Abstract
Purpose
Concurrent chemoradiotherapy (CRT) is the standard treatment for advanced head and neck squamous cell carcinoma. In this phase II randomized study, the efficacy and toxicity of docetaxel, cisplatin and 5-fluorouracil induction chemotherapy (ICT) followed by concurrent CRT was compared with those after standard CRT alone in patients with locally advanced, unresectable head and neck cancer.
Patients and methods
Between January 2007 and June 2009, 66 patients with advanced (stage III or IV) unresectable squamous cell carcinoma of the head and neck (oral cavity, oropharynx, hypopharynx, and larynx) were randomly assigned to two groups: one receiving two cycles of docetaxel, cisplatin, and 5-fluorouracil ICT followed by CRT with three cycles of cisplatin and one treated by CRT alone. Response rate, local tumor control (LTC), locoregional tumor control (LRTC), overall survival (OS), progression-free survival (PFS), and toxicity results were assessed.
Results
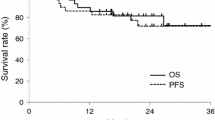
Three patients from the ICT + CRT group did not appear at the first treatment, so a total of 63 patients were evaluated in the study (30 ICT + CRT group and 33 CRT group). Three patients died of febrile neutropenia after ICT. The median follow-up time for surviving patients was 63 months (range 53–82 months). The rate of radiologic complete response was 63 % following ICT + CRT, whereas 70 % after CRT alone. There were no significant differences in the 3-year rates of LTC (56 vs. 57 %), LRTC (42 vs. 50 %), OS (43 vs. 55 %), and PFS (41 vs. 50 %) in the ICT + CRT group and in the CRT group, respectively. The rate of grade 3–4 neutropenia was significantly higher in the ICT + CRT group than in the CRT group (37 and 12 %; p = 0.024). Late toxicity (grade 2 or 3 xerostomia) developed in 59 and 42 % in the ICT + CRT and CRT groups, respectively.
Conclusion
The addition of ICT to CRT did not show any advantage in our phase II trial, while the incidence of adverse events increased. The three deaths as a consequence of ICT call attention to the importance of adequate patient selection if ICT is considered.
Zusammenfassung
Hintergrund
Simultane Chemoradiotherapie (CRT) ist eine Standardtherapie beim fortgeschrittenen Plattenepithelkarzinom im Kopf-Hals-Bereich. In dieser randomisierten Phase-II-Studie wurden die Wirksamkeit und Toxizität einer Induktionschemotherapie (ICT) mit Docetaxel, Cisplatin und 5-Fluorouracil gefolgt von simultaner CRT mit der CRT allein bei Patienten mit lokal fortgeschrittenen, irresektablen Kopf-Hals-Tumoren verglichen.
Patienten und Methoden
Zwischen Januar 2007 und Juni 2009 wurden 66 Patienten mit fortgeschrittenem (Stadium III oder IV), inoperablem Plattenepithelkarzinom im Kopf-Hals-Bereich (Mundhöhle, Oropharynx, Hypopharynx, Larynx) nach dem Zufallsprinzip in 2 Gruppen eingeteilt. Die eine Gruppe erhielt 2 Zyklen der Docetaxel-, Cisplatin- und 5-Fluorouracil-ICT gefolgt von CRT mit 3 Zyklen Cisplatin, die andere Gruppe erhielt nur CRT. Ansprechrate, lokale Tumorkontrolle (LTC), lokoregionale Tumorkontrolle (LRTC), Gesamtüberleben (OS), progressionsfreies Überleben (PFS) und toxischer Effekt wurden verglichen.
Ergebnisse
Drei Patienten der Gruppe mit ICT + CRT erschienen bei der ersten Behandlung nicht, so dass insgesamt 63 Patienten in der Studie ausgewertet wurden (30 in der Gruppe ICT + CRT, 33 in der CRT-Gruppe). Drei Patienten starben an febriler Neutropenie nach ICT. Die mediane Nachbeobachtungszeit der überlebenden Patienten betrug 63 Monate (Spanne 53–82 Monate). Die Rate des radiologischen vollständigen Ansprechens war 63 % nach ICT + CRT vs. 70 % nach CRT allein. Es gab keinen signifikanten Unterschied in der 3-Jahres-Rate bei LTC (56 vs. 57 %), LRTC (42 vs. 50 %), OS (43 vs. 55 %) und PFS (41 vs. 50 %) zwischen der Gruppe mit ICT + CRT und den mit CRT behandelten Patienten. Die Rate von Neutropenie mit einem Grad 3–4 lag in der Gruppe mit ICT + CRT deutlich höher als in der CRT-Gruppe (37 und 12 %; p = 0,024). Späte Toxizität (Grad-2- und Grad-3-Xerostomie) ereignete sich in der Gruppe mit ICT + CRT und in der CRT-Gruppe in jeweils 59 vs. 42 %.
Schlussfolgerung
Die Kombination von ICT und CRT erbrachte in unserer Phase-II-Studie keine Vorteile, wobei die Gesamtinzidenz der unerwünschten Ereignisse stieg. Die 3 Todesfälle infolge ICT weisen auf die Wichtigkeit der Patientenauswahl im Falle einer ICT-Behandlung hin.


Similar content being viewed by others
References
Pignon JP, le Maître A, Maillard E et al; MACH-NC Collaborative Group (2009) Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 92:4–14
Argiris A (2013) Current status and future directions in induction chemotherapy for head and neck cancer. Crit Rev Oncol Hematol 88:57–74
Pointreau Y, Garaud P, Chapet S et al (2009) Randomized trial of induction chemotherapy with cisplatin and 5-fluorouracil with or without docetaxel for larynx preservation. J Natl Cancer Inst 101:498–506
Posner MR, Hershock DM, Blajman CR et al; TAX 324 Study Group (2007) Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med 357:1705–1715
Blanchard P, Bourhis J, Lacas B et al (2013) Meta-Analysis of Chemotherapy in Head and Neck Cancer, Induction Project, Collaborative Group. Taxane–cisplatin–fluorouracil as induction chemotherapy in locally advanced head and neck cancers: an individual patient data meta-analysis of the meta-analysis of chemotherapy in head and neck cancer group. J Clin Oncol 31:2854–2860
Balermpas P, Bauer C, Fraunholz I et al (2014) Concomitant chemoradiotherapy versus induction chemotherapy followed by chemoradiotherapy as definitive, first line treatment of squamous cell carcinoma of the head and neck: a retrospective single center analysis. Strahlenther Onkol 190:256–262
Paccagnella A, Ghi MG, Loreggian L et al; Gruppo di Studio Tumori della Testa e del Collo XRP 6976 F/2501 Study (2010) Concomitant chemoradiotherapy versus induction docetaxel, cisplatin and 5 fluorouracil (TPF) followed by concomitant chemoradiotherapy in locally advanced head and neck cancer: a phase II randomized study. Ann Oncol 21:1515–1522
Cohen EEW, Karrison T, Kocherginsky M et al (2012) DeCIDE: a phase III randomized trial of docetaxel (D), cisplatin (P), 5-fluorouracil (F) (TPF) induction chemotherapy (IC) in patients with N2/N3 locally advanced squamous cell carcinoma of the head and neck (SCCHN). J Clin Oncol 30(Suppl. abstr):5500
Haddad R, O’Neill A, Rabinowits G et al (2013) Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol 14:257–264
Hitt R, Grau JJ, López-Pousa A et al (2014) Spanish Head and Neck Cancer Cooperative Group (TTCC). A randomized phase III trial comparing induction chemotherapy followed by chemoradiotherapy versus chemoradiotherapy alone as treatment of unresectable head and neck cancer. Ann Oncol 25:216–225
Wiggenraad R, Mast M, van Santvoort J et al (2005) ConPas: a 3-D conformal parotid gland-sparing irradiation technique for bilateral neck treatment as an alternative to IMRT. Strahlenther Onkol 18:673–682
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors: European Organisation for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Adelstein DJ, Li Y, Adams GL et al (2003) An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 21:92–98
Forastiere AA, Goepfert H, Maor M et al (2003) Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 349:2091–2098
Ang KK, Harris J, Garden AS et al (2005) Concomitant boost radiation plus concurrent cisplatin for advanced head and neck carcinomas: radiation therapy oncology group phase II trial 99–14. J Clin Oncol 23:3008–3015
Brömme JO, Schmücking M, Arnold A et al (2013) Taxane-containing induction chemotherapy followed by definitive chemoradiotherapy. Outcome in patients with locally advanced head and neck cancer. Strahlenther Onkol 189:618–624
Greene FL, Page DL, Fleming ID et al (2002) AJCC Cancer Staging Handbook. TNM Classification of Malignant Tumors. Springer-Verlag, New York, pp 27–60
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Z. Takácsi-Nagy, E. Hitre, É. Remenár, F. Oberna, C. Polgár, T. Major, M. Gödény, J. Fodor, and M. Kásler state that there are no conflict of interest.
All studies on humans described in the present manuscript were carried out with the approval of the responsible ethics committee and in accordance with national law and the Helsinki Declaration of 1975 (in its current, revised form). Informed consent was obtained from all patients included in studies.
Rights and permissions
About this article
Cite this article
Takácsi-Nagy, Z., Hitre, E., Remenár, É. et al. Docetaxel, cisplatin and 5-fluorouracil induction chemotherapy followed by chemoradiotherapy or chemoradiotherapy alone in stage III–IV unresectable head and neck cancer. Strahlenther Onkol 191, 635–641 (2015). https://doi.org/10.1007/s00066-015-0829-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-015-0829-z