylthiosemicarbazone) PET imaging to predict highly malignant tumor grades and hypoxia-inducible factor-1alpha expression in patients with glioma. AJNR Am J Neuroradiol 34:92
Thomas A, Pommier Y (2019) Targeting topoisomerase I in the era of precision medicine. Clin Cancer Res 25:6581
Tsang T, Posimo JM, Gudiel AA, Cicchini M, Feldser DM, Brady DC (2020) Copper is an essential regulator of the autophagic kinases ULK1/2 to drive lung adenocarcinoma. Nat Cell Biol 22:412
Tsvetkov P, Coy S, Petrova B, Dreishpoon M, Verma A, Abdusamad M, Rossen J, Joesch-Cohen L, Humeidi R, Spangler RD et al (2022) Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 375:1254
Unsoeld M, Lamprecht U, Traub F, Hermes B, Scharpf M, Potkrajcic V, Zips D, Paulsen F (2020) MR thermometry data correlate with pathological response for soft tissue sarcoma of the lower extremity in a single center analysis of prospectively registered patients. Cancers 12:959
Ünver H, Dıkmen G, Kiyan HT (2022) Synthesis, X-ray characterization and evaluation of potent anti-angiogenic activity of a novel copper (II)-imidazole-bipyridyl complex. Inorg Nano-Metal Chem 52:1153–1160
Vakili-Samiani S, Khanghah OJ, Gholipour E, Najafi F, Zeinalzadeh E, Samadi P, Sarvarian P, Pourvahdani S, Kelaye SK, Hamblin MR et al (2022) Cell cycle involvement in cancer therapy; WEE1 kinase, a potential target as therapeutic strategy. Mutat Res 824:111776
Wang J, Xu M, Wang D, Li Z, Primo FL, Tedesco AC, Bi H (2019a) Copper-doped carbon dots for optical bioimaging and photodynamic therapy. Inorg Chem 58:13394
Wang R, He Z, Cai P, Zhao Y, Gao L, Yang W, Zhao Y, Gao X, Gao F (2019b) Surface-functionalized modified copper sulfide nanoparticles enhance checkpoint blockade tumor immunotherapy by photothermal therapy and antigen capturing. ACS Appl Mater Interfaces 11:13964
Wang Y, Zhang L, Zhou F (2022) Cuproptosis: a new form of programmed cell death. Cell Mol Immunol 19:867
Wang Y, Chen Y, Zhang J, Yang Y, Fleishman JS, Wang Y, Wang J, Chen J, Li Y, Wang H (2023a) Cuproptosis: a novel therapeutic target for overcoming cancer drug resistance. Drug Resist Updat 72:101018
Wang C, Yang X, Dong C, Chai K, Ruan J, Shi S (2023b) Cu-related agents for cancer therapies. Coord Chem Rev 487:215156
Wang X, Zhu M, Li S, Xu G, Zhang Z, Yang F (2024) Novel mono-, bi-, tri-, and tetra-nuclear copper complexes that inhibit tumor growth through apoptosis and anti-angiogenesis. J Inorg Biochem 250:112403
Wee NKY, Weinstein DC, Fraser ST, Assinder SJ (2013) The mammalian copper transporters CTR1 and CTR2 and their roles in development and disease. Int J Biochem Cell Biol 45:960
Wei J, Fang D (2021) Endoplasmic reticulum stress signaling and the pathogenesis of hepatocarcinoma. Int J Mol Sci 22:1799
White C, Kambe T, Fulcher YG, Sachdev SW, Bush AI, Fritsche K et al (2009) Copper transport into the secretory pathway is regulated by oxygen in macrophages. J Cell Sci 122:1315
Wooton-Kee CR, Robertson M, Zhou Y, Dong B, Sun Z, Kim KH et al (2020) Metabolic dysregulation in the Atp7b-/- wilson’s disease mouse model. Proc Natl Acad Sci USA 117:2076
Wózniak-Budych MJ, Staszak K, Staszak M (2023) Copper and copper-based nanoparticles in medicine-perspectives and challenges. Molecules 28:6687
Wu Z, Zhang W, Kang YJ (2019) Copper affects the binding of HIF-1α to the critical motifs of its target genes. Metallomics 11:429
Xie F, Peng F (2017) Radiopharmaceuticals for assessment of altered metabolism and biometal fluxes in brain aging and Alzheimer’s disease with positron emission tomography. J Alzheimers Dis 59:527–536
Xie F, Wei W (2022) [64Cu]Cu-ATSM: an emerging theranostic agent for cancer and neuroinflammation. Eur J Nucl Med Mol Imaging 49:3964
Xie J, Yang Y, Gao Y, He J (2023) Cuproptosis: mechanisms and links with cancers. Mol Cancer 22:46
Xu Y, Zhou Q, Feng X, Dai Y, Jiang Y, Jiang W, Liu X, Xing X, Wang Y, Ni Y et al (2020) Disulfiram/copper markedly induced myeloma cell apoptosis through activation of JNK and intrinsic and extrinsic apoptosis pathways. Biomed Pharmacother 126:110048
Xu Y, Liu S-Y, Zeng L, Ma H, Zhang Y, Yang H, Liu Y, Fang S, Zhao J, Xu Y et al (2022) An enzyme-engineered nonporous copper(I) coordination polymer nanoplatform for cuproptosis-based synergistic cancer therapy. Adv Mater 34:e2204733
Xu X, Ding C, Zhong H, Qin W, Shu D, Yu M et al (2023) Integrative analysis revealed that distinct cuprotosis patterns reshaped tumor microenvironment and responses to immunotherapy of colorectal cancer. Front Immunol 14:1165101
Yan T, Yang K, Chen C, Zhou Z, Shen P, Jia Y, Xue Y, Zhang Z, Shen X, Han X (2021) Synergistic photothermal cancer immunotherapy by Cas9 ribonucleoprotein-based copper sulfide nanotherapeutic platform targeting PTPN2. Biomaterials 279:121233
Yang Y (2015) Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest 125:3335
Yang L, Yang P, Lip G, Ren J (2023a) Copper homeostasis and cuproptosis in cardiovascular disease therapeutics. Trends Pharmacol Sci 44:573–585
Yang W, Wang Y, Huang Y, Yu J, Wang T, Li C, Yang L, Zhang P, Shi L, Yin Y, Tao K, Li R (2023b) 4-Octyl itaconate inhibits aerobic glycolysis by targeting GAPDH to promote cuproptosis in colorectal cancer. Biomed Pharmacother 159:114301
Yang Y, Huang J, Liu M, Qiu Y, Chen Q, Zhao T, Xiao Z, Yang Y, Jiang Y, Huang Q, Ai K (2023c) Emerging sonodynamic therapy-based nanomedicines for cancer immunotherapy. Adv Sci 10:e2204365
Yang Z, Zhao Z, Cheng H, Shen Y, Xie A, Zhu M (2023d) In-situ fabrication of novel Au nanoclusters-Cu(2+)@sodium alginate/hyaluronic acid nanohybrid gels for cuproptosis enhanced photothermal/photodynamic/chemodynamic therapy via tumor microenvironment regulation. J Colloid Interface Sci 641:215–228
Yokoi K, Yamaguchi K, Umezawa M, Tsuchiya K, Aoki S (2022) Induction of paraptosis by cyclometalated iridium complex-peptide hybrids and CGP37157 via a mitochondrial Ca2+ overload triggered by membrane fusion between mitochondria and the endoplasmic reticulum. Biochem 61(8):639–655
Yoshii Y, Matsumoto H, Yoshimoto M, Furukawa T, Morokoshi Y, Sogawa C et al (2014) Controlled administration of penicillamine reduces radiation exposure in critical organs during 64Cu-ATSM internal radiotherapy: a novel strategy for liver protection. PLoS ONE 9:e86996
Yoshii Y, Matsumoto H, Yoshimoto M, Zhang MR, Oe Y, Kurihara H et al (2018) Multiple administrations of (64)Cu-ATSM as a novel therapeutic option for glioblastoma: a translational study using mice with xenografts. Transl Oncol 11:24
Yu Z, Hu Y, Sun Y, Sun T (2021) Chemodynamic therapy combined with multifunctional nanomaterials and their applications in tumor treatment. Chemistry 27:13953
Zhang S, Zhu Y, Tu C, Wei H, Yang Z, Lin L, Ding J, Zhang J, Guo Z (2004) A novel cytotoxic ternary copper(II) complex of 1,10-phenanthroline and L-threonine with DNA nuclease activity. J Inorg Biochem 98:2099
Zhang Q, Guo X, Cheng Y, Chudal L, Pandey NK, Zhang J et al (2020) Use of copper-cysteamine nanoparticles to simultaneously enable radiotherapy, oxidative therapy and immunotherapy for melanoma treatment. Signal Transduct Target Ther 5:58
Zhao P, Wang Y, Kang X, Wu A, Yin W, Tang Y, Wang J, Zhang M, Duan Y, Huang Y (2018) Dual-targeting biomimetic delivery for anti-glioma activity via remodeling the tumor microenvironment and directing macrophage-mediated immunotherapy. Chem Sci 9:2674
Zhao D, Wu Y, Huang W, Gong S, Chen Z (2022) DNA binding, DNA cleavage, cellular uptake, cytotoxicity, and apoptosis-inducing ability of a binuclear Schiff base copper (II) complex. New J Chem 46:15219
Zhao L, Zhong B, Zhu Y, Zheng H, Wang X, Hou Y et al (2023) Nitrovin (Difurazone), an antibacterial growth promoter, induces ROS-mediated paraptosis-like cell death by targeting thioredoxin reductase 1 (TrxR1). Biochem Pharmacol 210:115487
Zheng H, Hu C, Quan Y, Ye X, Shi X, Guo Z, Wang X (2023) A copper complex that combats triple negative breast cancer by restraining angiogenesis. Dalton Trans 52:7626–7634
Zhong X, Wei H-L, Liu W-S, Wang D-Q, Wang X (2007) The crystal structures of copper(II), manganese(II), and nickel(II) complexes of a (Z)-2-hydroxy-N’-(2-oxoindolin-3-ylidene)benzohydrazide-potential antitumor agents. Bioorg Med Chem Lett 17:3774
Zhou H, Zheng C, Zou G, Tao D, Gong J (2002) G1-phase specific apoptosis in liver carcinoma cell line induced by copper-1,10-phenanthroline. Int J Biochem Cell Biol 34:678
Zhuang X, Kang Y, Zhao L, Guo S (2022) Design and synthesis of copper nanoparticles for the treatment of human esophageal cancer: introducing a novel chemotherapeutic supplement. J Exp Nanosci 17:274
Zhuo X, Liu Z, Aishajiang R, Wang T, Yu D (2023) Recent progress of copper-based nanomaterials in tumor-targeted photothermal therapy/photodynamic therapy. Pharmaceutics 15:2293
Zohrevandi M, Abdolmaleki S, Ghadermazi M, Gholiee Y, Aliabadi A, Motieiyan E, Hakimi M, Marabello D (2022) Synthesis, characterization, crystallographic structure, theoretical studies, and in vitro cytotoxicity assessment of two Gd (III) and Ce (IV) complexes containing pyridine-2, 6-dicarboxylate. Polyhedron 211:115561
Acknowledgements
We would like to thank the University of Georgia for its financial support.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
S.A. wrote the main manuscript text. A.A and S.Kh. prepared figures 1-11. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdolmaleki, S., Aliabadi, A. & Khaksar, S. Unveiling the promising anticancer effect of copper-based compounds: a comprehensive review. J Cancer Res Clin Oncol 150, 213 (2024). https://doi.org/10.1007/s00432-024-05641-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00432-024-05641-5