Abstract
Purpose
To compare the toxicity and clinical efficacy of TL (docetaxel + lobaplatin) induction chemotherapy combined with lobaplatin concurrent chemoradiotherapy and TPF (docetaxel + cisplatin + 5-fluorouracil) induction chemotherapy combined with cisplatin concurrent chemoradiotherapy in the treatment of locally advanced head and neck squamous cell carcinoma.
Methods and patients
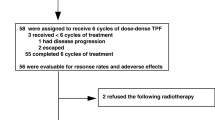
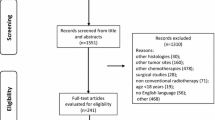
In total, 128 patients with locally advanced head and neck cancer were prospectively enrolled between August 2016 and April 2021. They were randomly divided into trial group and control group, all using chronological dosage mode. The trial group used TL regimen induction chemotherapy combined with lobaplatin concurrent chemoradiotherapy; the control group used TPF regimen induction chemotherapy and cisplatin concurrent chemotherapy. The endpoints were adverse events and survival rates at 1, 3 and 5 years.
Results
Median follow-up was 42 months (20–71 months). (1) Adverse events: During induction chemotherapy, compared with TPF group, grade 3–4 leukocytes and neutrophils, diarrhea, 1–2 hyperbilirubinemia, nausea / vomiting, oral mucositis, fatigue, anorexia, hyponatremia were significantly lower in TL group (p<0. 05): 6% vs. 35%, 14% vs. 53%, 0% vs. 6%, 15% vs. 40%, 9% vs. 56%, 0% vs. 10%, 3% vs. 13%, 2% vs. 23%, 15% vs. 74%. During chemoradiotherapy, the incidence of hyponatremia, hypokalaemia and grade 1–2 nausea was significantly lower in the TL group (p<0. 05), with 24% vs. 69%, 20% vs. 65% and 24% vs. 44%, respectively. However, more grade 3–4 thrombocytopenia were observed in the TL group (15% vs. 3%, p<0. 05). (2) There was no significant difference in the recent objective response rate (ORR) between patients with TL group and TPF group (p=0.961). (3) There was no statistical difference in 1, 3 and 5 years OS between TL group and TPF group, respectively, (71.0% vs. 67.5%, p=0.573), (56.6% vs. 56.9%, p=0.814), (52.5% vs. 52.9%, p=0.841); 1, 3 and 5 years PFS are: (63.4% vs. 64.0%, p=0.883), (51.1% vs. 54.0%, p=0.705) and (47.3% vs. 45.9%, p=0.887), None of them were significantly different. Multivariate analysis of COX regression showed that T stage (p=0.01) and surgery (p=0.046) were independent factors affecting PFS and OS, respectively. OS subgroup analysis shows that people receiving the TL regimen in postoperative and nodal stage N1 and N2 patients tended to survive longer than those receiving the TPF regimen.
Conclusion
Patients with postoperative, N1 or N2 stage locally advanced head and neck squamous cell carcinoma (HNSCC) may have more significant clinical benefits when treated with TL regimen. TL regimen has advantages in reducing toxic side effects and can be used as one of the first-line treatment options.
Trial registration
ClinicalTrials.gov (No. NCT03117257).


Similar content being viewed by others
Data availability
The datasets generated and/or analysed during the current study are available in https://figshare.com/, https://doi.org/10.6084/m9.figshare.19122641
Abbreviations
- LBP:
-
Lobaplatin
- CRT:
-
Chemoradiotherapy
- HNSCC:
-
Head and neck squamous cell carcinoma
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- IMRT:
-
Intensity-modulated radiotherapy
- PTV:
-
Planned target volume
References
Blanchard P, Bourhis J, Lacas B, Posner MR, Vermorken JB, Cruz Hernandez JJ et al (2013) Taxane-cisplatin-fluorouracil as induction chemotherapy in locally advanced head and neck cancers: an individual patient data meta-analysis of the meta-analysis of chemotherapy in head and neck cancer group. J Clin Oncol 31:2854–2860
Blanchard P, Landais C, Petit C et al (2016) Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 100 randomized trials and 19,248 patients, on behalf of MACH-NC group. Ann Oncol 27:1
Chen Y, Ismaila N, Chua M et al (2021) Chemotherapy in combination with radiotherapy for definitive-intent treatment of stage II-IVA nasopharyngeal carcinoma: CSCO and ASCO guideline[J].J Clin Oncol. https://doi.org/10.1200/JCO.20.03237
Cohen Ezra E.W., Karrison Theodore G, Kocherginsky Masha, Mueller Jeffrey, Egan Robyn, Huang Chao H, Brockstein Bruce E, Agulnik Mark B, Mittal Bharat B, Yunus Furhan, Samant Sandeep, Raez Luis E, Mehra Ranee, Kumar Priya, Ondrey Frank, Marchand Patrice, Braegas Bettina, Seiwert Tanguy Y, Villaflor Victoria M, Haraf Daniel J, Vokes Everett E (2014) Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol Off J Am Soc Clin Oncol 32(25):2735–43
Deng QQ, Huang XE, Ye LH, Lu YY, Liang Y, Xiang J (2013) Phase II trial of Loubo® (Lobaplatin) and pemetrexed for patients with metastatic breast cancer not responding to anthracycline or taxanes. Asian Pac J Cancer Prev 14:413–417
Hao T, Yesong G, Jiang M et al (2013) Radiosensitization of lobaplatin on human nasopharyngeal cancer cell line CNE2 in vitro. Chinese J Radiol Med Prot 33:602–606
Hitt R, Grau JJ, López-Pousa A, Berrocal A, García-Girón C, Irigoyen A et al (2014) A randomized phase III trial comparing induction chemotherapy followed by chemoradiotherapy versus chemoradiotherapy alone as treatment of unresectable head and neck cancer. Ann Oncol 25:216–225
Keil FelixHartl, Altorjai Maximilian et al (2021) Docetaxel cisplatin and 5-FU compared with docetaxel cisplatin and cetuximab as induction chemotherapy in advanced squamous cell carcinoma of the head and neck: Results of a randomised phase II AGMT trial. Eur J Cancer: Off J Eur Org Res Treat Cancer (EORTC) Eur Assoc Cancer Res (EACR) 151:1
Lu H, Daying M (2019) Clinical effect of docetaxel combined with lobaplatin in the treatment of cervical cancer. Chinese J Cancer Prev Treat 26:125–126
Lee N, Harris J, Garden AS, Straube W, Glisson B, Xia P, Bosch W, Morrison WH, Quivey J, Thorstad W, Jones C, Ang KK (2009) Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol. 27(22):3684–90
Lee AW, Tung SY, Chua DT, Ngan RK, Chappell R, Tung R et al (2010) Randomized trial of radiotherapy plus concurrent-adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst 102:1188–1198
Lee YG, Kang EJ, Keam B, Choi JH, Kim JS, Park KU et al (2020) Treatment strategy and outcomes in locally advanced head and neck squamous cell carcinoma: a nationwide retrospective cohort study (KCSG HN13-01). BMC Cancer 20:813
Ock CY, Keam B, Lim Y, Kim TM, Lee SH, Kwon SK et al (2016) Effect of induction chemotherapy on survival in locally advanced head and neck squamous cell carcinoma treated with concurrent chemoradiotherapy: Single center experience. Head Neck 38:277–284
Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC et al (2020) Colorectal cancer statistics, 2020. CA Cancer J Clin 70:145–164
Takácsi-Nagy Z, Hitre E, Remenár É, Oberna F, Polgár C, Major T et al (2015) Docetaxel, cisplatin and 5-fluorouracil induction chemotherapy followed by chemoradiotherapy or chemoradiotherapy alone in stage III-IV unresectable head and neck cancer: Results of a randomized phase II study. Strahlenther Onkol 191:635–641
Thanikachalam K, Krishnan J, Siddiqui F, Ali Haythem Y, Sheqwara J (2020) Carboplatin versus cetuximab chemoradiation in cisplatin ineligible locally advanced head and neck squamous cell carcinoma. J Clin Oncol 38:e18555–e18555
Trendowski MR, El Charif O, Dinh PC Jr, Travis LB, Dolan ME (2019) Genetic and Modifiable Risk Factors Contributing to Cisplatin-induced Toxicities. Clin Cancer Res 25:1147–1155
Winquist E, Agbassi C, Meyers BM, Yoo J, Chan KKW (2017) Systemic therapy in the curative treatment of head-and-neck squamous cell cancer: Cancer Care Ontario clinical practice guideline. Curr Oncol 24:e157–e162
Yanqun X, Weixiong X, Sun X et al (2013) Inhibitory effect of lobaplatin on proliferation of head and neck squamous cell carcinoma cell lines in vitro. J Int Oncol 40:479-480
Yong H, Shukui Q (2015) Experimental and clinical research current situation and progress of lobaplatin in the treatment of nonsmall cell lung cancer[J].Chinese Clin Oncol 20(10):937–941
Yu P, Jiang K et al (2014) Clinical progression of lobaplatin in combination chemotherapy for patients with recurrence or metastatic cancer. Chinese-German J Clin Oncol (English Edition) 000:386–391
Zhang S, Lin S, Hu L (2016) Lobaplatin combined with docetaxel neoadjuvant chemotherapy followed by concurrent lobaplatin with intensity-modulated radiotherapy increases the survival of patients with high-risk lymph node positive nasopharyngeal carcinoma. J Buon 21:161–167
Funding
This study was also supported by the Major Research Project of Innovation Groups of Guizhou Education Department (Qian Jiao He KY [2017]038), and the Basic Science and Technology Research Program of Guizhou Province (Qian Ke He Ji [2017] 1148).
Author information
Authors and Affiliations
Contributions
FJ and WW participated in the design and supervision of the study. YL, JL, XL, XG, XC, LL, HT and YC were responsible for the clinical work in the study, MZ, ZW were responsible for data sorting and analysis, and were the major contributors in writing the manuscript. WW was responsible for the review and revision of the first draft. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval and consent to participate
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study has been performed in accordance with the Declaration of Helsinki and has been approved by the Ethics Committee, the approval number is 2017-01, and all patients signed informed consent.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, M., Chen, Y., Wu, W. et al. A prospective phase II randomized study of docetaxel combined with lobaplatin versus TPF regimen induction chemotherapy followed by concurrent chemoradiotherapy for locally advanced head and neck squamous cell carcinoma. J Cancer Res Clin Oncol 149, 18081–18091 (2023). https://doi.org/10.1007/s00432-023-05497-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-05497-1