Abstract
Background
Lymph node metastasis (LNM) is a critical prognostic factor in resectable pancreatic cancer (PC) patients, determining treatment strategies. This study aimed to develop a clinical model to adequately and accurately predict the risk of LNM in PC patients.
Methods
13,200 resectable PC patients were enrolled from the SEER (Surveillance, Epidemiology, and End Results) database, and randomly divided into a training group and an internal validation group at a ratio of 7:3. An independent group (n = 62) obtained from The First Affiliated Hospital of Xinxiang Medical University was enrolled as the external validation group. The univariate and multivariate logistic regression analyses were used to screen independent risk factors for LNM. The minimum Akaike’s information criterion (AIC) was performed to select the optimal model parameters and construct a nomogram for assessing the risk of LNM. The performance of the nomogram was assessed by the receiver operating characteristics (ROC) curve, calibration plot, and decision curve analysis (DCA). In addition, an online web calculator was designed to assess the risk of LNM.
Result
A total of six risk predictors (including age at diagnosis, race, primary site, grade, histology, and T-stage) were identified and included in the nomogram. The areas under the curves (AUCs) [95% confidential interval (CI)] were 0.711 (95%CI: 0.700–0.722), 0.700 (95%CI: 0.683–0.717), and 0.845 (95%CI: 0.749–0.942) in the training, internal validation and external validation groups, respectively. The calibration curves showed satisfied consistency between nomogram-predicted LNM and actual observed LNM. The concordance indexes (C-indexes) in the training, internal, and external validation sets were 0.689, 0.686, and 0.752, respectively. The DCA curves of the nomogram demonstrated good clinical utility.
Conclusion
We constructed a nomogram model for predicting LNM in pancreatic cancer patients, which may help oncologists and surgeons to choose more individualized clinical treatment strategies and make better clinical decisions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
PC is the most aggressive and lethal malignancy in gastrointestinal cancers. The overall 5-year survival rate is less than 10%, with few significant improvements for years (Ansari et al. 2016; Siegel et al. 2022). The primary treatment for PC including surgery, neoadjuvant therapy, and postoperative therapy, surgical resection is considered to be the only potentially curative treatment among those treatments (Stott et al. 2022). However, most PC patients underwent surgical resection with inadequate number and extent of lymph node dissection (Groot et al. 2017; Kovac et al. 2019). Mostly, it is difficult to get R0 excision and patients diagnosed with PC usually experience early local recurrence and metastasis after surgery (Suto et al. 2022; Torphy et al. 2020). Besides, it is insufficient to evaluate the preoperative LNM on the imaging appearance solely. Therefore, the evaluation of preoperative LNM is an important prognostic determinant factor for resectable PC, which determined the surgical resection type and the implementation of preoperative neoadjuvant therapy and aggressive postoperative adjuvant therapy (Shi et al. 2019; Suto et al. 2022).
The nomogram model has been widely used in the prediction of lymph node metastasis. However, there is a lack of nomograms for predicting LNM in resectable PC patients preoperatively. In this study, the clinical characteristics of cases diagnosed with PC were analyzed, and a nomogram for predicting LNM was developed, which contributes to providing personalized guidance for resectable PC patients.
Materials and methods
Data collection
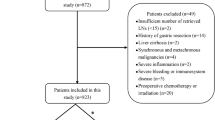
In our study, patients diagnosed with pancreatic cancer from 2000 to 2019 were collected from the SEER database. The exclusion criteria were as follows: diagnosed confirmation with clinical diagnosis only, radiography without microscopic confirm, direct visualization without microscopic confirmation or unknown; more than 2 primaries; SEER cause-specific death unknown; survival months equal to zero or unknown; grade unknown; stage or T, N, M Stage unknown; surgery unknown; tumor size unknown; regional nodes examined or positive unknown; age < 18 years old; race unknown; confirmed distant metastasis during surgery (stage M1); unresectable pancreatic cancer; death within one month after surgery. The following clinicopathological variables of gender, age at diagnosis, race, grade, primary site, histology, T-stage, and lymph node status were collected. The screening flowchart is shown in Fig. 1.
A total of 62 patients diagnosed with PC from December 2018 to February 2022 in The First Affiliated Hospital of Xinxiang Medical University were used to further validate the constructed nomogram externally. The inclusion and exclusion criteria were the same as the training set. The time of the last follow-up was March 2023. This study was approved by the institutional review board of our hospital.
Statistical analysis
The median (IQR), frequency (proportions), Mann–Whitney U tests, independent t-tests, Pearson’s chi-square test, Fisher’s exact test, and univariate and multivariate binary logistic regression analysis were calculated by SPSS (SPSS Inc., Chicago, USA). Nomograms, ROC curves, calibration plots, the nutrition risk index (NRI), integrated discrimination improvement (IDI), DCA curves, and Kaplan–Meier plots, were conducted by R software (version 4.2.2). P < 0.05 was considered statistically significant.
Construction, validation, and clinical usefulness of the nomogram
Univariate and multivariate logistic regression analyses were utilized to find the independent factors in predicting LNM and the minimum Akaike’s information criterion (AIC) was performed to choose the optimal model parameters and construct a nomogram for evaluating the risk of LNM. The predictors include age at diagnosis, race, primary site, grade, histology, and T-stage. Nomogram was constructed based on these variables (a dynamic nomogram was also provided in our study). The accuracy and discrimination of the nomogram were assessed by the ROC curve and the C-index. The calibration curves were utilized to evaluate the consistency between the actual outcomes and the predicted probabilities. The NRI and IDI were calculated to compare the performance between the nomogram and the clinical predictors. Additionally, the clinical utility in decision-making was assessed by DCA.
Results
Patient characteristics
A total of 13,200 resectable pancreatic cancer patients were enrolled in our research between 2000 and 2019 according to the screening flowchart from the SEER database and randomly divided into a training group (n = 9279) and internal validation group (n = 3921) at a ratio of 7:3 (Fig. 1). Meanwhile, 62 patients who underwent surgical resection with PC were obtained from the First Affiliated Hospital of Xinxiang Medical University and applied as the external validation group. The detailed clinicopathological features of all patients are presented in Table 1. There was no significant difference in the three groups except the race (P < 0.001) (Table 1).
Univariate and multivariate logistic regression results
The clinicopathological factors associated with LNM were revealed by the univariate and multivariate logistic regression analysis. Univariate logistic regression analysis showed that age at diagnosis, race, primary site, grade, histology, and T-stage were significant factors for LNM in PC patients (Table 2). Consequently, we figured out independent factors by multivariate logistic regression analysis, including race [Asian: odds ratio (OR) = 0.807 (95%CI = 0.686–0.949), P = 0.009], primary site [Body of pancreas: OR = 0.479 (95%CI = 0.404–0.568), P < 0.001], grade [G3: OR = 1.904 (95%CI = 1.642–2.208), P < 0.001], histology [Neuroendocrine carcinoma: OR = 5.465 (95%CI = 4.586–6.513), P < 0.001], and T-stage [T4: OR = 4.892 (95%CI = 3.694–6.408), P < 0.001] (Table 2).
Construction and validation of the nomogram based on predictors of lymph nodes metastasis
The minimum Akaike’s information criterion (AIC) was used to select the optimal model parameters and construct a nomogram for assessing the risk of LNM (Arunajadai 2009; Coles et al. 1980; Wang et al. 2004; Zhang 2016), and a total of six predictors including age at diagnosis, race, primary site, grade, histology, and T-stage were integrated to construct the nomogram (Fig. 2). The AUC was 0.711 (95%CI: 0.700–0.722) in the training, 0.700 (95%CI: 0.683–0.717) in the internal validation group, and 0.845 (95%CI: 0.749–0.942) in the external validation group, which proved a superior performance than the single factor (Fig. 3). The AUC of the T-stage and grade alone were lower than that of the nomogram. The AUC for T-stage was 0.645 (95%CI: 0.635–0.656), 0.649 (95%CI: 0.634–0.665), and 0.704 (95%CI: 0.587–0.821) in the training set, internal validation set and external validation set. Moreover, the AUC for the grade was 0.619 (95% CI: 0.608–0.630), 0.615 (95%CI: 0.598–0.632), and 0.601 (95%CI: 0.472–0.729) in the training, internal validation, and external validation groups, separately. Furthermore, the calibration plots show good consistency in the training set (C-index: 0.689), internal validation set (C-index: 0.686), and external validation set (C-index: 0.752) (Fig. 4). We also designed an online web calculator: https://xxlchxjh.shinyapps.io/DynNomappforLNMinpancreaticcancer/.
The clinical application value was determined by DCA which calculates the net benefits at different risk threshold probabilities. The net benefit of the nomogram was the largest in comparison to the grade and T-stage, which indicated the nomogram was a reliable clinical tool for predicting LNM in PC patients who underwent surgical resection (Fig. 5).
Additionally, the accuracy of the nomogram compared with the T-stage was demonstrated by the NRI and IDI. The NRI was 0.370 (95%CI: 0.329–0.411) and the IDI was 0.044 (95%CI: 0.039–0.048, P < 0.001) in the training group. The NRI and IDI in the internal validation group were 0.274 (95%CI: 0.211–0.337) was 0.035 (95%CI: 0.029–0.041, P < 0.001). In the external group, the NRI and IDI were 0.577 (95%CI: 0.091–1.063) was 0.062 (95%CI: 0.004–0.120, P = 0.037). The accuracy for predicting LNM by the nomogram was greater than the T-stage.
The Kaplan–Meier overall survival curves of training and internal/external validation groups are plotted in Fig. 6. The prognosis of PC patients with positive LNM was significantly lower in both training and internal/external validation groups. (P < 0.01).
Discussion
PC is one of the most lethal of all cancers with high mortality, which is the seventh leading cause of cancer death worldwide (Sung et al. 2021). Even after surgical resection, early recurrence rates were reported to be 50% to 60%, with 5-year survival rates of only 20% to 30% (Gupta et al. 2017; Shin et al. 2018). PC patients with positive LNM have a worse prognosis with or without surgical resection. The status of LNM is a significant prognostic factor in PC patients, which is also important for the choice of treatment decisions. PC patients with positive lymph node metastasis should accept neoadjuvant chemotherapy or immunotherapy before surgical resection (Barrak et al. 2022; Kanda et al. 2011; Roland et al. 2015). Therefore, it is important to distinguish the status of lymph nodes before surgical resection in the clinic. At present, there are low sensitivities and specificities in evaluating lymph node metastasis by imageological examinations, and it is difficult to identify the LNM before surgical resection. Therefore, it is important to construct a sensitive and efficient prediction model for assessing the status of LNM preoperatively in PC patients.
In our study, a total of six clinicopathological factors were considered as risk factors associated with LNM in PC patients, including age at diagnosis, grade, histology, T-stage, primary site, and race, which was largely consistent with previous analyses (Huang et al. 2023; Song et al. 2018). The convenient preoperative nomogram prediction model was constructed by those independent predictors. This is the first research to construct and validate a nomogram for predicting LNM in resectable PC patients based on large populations. Previously, researchers pay more attention to the status of lymph node metastasis in pancreatic head cancer. Xingren Guo et al. developed a nomogram for predicting the lymphatic metastasis in pancreatic head cancer based on 191 pancreatic head cancer patients who received laparoscopic pancreaticoduodenectomy (Guo et al. 2023). Yi-Nan Shen et al. constructs a nomogram for predicting the peripancreatic vein invasion in pancreatic head cancer patients. Additionally, the other tumor sites of PC such as the body and tail of the pancreas also occur lymphatic metastasis (Shi et al. 2022; Tanaka et al. 2022, 2020), and a model for predicting the status of LNM in those tumor sites of PC is in need. The nomogram model constructed in our study could satisfy this requirement. In our study, it is obvious that PC patients with the tumor site in the head have more potential LNM compared with the tail and body of the pancreas, which was consistent with previous studies and clinical practice (Guo et al. 2023; Kobayashi et al. 2022).
Various studies demonstrated that race was related to lymph node metastasis and prognosis (Oweira et al. 2017; Zheng-Pywell et al. 2022). Rui Zheng-Pywell et al. reveals that black patients had a higher risk of LNM in tumors less than 2 cm in size compared with white patients (Zheng-Pywell et al. 2022). In our study, Asian PC patients such as Chinese, Japanese, and Korean were less likely to undergo LNM. Moreover, a higher positive rate of LNM was observed in black PC patients, which is consistent with the previous conclusion.
The correlation between grade and LNM in PC patients has been revealed in previous studies widely. Harimoto Norifumi et al. shows that lymph node metastasis was significantly associated with higher tumor grade in pancreatic neuroendocrine neoplasm (Harimoto et al. 2019). Similarly, our study found that grade was an independent risk factor associated with LNM in PC patients. LNM is more likely to occur in poorly differentiated or undifferentiated PC patients.
The histological type is commonly considered an important predictor of the prognosis in PC patients. Bi-Yang Cao et al. found that adenocarcinoma was the independently associated risk factor for poor prognosis in patients with liver metastasis in PC patients (Cao et al. 2023). Until now, there were few studies focused on the association between histological type and risk of LNM. In this study, there is a higher risk of LNM in PC patients with infiltrating duct carcinoma, while, PC patients with the histological type of neuroendocrine carcinoma have less LNM. Furthermore, the T-stage was a significant prognostic factor in PC, including the tumor size and infiltrating scope. In 2022, Xi-Tai Huang et al. showed that the T-stage was significantly associated with LNM in pancreatic neuroendocrine tumors (Huang et al. 2022). In this study, PC patients with T4 indicate more potential risk of LNM in comparison with T1 or T2.
The nomogram for evaluating the risk of LNM in PC patients was developed by easily available clinicopathological factors, including age at diagnosis, race, grade, histology, T-stage, and tumor location. The AUC and the calibration curves demonstrated excellent discrimination and consistency of this nomogram model. The risk of LNM in PC patients could be conveniently and accurately calculated by those accessible variables. Furthermore, DCA curves were utilized to estimate the clinical utility, which shows good net benefit. In summary, the risk of LNM in preoperative PC patients can be easily and accurately predicted by the newly established nomogram model.
Although the nomogram model had good accuracy for predicting the risk of LNM in PC patients, there are several limitations to this study. First of all, the selection bias could not be avoided due to the nature of retrospective analyses. For example, patients with missing data were excluded from our study, which may cause selection bias. Secondly, variables such as age, tumor size, leucocyte, albumin, and lymphocytes/monocytes have been identified as independent predictors of LNM in pancreatic head cancer (Guo et al. 2023). The serum CA 19–9, PC.ae.C42_5, and PC.aa.C38_4 were considered the powerful preoperative clinical variables in predicting the early recurrence of pancreatic cancer (Rho et al. 2019). However, those variables were not supplied in the SEER database. Therefore, those important variables cannot be incorporated into the nomogram model. Finally, the external validation data from our hospital are very little, which may lead to underfitting the model and more external validations are needed.
Conclusion
In summary, the nomogram for predicting the preoperative LNM in PC patients was developed based on the SEER database, which shows good performance and clinical application.
Data availability
The data of this study are available for all authors.
Abbreviations
- PC:
-
Pancreatic cancer
- LNM:
-
Lymph node metastasis
- SEER:
-
Surveillance, epidemiology, and end results
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the curve
- DCA:
-
Decision curve analysis
- CI:
-
Confidential interval
- OR:
-
Odds ratios
- IQR:
-
Inter-quartile range
- NRI:
-
The nutrition risk index
- IDI:
-
Integrated discrimination improvement
References
Ansari D, Tingstedt B, Andersson B, Holmquist F, Sturesson C, Williamsson C, Sasor A, Borg D, Bauden M, Andersson R (2016) Pancreatic cancer: yesterday, today and tomorrow. Future Oncol 12:1929–1946
Arunajadai SG (2009) Stepwise logistic regression. Anesth Analg 109:285–286
Barrak D, Villano AM, Moslim MA, Hopkins SE, Lefton MD, Ruth K, Reddy SS (2022) Total neoadjuvant treatment for pancreatic ductal adenocarcinoma is associated with limited lymph node yield but improved ratio. J Surg Res 280:543–550
Cao BY, Tong F, Zhang LT, Kang YX, Wu CC, Wang QQ, Yang W, Wang J (2023) Risk factors, prognostic predictors, and nomograms for pancreatic cancer patients with initially diagnosed synchronous liver metastasis. World J Gastrointest Oncol 15:128–142
Coles LS, Brown BW, Engelhard C, Halpern J, Fries JF (1980) Determining the most valuable clinical variables: a stepwise multiple logistic regression program. Methods Inf Med 19:42–49
Groot VP, van Santvoort HC, Rombouts SJ, Hagendoorn J, Borel Rinkes IH, van Vulpen M, Herman JM, Wolfgang CL, Besselink MG, Molenaar IQ (2017) Systematic review on the treatment of isolated local recurrence of pancreatic cancer after surgery; re-resection, chemoradiotherapy and SBRT. HPB (oxford) 19:83–92
Guo X, Song X, Long X, Liu Y, Xie Y, Xie C, Ji B (2023) New nomogram for predicting lymph node positivity in pancreatic head cancer. Front Oncol 13:1053375
Gupta R, Amanam I, Chung V (2017) Current and future therapies for advanced pancreatic cancer. J Surg Oncol 116:25–34
Harimoto N, Hoshino K, Muranushi R, Hagiwara K, Yamanaka T, Ishii N, Tsukagoshi M, Igarashi T, Tanaka H, Watanabe A et al (2019) Significance of lymph node metastasis in resectable well-differentiated pancreatic neuroendocrine tumor. Pancreas 48:943–947
Huang XT, Xie JZ, Huang CS, Li JH, Chen W, Liang LJ, Yin XY (2022) Development and validation of nomogram to predict lymph node metastasis preoperatively in patients with pancreatic neuroendocrine tumor. HPB (oxford) 24:2112–2118
Huang J, Li X, Jiang Q, Qiu H, Rong Y, Cui B, Guo G (2023) Analysis of risk factors for distant metastasis of pancreatic ductal adenocarcinoma without regional lymph node metastasis and a nomogram prediction model for survival. Evid Based Complement Alternat Med 2023:2916974
Kanda M, Fujii T, Nagai S, Kodera Y, Kanzaki A, Sahin TT, Hayashi M, Yamada S, Sugimoto H, Nomoto S et al (2011) Pattern of lymph node metastasis spread in pancreatic cancer. Pancreas 40:951–955
Kobayashi K, Ono Y, Sato S, Kato T, Oba A, Sato T, Ito H, Inoue Y, Takamatsu M, Saiura A, Takahashi Y (2022) Evaluation of local recurrence after pancreaticoduodenectomy for borderline resectable pancreatic head cancer with neoadjuvant chemotherapy: can the resection level change after chemotherapy? Surgery 173(5):1220
Kovac JD, Mayer P, Hackert T, Klauss M (2019) The time to and type of pancreatic cancer recurrence after surgical resection: is prediction possible? Acad Radiol 26:775–781
Oweira H, Petrausch U, Helbling D, Schmidt J, Mannhart M, Mehrabi A, Schob O, Giryes A, Decker M, Abdel-Rahman O (2017) Prognostic value of site-specific metastases in pancreatic adenocarcinoma: a surveillance epidemiology and end results database analysis. World J Gastroenterol 23:1872–1880
Rho SY, Lee SG, Park M, Lee J, Lee SH, Hwang HK, Lee MJ, Paik YK, Lee WJ, Kang CM (2019) Developing a preoperative serum metabolome-based recurrence-predicting nomogram for patients with resected pancreatic ductal adenocarcinoma. Sci Rep 9:18634
Roland CL, Yang AD, Katz MH, Chatterjee D, Wang H, Lin H, Vauthey JN, Pisters PW, Varadhachary GR, Wolff RA et al (2015) Neoadjuvant therapy is associated with a reduced lymph node ratio in patients with potentially resectable pancreatic cancer. Ann Surg Oncol 22:1168–1175
Shi W, Jiang R, Liang F, Yu G, Long J, Zhao J (2019) Definitive chemoradiotherapy and salvage chemotherapy for patients with isolated locoregional recurrence after radical resection of primary pancreatic cancer. Cancer Manag Res 11:5065–5073
Shi H, Chen Z, Dong S, He R, Du Y, Qin Z, Zhou W (2022) A nomogram for predicting survival in patients with advanced (stage III/IV) pancreatic body tail cancer: a SEER-based study. BMC Gastroenterol 22:279
Shin SH, Kim SC, Song KB, Hwang DW, Lee JH, Park KM, Lee YJ (2018) Chronologic changes in clinical and survival features of pancreatic ductal adenocarcinoma since 2000: a single-center experience with 2,029 patients. Surgery 164:432–442
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics, 2022. CA Cancer J Clin 72:7–33
Song W, Miao DL, Chen L (2018) Nomogram for predicting survival in patients with pancreatic cancer. Onco Targets Ther 11:539–545
Stott MC, Oldfield L, Hale J, Costello E, Halloran CM (2022) Recent advances in understanding pancreatic cancer. Fac Rev 11:9
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249
Suto H, Okano K, Oshima M, Ando Y, Matsukawa H, Takahashi S, Shibata T, Kamada H, Masaki T, Suzuki Y (2022) Prediction of local tumor control and recurrence-free survival in patients with pancreatic cancer undergoing curative resection after neoadjuvant chemoradiotherapy. J Surg Oncol 126:292–301
Tanaka K, Nakamura T, Asano T, Nakanishi Y, Noji T, Tsuchikawa T, Okamura K, Shichinohe T, Hirano S (2020) Pancreatic body and tail cancer and favorable metastatic lymph node behavior on the left edge of the aorta. Pancreatology 20:1451–1457
Tanaka K, Kimura Y, Hayashi T, Ambo Y, Yoshida M, Umemoto K, Murakami T, Asano T, Nakamura T, Hirano S (2022) Appropriate lymph node dissection sites for cancer in the body and tail of the pancreas: a multicenter retrospective study. Cancers (basel) 14(18):4409
Torphy RJ, Fujiwara Y, Schulick RD (2020) Pancreatic cancer treatment: better, but a long way to go. Surg Today 50:1117–1125
Wang D, Zhang W, Bakhai A (2004) Comparison of Bayesian model averaging and stepwise methods for model selection in logistic regression. Stat Med 23:3451–3467
Zhang Z (2016) Variable selection with stepwise and best subset approaches. Ann Transl Med 4:136
Zheng-Pywell R, Lopez-Aguiar A, Fields RC, Vickers S, Yates C, Dudeja V, Chen H, Reddy S, Maithel SK, Rose JB (2022) Are we undertreating black patients with nonfunctional pancreatic neuroendocrine tumors? critical analysis of current surveillance guidelines by race. J Am Coll Surg 234:599–606
Acknowledgements
All the researchers and staff of the SEER program should be highly appreciated.
Funding
The article received support from the fund from the Joint Project of Henan Province and Ministry (LHGJ20200516, and LHGJ20200500).
Author information
Authors and Affiliations
Contributions
HC, XX, XK, and QZ designed this research and revised the manuscript. HC and JX collected the data. Statistical analysis was conducted by HC and XX. Figures and tables were generated by HC, XX, and JX. HC wrote the original manuscript. XX, XK, and QZ supervised this study. All authors contributed to this article and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Ethical approval
Data from our hospital have been approved by the Ethics Committee of the First Affiliated Hospital of Xinxiang Medical University. While, data from the SEER database were in no need of informed consent and medical ethics review.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cheng, H., Xu, JH., Kang, XH. et al. Nomogram for predicting the preoperative lymph node metastasis in resectable pancreatic cancer. J Cancer Res Clin Oncol 149, 12469–12477 (2023). https://doi.org/10.1007/s00432-023-05048-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-05048-8