Abstract
Background
As the form of World Health Organization Central Nervous System (WHO CNS) tumor classifications is updated, there is a lack of research on outcomes for intracranial combined solitary-fibrous tumor and hemangiopericytoma (SFT/HPC). This study aimed to explore conditional survival (CS) pattern and develop a survival prediction tool for intracranial SFT/HPC patients.
Methods
Data of intracranial SFT/HPC patients was gathered from the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute. The patients were split into training and validation groups at a 7:3 ratio for our analysis. CS is defined as the likelihood of surviving for a specified period of time (y years), given that the patient has survived x years after initial diagnosis. Then, we used this definition of CS to analyze the intracranial SFT/HPC patients. The least absolute shrinkage and selection operator (LASSO) regression and best subset regression (BSR) were employed to identify predictive factors. The Multivariate Cox regression analysis was applied to establish a novel CS-based nomogram, and a risk stratification system was developed using this model.
Results
From the SEER database, 401 patients who were diagnosed with intracranial SFT/HPC between 2000 and 2019 were identified. Among them, 280 were included in the training group and 121 were included in the internal validation group for analysis. Our study revealed that in intracranial SFT/HPC, 5-year survival rates saw significant improvement ranging from 78% at initial diagnosis to rates of 83%, 87%, 90%, and 95% with each successive year after surviving for 1–4 years. The LASSO regression and BSR identified patient age, tumor behavior, surgery and radiotherapy as predictors of CS-based nomogram development. A risk stratification system was also successfully constructed to facilitate the identification of high-risk patients.
Conclusion
The CS pattern of intracranial SFT/HPC patients was outlined, revealing a notable improvement in 5-year survival rates after an added period of survival. Our newly-established CS-based nomogram and risk stratification system can provide a real-time dynamic survival estimation and facilitate the identification of high-risk patients, allowing clinicians to better guide treatment decision for these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Solitary-fibrous tumor and hemangiopericytoma are a rare type of meningeal tumor that occurs at a rate of 3.8 cases per 10,000,000 individuals per year in the US (Kinslow and Wang 2020; Kinslow et al. 2018). Since the recent update of the World Health Organization Central Nervous System (WHO CNS) tumor classifications, the solitary-fibrous tumor and hemangiopericytoma have been grouped together due to their common NAB2/STAT6 fusion gene, indicating a likely shared genetic origin and potential variability in clinical behaviors (Sung et al. 2016; Lu et al. 2022; Fritchie et al. 2016; Louis et al. 2016). The recent studies had reported that the incidence of this tumor is increasing, and it required specialized medical attention (Lu et al. 2022). As the form of classification is updated, there is a lack of research on prognostic factors and outcomes for combined SFT and HPC tumors, with previous studies often limited by small sample sizes and an inability to validate findings (Swaminathan et al. 2022a; Mena et al. 1991; Rutkowski et al. 2012; Zeng et al. 2017; Wu et al. 2021). Moreover, there is inadequate description regarding the survival pattern of SFT/HPC patients in contemporary time. The survival description of this tumor is often based on a relatively small sample size and is generally targeted at overall survival (OS) (Wu et al. 2021; Swaminathan et al. 2022b). Further investigation is required to determine the conditional survival (CS) of SFT/HPC.
Currently, surgery with or without adjuvant radiotherapy is the standard of care for with SFT/HPC patients (Kinslow et al. 2018, 2023a). However, due to a lack of controlled trials and prospective studies, the use of radiotherapy varies by institutions and remains controversial (Kinslow et al. 2023b; Sonabend et al. 2014; Prado et al. 2012; Combs et al. 2005; Gou et al. 2022). Also, as tumors are infrequent, past investigations have frequently combined tumors situated in the intracranial and spinal areas, leading to significant dissimilarity in the findings. This suggests that further research should be intensified to focus on intracranial lesions in order to gain greater insight into this type of tumor.
In modern medicine, many studies have suggested that utilizing dynamic survival estimation may aid in the development of effective treatment plans and follow-up strategies that could benefit individuals with cancer (Qian et al. 2023; Luo et al. 2023; Meng et al. 2023). However, no survival prediction tool has been developed thus far for patients with intracranial SFT/HPC. Nomograms are currently the most effective method with graphical algorithms for predicting cancer patient outcomes (Gafita et al. 2021; Sharouni et al. 2021; Berardi et al. 2020). Traditional nomogram incorporated individual predictors but failed to account for survival time, rendering them incapable of delivering real-time survival estimations (Meng et al. 2022). At the same time, CS analysis is a statistical tool that can estimate survival rates over time, thus providing real-time updates to convey the changing dynamics of survival rates (Hieke et al. 2015; Zabor et al. 2013; Jung et al. 2018). Hence, we attempted to combine the nomogram with CS to develop a novel dynamic survival prediction tool, aiming to aid in clinical practice.
The Surveillance, Epidemiology, and End Results (SEER) database is a valuable resource for clinical cancer research as it covers approximately 27.8% of the US population, and it has been widely advocated for the rare tumor study (Harlan and Hankey 2003). Therefore, we conducted this population-based cohort analysis to explore survival pattern and develop a survival prediction tool for SFT/HPC patients.
Methods
Data source and patient selection
The SEER program is the National Cancer Institute’s (NCI) authoritative source for research on cancer incidence and survival and it covers approximately 27.8% of the United States population. The vital status of collected patients are regularly reviewed on an annual basis, and the database is subject to frequent quality control checks, thereby supplying clinical researchers with high-quality research samples.
We queried the SEER database to identify cases of SFT/HPC (ICD-O-3 code 8815 and 9150) within the brain (ICD O-3 codes C70.1–C72.9). The following exclusion criteria were used: (1) disease not diagnosed between 2000 and 2019; (2) treatment information was unknown; and (3) the follow-up period was missing. The study gathered patient demographic information from the SEER database, encompassing factors such as age at diagnosis, sex, race, marital status, rural/urban residence, and household income. In addition, the data on tumor site, histological type, tumor size and tumor behavior code ICD-O-3 were collected. The study also acquired treatment and survival information for the patients. Our study utilized OS as the endpoint measuring the period between the patient's diagnosis of the tumor and their death. And OS was analyzed via the Kaplan–Meier method (Stel et al. 2011).
Statistical analysis
The identified SFT/HPC patients were split into a 7:3 ratio for training and validation groups. Descriptive statistics were used to exhibit the characteristics of the patients, tumors, and treatments for the total cohort, as well as for the training and validation groups.
CS(y|x) represents the probability that a patient who has not succumbed to SFT/HPC at a certain time x after diagnosis will survive an additional y years. Calculations of CS were conducted via standard definition of conditional probability(Skuladottir and Olsen 2003):
OS(x) and OS(y + x) were survival probability calculated by the Kaplan − Meier methods for x- and (x + y)-years. For example, CS(2|3) represents the probability of a patient surviving for an additional 2 years after surviving for 3 years following their initial diagnosis.
Our study utilized two methods for identifying predictors of prognosis: the least absolute shrinkage and selection operator (LASSO) regression with tenfold cross-validation (lambda.min used as the screening criterion) (Tibshirani 1997), and the best subset regression (BSR) with the maximum adjusted R squared used as the screening criterion (Zhang 2016). The interaction of the variables screened by the two methods was taken as the prognostic factors of the final modeling. A multivariate Cox regression analysis was performed to validate the prognostic significance of selected variables and integrate those predictors to develop a novel CS-based nomogram model.
All variables in our nomogram were quantified as points and upon input of prognostic factors, a personalized OS and CS rates was determined through calculation of total risk points. We also used the maximum standardized log-rank statistic to stratify the risk of patients according to their total risk score for optimization of clinical management. The Kaplan–Meier method was utilized to evaluate the difference in OS between risk groups.
The performance of the model was assessed in both training and validation cohorts. Calibration plots were used to evaluate the consistency between predicted probabilities and observed outcomes. Discrimination in medical terminology refers to the capacity of a nomogram to distinguish between patient prognoses using the Harrell C-index as a quantification measure. The time-dependent receiver operating characteristic (ROC) curves with the area under the curves (AUCs) were employed to assess the accuracy of the survival prediction for the final model. Furthermore, the decision curve analysis (DCA) was adopted to gauge the effectiveness of our nomogram as a medical intervention, in terms of net benefit. R (version 4.1.0) was used for statistical analysis in our study and statistical significance was determined by p values less than 0.05 in a two-tailed test.
Results
Clinicopathological characteristics
We identified 401 patients with intracranial SFT/HPC from the SEER database, and these patients were assigned randomly to either the training cohort (n = 280) or the validation cohort (n = 121). The vast majority of the cohort fell within the less than 60 age range (67.1%), identified as white (81.0%), and resided in a metropolitan county at the time of diagnosis (94.0%). There was no discrepancy in prevalence between the genders. The majority of intracranial SFT/HPC were found in the supratentorial region (60.1%) and diagnosed as HPC type (80.3%). 58.6% of tumors documented by SEER were found to be either benign or borderline in nature. In terms of treatment status, the data suggested that a large proportion of patients underwent surgery procedure (94.0%), and 50.9% of patients received radiotherapy. See Table 1 for details.
Conditional survival analysis
The Kaplan–Meier method was used to predict OS probability in SFT/HPC patients and found an OS rate of 86% at the 3-year mark and 78% at 5 years. Through CS analysis, The CS curve for SFT/HPC depicted a rise in 5-year survival rate among these patients for every additional year of survival (Fig. 1). The 5-year survival rate for patients improved gradually from 78% at initial diagnosis to rates of 83%, 87%, 90%, and 95% with each successive year after surviving for 1–4 years, respectively (Fig. 1).
Conditional survival analysis of intracranial SFT/HPC patients. Kaplan–Meier estimates of survival at diagnosis (0 years) and conditional survival based on years already survived after diagnosis (1–4 years). Conditional survival curves (A); and updated survival data (B) and patient numbers (C) adjusted for survived time. SFT/HPC, solitary-fibrous tumor/hemangiopericytoma
The CS-based nomogram and risk stratification system construction
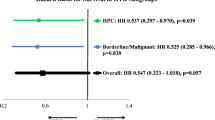
Based on the training cohort, two methods were adopted to screen predictors of SFT/HPC prognosis for prediction model construction: the LASSO regression with tenfold cross-validation identified 4 non-zero variables with lambda.min as screening criteria (Fig. 2A and B), and 8-variable (8/12) combination was selected with the highest adjusted R-squared in BSR analysis (Fig. 2C). Finally, by taking the intersection of the variables selected by the two methods, the subset of 4 variables including age at diagnosis, tumor behavior code, surgery and radiotherapy were screened for nomogram model development (Fig. 2D). The Multivariate Cox regression forest plot further confirmed a significant association between these predictive factors and the prognosis of SFT/HPC (Age, ≥ 60y vs < 60y, HR 3.315, P < 0.001; Tumor behavior, Malignant vs Benign and borderline, HR 1.344, P = 0.167; Surgery, GTR vs No surgery, P = 0.034; Radiotherapy, Yes vs No, P = 0.005; Fig. 2E).
Then, by combining the CS formula and traditional nomogram model, we successfully integrated above 4 variables to establish a CS-based nomogram model using the Multivariate Cox regression method in training set to estimate 3- and 5-year OS and 5-year CS individually for SFT/HPC patients (Fig. 3). In addition, we utilized the nomogram model to determine the total risk score for each patient and create a system for stratifying their risk. The maximum standardized log-rank statistic indicated that the most appropriate risk cutoff point on survival was at 80, thereby stratifying patients into low- and high-risk groups (Fig. 4A and B). The Kaplan–Meier curves demonstrated that low-risk patients had a significantly better survival advantage than those in the high-risk group in training group (Fig. 4C). In the validation cohort, the risk score also demonstrated a potential association with prognosis, approaching statistical significance with a borderline P value (Fig. 4D).
The CS-nomogram evaluation and validation
The C-index values of the CS-nomogram were 0.722 and 0.608 in the training and validation groups respectively, indicating that our innovative model displayed good prognostic value. The calibration plots showed this model was well calibrated with good concordance between predicted and observed 3- and 5-year OS probability in both training and validation sets (Fig. 5A and B). ROC analysis revealed that the nomogram exhibited strong discriminatory power in both groups. The 3- and 5-year AUC values were 0.76 in the training group, and 0.64 and 0.76 in the validation group, respectively (Fig. 5C and D). In terms of clinical usefulness, the DCA analysis revealed that the nomograms demonstrated a significant positive net benefit in both training and validation groups (Fig. 5E and F). In DCA curves, the horizontal axis represents the probability threshold and the vertical axis represents the net benefit after subtracting the disadvantage. Both the training and validation cohorts showed improved 3- and 5-year DCA curves for the nomogram, demonstrating its favorable clinical utility.
The CS-based nomogram validation. Calibration plots of the nomogram for training (A) and validation groups (B); Time-dependent area under curve (AUC) curves for training (C) and validation groups (D). Decision curve analysis (DCA) curves for evaluating clinical usefulness of the nomogram in training (E) and validation groups (F)
Discussion
There have been few studies that have analyzed SFT/HPC together since the update of the WHO guidelines in 2016, likely due to the rarity of these tumors. Those who have conducted mixed tumors in various regions have obtained conflicting findings. Boyett et al. also reported that tumor location was significantly associated with the prognosis of SFT/HPC patients (Boyett et al. 2019). Thus, we conducted a population-based investigation of SFT/HPC with a specific emphasis on intracranial lesions. A CS-based prediction tool was also successfully established for these patients, with the aim of optimizing the clinical management of this disease.
We first described the OS and CS pattern of intracranial SFT/HPC patients and found the 5-year OS of SFT/HPC was 78%. The CS method provides a unique way to assess survival by estimating changes in survival in real-time based on the amount of time already survived. In the recent years, this approach has gained popularity in clinical trials of various cancers, where it is used to analyze changes in the distribution of survival as the disease progresses. Our study revealed that in intracranial SFT/HPC, 5-year survival rates saw significant improvement ranging from 78% at initial diagnosis to rates of 83%, 87%, 90%, and 95% with each successive year after surviving for 1–4 years, respectively. CS prediction analysis provides patients with a visual representation of the dynamic change in their survival probability which can alleviate anxiety in SFT/HPC patients, and facilitate communication and collaboration between doctors and patients.
The alteration of real-time survival estimation depends not solely on the duration of survival, but also on the clinicopathological attributes of the individual (Balachandran et al. 2015; Iasonos et al. 2008). Therefore, we developed a CS-based prognosis prediction tool by combining the traditional model and CS analysis. The LASSO regression analysis and BSR analysis were implemented to screen the variables most significantly related to SFT/HPC’s prognosis and prevent overfitting or underfitting the model. After rigorous selection, a novel CS-based nomogram model was successfully developed integrating four predictive factors including age at diagnosis, tumor behavior code, surgery and radiotherapy. And this model can offer SFT/HPC patients a constantly updated estimation of their chances of survival, with a favorable prediction performance. Furthermore, as a feature of its implementation, this model can be employed for risk stratification by assigning risk scores for SFT/HPC patients. And those patients with high-risk score may require more intensive monitoring and a more proactive course of treatment.
Our multivariate Cox regression analysis also confirmed a significant association between these four predictive factors and the prognosis of SFT/HPC. Our study confirmed prior findings that patients with older age and malignant tumor phenotype were significantly associated with worse outcomes (Kinslow et al. 2018; Lu et al. 2022; Mena et al. 1991; Zeng et al. 2017; Sonabend et al. 2014; Schiariti et al. 2011; Ghia et al. 2013). As the first-line treatment choice for these patients, it is unsurprising that complete surgical removal of tumor was shown to have a significant impact on prognosis, in agreement with previous studies (Kinslow et al. 2018; Lu et al. 2022). It is noteworthy that radiotherapy reception conferred a remarkable survival advantage in our study. Ghose et al. found that survival is improved when complete resection is followed by adjuvant radiation after they systematic reviewed 523 CNS hemangiopericytoma patients (Ghose et al. 2017). Kinslow et al. also supported that gross total resection plus radiotherapy might be optimal in the management CNS SFT/HPC tumors with borderline/malignant feature (Kinslow et al. 2018). However, some researchers reported that the addition of postoperative adjuvant radiation did not seem to confer a survival benefit (Rutkowski et al. 2012, 2010). Owing to the aggressive nature of SFT/HPC, we believe that further radiotherapy may need to be comprehensively evaluated and considered in the high-risk population identified by our nomogram model. Further prospective trials should be conducted to evaluate the effect the radiotherapy.
There are several limitations of our study that require attention. Firstly, due to its retrospective design, there may have been some bias in the selection of data. Prospective cohort studies may be needed to further strengthen the study of this type of tumor. Secondly, the SEER database did not contain some data, including tumor molecular bioinformation, detailed treatment information, and patient complications. Thirdly, we were unable to assess the progression-free survival of these patients due to the limitations of the SEER database. Lastly, our nomogram model needs further externally validation. In the future, it may be necessary to conduct multicenter studies for comprehensive collection of clinicopathological and therapeutic features, in order to determine related prognostic factors and analyze multiple outcome indicators.
Conclusion
In our study, the CS pattern of intracranial SFT/HPC patients was outlined, revealing a notable improvement in 5-year survival rates after an added period of survival. We also successfully developed the first novel CS-based nomogram model and a risk stratification system for SFT/HPC patients. This model with favorable predictive performance can deliver precise and timely prognostic data, facilitating individualized and economical post-treatment approach and crucial treatment suggestions for patients.
Data availability
Publicly available datasets were analyzed in this study. This data can be found here: https://seer.cancer.gov/data/.
References
Balachandran VP, Gonen M, Smith JJ, DeMatteo RP (2015) Nomograms in oncology: more than meets the eye. Lancet Oncol 16:e173-180
Berardi G, Morise Z, Sposito C, Igarashi K, Panetta V, Simonelli I, Kim S, Goh B, Kubo S, Tanaka S, Takeda Y, Ettorre G, Wilson G, Cimino M, Chan C, Torzilli G, Cheung T, Kaneko H, Mazzaferro V, Geller D, Han H, Kanazawa A, Wakabayashi G, Troisi R (2020) Development of a nomogram to predict outcome after liver resection for hepatocellular carcinoma in Child-Pugh B cirrhosis. J Hepatol 72:75–84
Boyett D, Kinslow C, Bruce S, Sonabend A, Rae A, McKhann G, Sisti M, Bruce J, Cheng S, Wang T (2019) Spinal location is prognostic of survival for solitary-fibrous tumor/hemangiopericytoma of the central nervous system. J Neurooncol 143:457–464
Combs S, Thilmann C, Debus J, Schulz-Ertner D (2005) Precision radiotherapy for hemangiopericytomas of the central nervous system. Cancer 104:2457–2465
El Sharouni M, Ahmed T, Varey A, Elias S, Witkamp A, Sigurdsson V, Suijkerbuijk K, van Diest P, Scolyer R, van Gils C, Thompson J, Blokx W, Lo S (2021) Development and validation of nomograms to predict local, regional, and distant recurrence in patients with thin (T1) melanomas. J Clin Oncol 39:1243–1252
Fritchie KJ, Jin L, Rubin BP, Burger PC, Jenkins SM, Barthelmeß S, Moskalev EA, Haller F, Oliveira AM, Giannini C (2016) NAB2-STAT6 gene fusion in meningeal hemangiopericytoma and solitary fibrous tumor. J Neuropathol Exp Neurol 75:263–271
Gafita A, Calais J, Grogan T, Hadaschik B, Wang H, Weber M, Sandhu S, Kratochwil C, Esfandiari R, Tauber R, Zeldin A, Rathke H, Armstrong W, Robertson A, Thin P, D’Alessandria C, Rettig M, Delpassand E, Haberkorn U, Elashoff D, Herrmann K, Czernin J, Hofman M, Fendler W, Eiber M (2021) Nomograms to predict outcomes after Lu-PSMA therapy in men with metastatic castration-resistant prostate cancer: an international, multicentre, retrospective study. Lancet Oncol 22:1115–1125
Ghia A, Allen P, Mahajan A, Penas-Prado M, McCutcheon I, Brown P (2013) Intracranial hemangiopericytoma and the role of radiation therapy: a population based analysis. Neurosurgery 72:203–209
Ghose A, Guha G, Kundu R, Tew J, Chaudhary R, Hemangiopericytoma CNS (2017) A systematic review of 523 patients. Am J Clin Oncol 40:223–227
Gou Q, Xie Y, Ai P (2022) Intracranial solitary fibrous tumor/hemangiopericytoma: role and choice of postoperative radiotherapy techniques. Front Oncol 12:994335
Harlan L, Hankey B (2003) The surveillance, epidemiology, and end-results program database as a resource for conducting descriptive epidemiologic and clinical studies. J Clin Oncol 21:2232–2233
Hieke S, Kleber M, König C, Engelhardt M, Schumacher M (2015) conditional survival: a useful concept to provide information on how prognosis evolves over time. Clin Cancer Res 21:1530–1536
Iasonos A, Schrag D, Raj GV, Panageas KS (2008) How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 26:1364–1370
Jung SH, Lee HY, Chow SC (2018) Statistical methods for conditional survival analysis. J Biopharm Stat 28:927–938
Kinslow CJ, Wang TJC (2020) Incidence of extrameningeal solitary fibrous tumors. Cancer 126:4067
Kinslow CJ, Bruce SS, Rae AI, Sheth SA, McKhann GM, Sisti MB, Bruce JN, Sonabend AM, Wang TJC (2018) Solitary-fibrous tumor/hemangiopericytoma of the central nervous system: a population-based study. J Neurooncol 138:173–182
Kinslow CJ, Rae AI, Kumar P, McKhann GM, Sisti MB, Bruce JN, Yu JB, Cheng SK, Wang TJC (2023) Risk stratification for management of solitary fibrous tumor/hemangiopericytoma of the central nervous system. Cancers. 15:876
Kinslow C, Rae A, Kumar P, McKhann G, Sisti M, Bruce J, Yu J, Cheng S, Wang T (2023) Risk stratification for management of solitary fibrous tumor/hemangiopericytoma of the central nervous system. Cancers 15:786
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820
Lu T, Xu H, Dong X, Jin Z, Wang Y (2022) Epidemiology and survival of patients with central nervous system solitary fibrous tumors: a population-based analysis. Front Oncol 12:977629
Luo J, Hu X, Ge X (2023) Conditional survival nomogram for monitoring real-time survival of young non-metastatic nasopharyngeal cancer survivors. J Cancer Res Clin Oncol 149:10181–10188
Mena H, Ribas J, Pezeshkpour G, Cowan D, Parisi J (1991) Hemangiopericytoma of the central nervous system: a review of 94 cases. Hum Pathol 22:84–91
Meng X, Hao F, Ju Z, Chang X, Guo Y (2022) Conditional survival nomogram predicting real-time prognosis of locally advanced breast cancer: analysis of population-based cohort with external validation. Front Public Health 10:953992
Meng X, Cai Y, Chang X, Guo Y (2023) A novel conditional survival nomogram for monitoring real-time prognosis of non-metastatic triple-negative breast cancer. Front Endocrinol 14:1119105
Prado C, Navarro-Martin A, Lucas A, Macià M, Guedea F (2012) Adyuvant fractionated radiotherapy after resection of intracranial hemangiopericytoma. Rep Pract Oncol Radiother 17:237–242
Qian H, Yang Z, Cai L, Chen H (2023) Conditional survival of elderly primary central nervous system lymphoma. J Cancer Res Clin Oncol. https://doi.org/10.1007/s00432-023-05200-4
Rutkowski MJ, Sughrue ME, Kane AJ, Aranda D, Mills SA, Barani IJ, Parsa AT (2010) Predictors of mortality following treatment of intracranial hemangiopericytoma. J Neurosurg 113:333–339
Rutkowski M, Jian B, Bloch O, Chen C, Sughrue M, Tihan T, Barani I, Berger M, McDermott M, Parsa A (2012) Intracranial hemangiopericytoma: clinical experience and treatment considerations in a modern series of 40 adult patients. Cancer 118:1628–1636
Schiariti M, Goetz P, El-Maghraby H, Tailor J, Kitchen N (2011) Hemangiopericytoma: long-term outcome revisited. Clinical article. J Neurosurg 114:747–755
Skuladottir H, Olsen J (2003) Conditional survival of patients with the four major histologic subgroups of lung cancer in Denmark. J Clin Oncol 21:3035–3040
Sonabend A, Zacharia B, Goldstein H, Bruce S, Hershman D, Neugut A, Bruce J (2014) The role for adjuvant radiotherapy in the treatment of hemangiopericytoma: a surveillance, epidemiology, and end results analysis. J Neurosurg 120:300–308
Stel V, Dekker F, Tripepi G, Zoccali C, Jager K (2011) Survival analysis I: the Kaplan–Meier method. Nephron Clin Pract 119:c83-88
Sung KS, Moon JH, Kim EH, Kang SG, Kim SH, Suh CO, Kim SH, Lee KS, Chang WS, Chang JH (2018) Solitary fibrous tumor/hemangiopericytoma: treatment results based on the 2016 WHO classification. J Neurosurg. https://doi.org/10.3171/2017.9.JNS171057
Swaminathan S, Ruzevick J, Venur V, Halasz L, Rockhill J, Gonzalez-Cuyar L, Cranmer L, Ferreira M (2022) Intracranial solitary fibrous tumor/hemangiopericytoma treated with microsurgical resection: retrospective cohort analysis of a single-center experience. Therap Clin Risk Manag. https://doi.org/10.2147/TCRM.S375064
Swaminathan S, Ruzevick J, Venur V, Halasz LM, Rockhill J, Gonzalez-Cuyar L, Cranmer LD, Ferreira M (2022) Intracranial solitary fibrous tumor/hemangiopericytoma treated with microsurgical resection: retrospective cohort analysis of a single-center experience. Ther Clin Risk Manag 18:901–912
Tibshirani R (1997) The lasso method for variable selection in the Cox model. Stat Med 16:385–395
Wu Y, Zhao T, Cai Y, Zheng M, Zhang Y, Qu Y, Cai Q (2023) Clinical outcomes of solitary fibrous tumors and hemangiopericytomas and risk factors related to recurrence and survival based on the 2021 WHO classification of central nervous system tumors. J Neurosurg 1:1–11
Zabor E, Gonen M, Chapman P, Panageas K (2013) Dynamic prognostication using conditional survival estimates. Cancer 119:3589–3592
Zeng L, Wang Y, Wang Y, Han L, Niu H, Zhang M, Ke C, Chen J, Lei T (2017) Analyses of prognosis-related factors of intracranial solitary fibrous tumors and hemangiopericytomas help understand the relationship between the two sorts of tumors. J Neurooncol 131:153–161
Zhang Z (2016) Variable selection with stepwise and best subset approaches. Ann Transl Med 4:136
Acknowledgements
The authors would like to thank the SEER database for the availability of the data.
Funding
None.
Author information
Authors and Affiliations
Contributions
DS and ZG designed the study; DS, ZY, LC, HH and ZG contributed to data analysis. DS wrote the initial draft of the manuscript; DS, ZY, LC, HH and ZG reviewed and edited the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Song, D., Yang, Z., Cai, L. et al. Conditional survival analysis and dynamic survival prediction for intracranial solitary-fibrous tumor/hemangiopericytoma. J Cancer Res Clin Oncol 150, 107 (2024). https://doi.org/10.1007/s00432-024-05629-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00432-024-05629-1