Abstract
Purpose
This study compared short- and long-term outcomes of robotic-assisted thoracoscopic surgery (RATS) versus video-assisted thoracoscopic surgery (VATS) for lobectomy in young adults aged ≤ 35 years with non-small cell lung cancer (NSCLC), aiming to assess the superiority of RATS over VATS for this special group of patients.
Methods
A total of 1355 consecutive NSCLC cases aged 18–35 years undergoing RATS (n = 105) or VATS (n = 1250) between 2014 and 2021 were retrospectively identified from a prospectively maintained database. Propensity score matching (PSM) was applied to establish a 1:3 RATS versus VATS ratio. Baseline clinicopathological characteristics, perioperative outcomes, lymph node (LN) assessment, and long-term survival were investigated.
Results
Following PSM, 105 and 315 cases were in the RATS and VATS groups, respectively. RATS led to a shorter postoperative hospital stay than VATS (4.0 ± 1.5 vs 4.3 ± 1.7 days, p = 0.02). The two groups were comparable in other perioperative outcomes and postoperative complications (all p > 0.05). Moreover, RATS assessed more LNs (9.4 ± 4.4 vs 8.3 ± 3.6, p = 0.03), especially N1 LNs (4.2 ± 3.1 vs 3.5 ± 2.2, p = 0.02), than VATS. By comparison, no difference in 5-year recurrence-free survival (RFS), overall survival (OS), or recurrence or mortality patterns was found between the two groups (all p > 0.05). Further subgroup analyses also observed similar long-term outcomes between the two groups regarding age, gender, and smoking history. Finally, Cox’s analyses found that the surgical approach was not independently correlated with RFS or OS.
Conclusion
RATS shortened postoperative hospital stay, assessed more N1 and total LNs, and achieved comparable long-term outcomes to VATS for very young NSCLC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-small cell lung cancer (NSCLC) is one of the most common malignancies and has become a major public health problem in the world (Siegel et al. 2023). NSCLC typically affects elderly individuals with a median age at the first diagnosis of approximately 70 years, and younger adults are uncommon to suffer from primary NSCLC (Garrana et al. 2021). Nevertheless, with the increased implementation of thin-section thoracic computed tomography (CT) and advances in diagnostic modalities which effectively contribute to assessing early-stage disease, a growing number of young adults have been diagnosed with NSCLC in recent years, with the most prevalent histology being adenocarcinoma and the preponderance of female (Thai et al. 2021; Galvez-Nino et al. 2020; Sacher et al. 2016). Therefore, the optimal surgical approach is of critical importance for young patients, especially very young adults aged ≤ 35 years who may be associated with high reproduction and financial concerns, to achieve better oncological outcomes, emotional and psychological conditions, and quality of life (Galvez-Nino et al. 2020; Subramanian et al. 2010; Landwehr et al. 2016).
Although traditional thoracotomy is still the standard approach in treating NSCLC, minimally invasive surgery (MIS) technics have been widely applied in recent years. Video-assisted thoracoscopic surgery (VATS) is the most prevalent adopted MIS approach which reduces operation-related complications, accelerates postoperative recoveries, and leads to better life qualities compared with thoracotomy for early-stage NSCLC patients (Bendixen et al. 2016). Since its first performance in 2002, robotic-assisted thoracoscopic surgery (RATS) has attracted the growing interest of thoracic surgeons and is becoming increasingly prevalent in treating NSCLC as an alternative to thoracotomy and VATS (Huang et al. 2021). The robotic-assisted surgical system provides a high-definition, three-dimensional (3D), and 10–15 times magnified operation field and highly flexible robotic arms with an increased degree of motion and rotational freedom, allowing operators to perform the surgery more conveniently and accurately (Jin et al. 2022). Previous studies have compared the feasibility and oncological efficacy of RATS versus VATS for NSCLC, suggesting that RATS assessed more lymph nodes (LNs), reduced operation-related pain, led to a higher postoperative quality of life, and achieved similar long-term outcomes (Veronesi et al. 2016; Kneuertz et al. 2019). Therefore, RATS might be especially suitable for very young patients pursuing a quick postoperative recovery and high quality of life. However, RATS usually increased expenditures with its superiority over VATS concerning short- and long-term outcomes remaining unrevealed specified for young adults with NSCLC (Jin et al. 2022). Consequently, debate persists on applying this novel modality to this special group of patients.
In the present study, we retrospectively identified a large cohort of NSCLC patients aged ≤ 35 years undergoing RATS or VATS lobectomy from a prospectively maintained database and compared the perioperative outcomes, LN dissection, and long-term survival between the two surgical approaches, aiming to assess the advantages of RATS over VATS.
Materials and methods
Patient selection and data collection
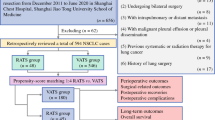
We retrospectively identified NSCLC patients aged 35 years or younger who underwent RATS or VATS lobectomy in Shanghai Chest Hospital, Shanghai Jiao Tong University School of Medicine during the period of November 2014 and March 2021 (Fig. 1). The inclusion criteria were as follows: (1) receiving RATS or VATS lobectomy; (2) aged 18–35 years; (3) pathology-confirmed NSCLC. The exclusion criteria were as follows: (1) surgery not for lung malignancy or cases with incomplete information; (2) History of lung surgery; (3) History of malignancy; (4) Undergoing segmentectomy, wedge resection, pneumonectomy, bi-lobectomies, bronchial sleeve resection, or bilateral operation; (5) pathology-proven adenocarcinoma in situ (AIS) or malignancy other than NSCLC; (6) clinical/pathological T4 or N3 disease; (7) R2 resection (residual macroscopic tumor) or without mediastinal LN assessment; (8) preoperative intra-pulmonary or distant metastasis. A total of 1355 patients were finally enrolled and further divided into the RATS (n = 105) and VATS (n = 1250) groups. The following data were collected: (1) baseline clinicopathological characteristics such as gender, age, history of smoke, body mass index (BMI), preoperative comorbidities, pulmonary functions [% of forced expiratory volume in 1 s (FEV1%), and % of diffusing capacity for carbon monoxide (DLCO%)], arterial blood gas analysis [oxygen pressure (PaO2), oxygen saturation (SaO2), and carbon dioxide pressure (PaCO2)], surgical location, tumor size, pathological T, N, and TNM stage of the disease, induction therapy, and adjuvant therapy; (2) perioperative outcomes including surgical duration, conversion, reoperation, intraoperative bleeding, postoperative blood transfusion, postoperative ICU admission, duration and volume of chest tube drainage, length of postoperative hospital stays, and postoperative complications; (3) LN assessment including the N1, N2, and the total number of dissected LNs and LN stations; (4) long-term outcomes including 1-, 3-, and 5-year recurrence-free survival (RFS), overall survival (OS), and recurrence and mortality patterns. All cases were staged following the 8th edition of the TNM staging system of the International Association for the Study of Lung Cancer.
Flow chart of the study population. NSCLC non-small cell lung cancer, AAH atypical adenomatous hyperplasia, AIS adenocarcinoma in situ, LN lymph node, MIS minimally invasive surgery, RATS robotic-assisted thoracoscopic surgery, VATS video-assisted thoracoscopic surgery, PSM propensity score matching, BMI body mass index, FEV1 forced expiratory volume in 1 s; DLCO diffusing capacity for carbon monoxide
Perioperative evaluation
Pulmonary and cardiac function tests were routinely performed before the operation to ensure the surgical tolerance of patients. For patients with impaired respiratory function symptoms or decreased FEV1, FEV1%, or DLCO% detected by the pulmonary function test, the arterial blood gas analysis was further performed. High-resolution thoracic CT and/or positron emission tomography/CT (PET/CT) were conducted to assess mediastinal and pulmonary LN status. PET/CT, bone scintigraphy, abdominal ultrasound, and enhanced cranial magnetic resonance imaging were carried out to determine distant metastasis.
Surgical techniques
RATS and VATS were performed according to the procedure reported by our surgical team previously (Huang et al. 2021, 2018; Hou et al. 2022). All patients received general intravenous (i.v.) anesthesia with double-lumen intubation and single-lung ventilation managed by dedicated thoracic anesthesiologists. RATS was conducted by adopting the Da Vinci Surgical System (Intuitive Surgical, CA, USA) via four minimal incisions without spreading the ribs. A 30-degree three-dimensional (3D) endoscope was inserted through the camera port located on the posterior axillary line's 7th or 8th intercostal space. Two incisions were symmetrically made at the 7th and 9th intercostal spaces along the mid-axillary and infrascapular lines, respectively. A utility port was created at the 3rd or 4th intercostal space on the anterior axillary line for lung retraction, operating field exposure, and specimen retrieval. Conventionally, VATS was performed via three or four minimal incisions with the non-rib spreading technic. The camera port was created at the 6th or 7th intercostal space along the anterior axillary line. Two incisions were made at the 3rd or 4th, and 8th intercostal spaces on the anterior and posterior axillary lines, respectively. The fourth port was created at the 9th intercostal space along the posterior axillary line, if needed, for assistance. A radical lobectomy with systematic mediastinal LN dissection was carried out. The interleaf fissures were divided, and hilar structures were dissected and identified individually, followed by the dissection of the target pulmonary artery and vein, and bronchus using endoscopic staplers. The resection margin was evaluated by the intraoperative frozen section for all patients. After ensuring the absence of active bleeding in the thoracic cavity and air leakage from the bronchial stump, the incisions were closed with one or two 24F chest tubes left. The conversion was defined as the operation starting with RATS or VATS dissection and finishing as the rib spreading thoracotomy. The representative operation video of RATS lobectomy is provided in Supplementary Video 1.
Postoperative management
All patients received the enhanced recovery protocol, including smoking cessation two weeks before surgery, breathing exercises, and early postoperative exercises. The 30 day postoperative complications were recorded, followed by being classified according to the Clavien–Dindo classification system as follows: I, any deviation from the ordinary postoperative course without requiring pharmacological treatment or surgical intervention or requiring drugs such as antipyretics, analgesics, diuretics, antiemetics, or electrolytes; II, complication requiring pharmacological intervention, including blood transfusion and total parenteral nutrition; III, comorbidities requiring operative or endoscopic intervention; IV, complication requiring ICU treatment; and V, death of the patient (Dindo et al. 2004). Specifically, pulmonary complications included pneumonia, acute respiratory distress syndrome (ARDS), respiratory failure requiring reintubation, empyema, and pulmonary embolism. Cardiac comorbidities included arrhythmia and myocardial ischemia or infarction. Anastomotic complications included prolonged air leaks, anastomotic dehiscence, and bronchopleural fistula. Other comorbidities included chylothorax, vocal cord paralysis, wound infection, and deep venous thrombosis.
The chest tube was removed when: (1) absence of apparent air leak and subcutaneous emphysema, 92) the drainage volume was less than 200 mL/day, 93) no cloudy, densely bloody, or purulent pleural effusion, and 4) the chest X-ray images indicated excellent resorption of the lung. Conventionally, patients were discharged the day or one day after removing drainage tubes unless there were comorbidities that still required intervention. Standard adjuvant therapy, including chemotherapy, immunotherapy, and radiotherapy, was recommended for patients if deemed necessary.
Follow-up
The lifelong follow-up assessment was planned for all patients one month after the operation and varied afterward: patients with histology of MIA were evaluated annually, while the other patients were measured every three months for the first two years, every half year in years three–five, and annually afterward. For postoperative follow-up of patients, thoracic CT, routine blood tests, serum tumor markers tests, and ultrasound were conventional approaches. Additionally, PET–CT was applied if suspected recurrence or metastasis were indicated during follow-up assessment. Moreover, head MRI and bone scintigraphy were applied for patients with suspected metastasis specified to the brain or bone. Telephone or Internet follow-ups were conducted yearly for patients who did not regularly come to the outpatient clinic until death or March 2023. The latest electronic medical data were recorded for patients lost to follow-up. The radiological recurrence tumors were defined as multiple new pulmonary lesions with the consolidation-to-tumor ratio (the ratio of the size of the solid component to the overall tumor diameter) of > 0.5 for all nodules, following the guidelines of radiologists (Yotsukura et al. 2021). RFS was defined as the duration from operation to any local or distant tumor recurrence, and patients with non-cancer-related death were deemed event free. OS was defined as the duration from operation to death.
Statistical analysis
Categorical variables were expressed using frequencies and percentages, while continuous variables were expressed using mean ± standard deviation (SD). To compare categorical variables, Pearson’s chi-square tests or Fisher’s exact tests were adopted. The homogeneity of variance and normality of distribution for continuous variables were determined using the Kolmogorov–Smirnov test. The student’s t-test was conducted for continuous variables with normal distribution and homogeneous variance, and the Mann–Whitney U test was performed otherwise. Kaplan–Meier curves log-rank (Mantel–Cox) test was carried out to analyze survival profiles. Cox hazard regression model analysis was further conducted to identify factors relevant to RFS and OS. To mitigate potential patient selection bias, propensity score matching (PSM) was carried out based on 14 critical baseline characteristics, including gender, age, history of smoke, BMI, FEV1%, DLCO%, surgical location, tumor size, tumor histology, pathological T stage, pathological N stage, pathological TNM stage, neoadjuvant therapy, and adjuvant therapy to establish a 1:3 RATS versus VATS ratio. SPSS version 26.0 (IBM Corporation, Armonk, NY, USA) was applied for statistical analysis and PSM, while GraphPad Prism-9 (GraphPad Software Inc., San Diego, CA, USA) was used for analyzing survival profiles. A two-tailed p value less than 0.10 was considered statistically significant for the Cox hazards regression model analysis, while less than 0.05 was considered statistically significant in all other tests.
Results
Baseline clinicopathological characteristics
The baseline clinicopathological characteristics of the included patients before PSM are shown in Table 1. Patients who received RATS or VATS were associated with a similar distribution of gender (p = 0.90), age (32.2 ± 3.0 vs 32.0 ± 2.8 years, p = 0.34), history of smoke (p = 0.76), BMI (22.0 ± 2.8 vs 21.8 ± 4.4 kg/m2, p = 0.62), preoperative comorbidities (p = 0.59), FEV1% (96.8 ± 11.5 vs 96.3 ± 12.2, p = 0.47), DLCO% (97.5 ± 13.5 vs 97.8 ± 16.9, p = 0.72), surgical location (p = 0.34), tumor histology (p = 0.68), tumor size (16.7 ± 9.9 vs 17.4 ± 10.1 mm, p = 0.69), pathological T (p = 0.78), N (p = 1.00), and TNM (p = 0.99) stage of the disease, neoadjuvant therapy (p = 1.00), and adjuvant therapy (p = 0.75). For patients receiving blood gas analysis, the PaO2, SaO2, and PaCO2 indexes were all similar between the two groups (all p > 0.05). PSM was then applied to establish a 1:3 RATS versus VATS ratio, and all baseline cofounding features of included cases were well balanced between the two groups following PSM (all p > 0.05).
Perioperative outcomes and LN dissection
The perioperative outcomes of matched patients are expressed in Table 2. By comparison, RATS and VATS were associated with a similar surgical duration (91.4 ± 23.4 vs 94.3 ± 34.6 min, p = 0.95), the incidence of conversion (p = 1.00) and reoperation (p = 1.00), intraoperative bleeding (82.38 ± 17.99 vs 84.24 ± 16.71 mL, p = 0.35), and postoperative blood transfusion (p = 1.00). Moreover, the RATS group had a shorter postoperative hospital stay (4.0 ± 1.5 vs 4.3 ± 1.7 days, p = 0.02) than the VATS group. Additionally, no difference was found between the two groups in postoperative ICU admission (6.7% vs 9.2%, p = 0.42) and chest tube drainage volume (686.9 ± 364.9 vs 701.8 ± 335.9 mL, p = 0.41) and duration (3.4 ± 1.3 vs 3.5 ± 1.6 days, p = 0.51), and postoperative complications (all p > 0.05). Meanwhile, no operation-related mortality was found in the RATS or VATS groups. Finally, patients receiving RATS or VATS were associated with comparable Clavien–Dindo postoperative complication scores (Fig. 2).
In terms of the LN dissection, RATS harvested significantly increased number of N1 (4.2 ± 3.1 vs 3.5 ± 2.2, p = 0.02) and total (9.4 ± 4.4 vs 8.3 ± 3.6, p = 0.03) LNs compared with VATS. Meanwhile, the two groups had a comparable number of harvested N2 LNs (5.2 ± 2.7 vs 4.8 ± 2.5, p = 0.08). Moreover, no difference was found in assessing N1 (2.5 ± 0.8 vs 2.4 ± 0.9, p = 0.07), N2 (3.2 ± 1.1 vs 3.1 ± 0.9, p = 0.45), and total (5.6 ± 1.4 vs 5.4 ± 1.4, p = 0.35) LN stations between two surgical approaches.
Long-term survival
The median follow-up of RATS and VATS groups was 62 months [range 24–99 months] and 65 months [range 24–98 months], respectively. In the RATS group, the 5-year RFS and OS of patients were 87.0 and 95.9%, respectively. In the VATS group, the 5-year RFS and OS were 86.2 and 95.7%, respectively (Fig. 3A, B). The two surgical approaches achieved comparable RFS (p = 0.86) and OS (p = 0.96) profiles. Furthermore, patients who underwent RATS or VATS were associated with comparable recurrence and mortality patterns (Fig. 3C, D). Additionally, patients were divided into subgroups concerning the histology type (MIA and the others), age (18–30 and 31–35 years), and gender (male and female), and the analysis within the subgroups also suggested that RATS and VATS led to similar long-term outcomes (Fig. 4A–D, Supplementary Fig. 1A-D, Supplementary Fig. 2A-D). Finally, multivariable Cox hazard regression analysis revealed that the surgical method (RATS vs VATS) was not independently correlated with RFS [hazard ratio (HR) = 0.96, p = 0.90, Supplementary Table 1) or OS (HR = 0.86, p = 0.80, Supplementary Table 2). Meanwhile, gender, age, and history of smoke were also not independent predictors of prognosis (all p > 0.05). Nevertheless, the advanced disease and LN metastasis were independently associated with the shortened DFS and OS.
Kaplan–Meier analysis of long-term outcomes of NSCLC patients aged ≤ 35 years who underwent RATS or VATS. Comparison of RFS A, OS B, recurrence pattern C, and mortality pattern D between the RATS and VATS groups. Regional recurrence was defined as tumor recurrence or metastasis confined to the chest cavity, while distant recurrence was defined as tumor metastasis beyond the chest cavity. RATS robotic-assisted thoracoscopic surgery, VATS video-assisted thoracoscopic surgery, RFS disease-free survival, OS overall survival, CNS central nervous system
Subgroup analysis of NSCLC patients aged ≤ 35 years who underwent RATS or VATS. Comparison of RFS A and OS B between the RATS and VATS groups in patients with MIA. Comparison of RFS C and OS D between the RATS and VATS groups in NSCLC patients with histology other than MIA. MIA minimally invasive adenocarcinoma, RATS robotic-assisted thoracoscopic surgery, VATS video-assisted thoracoscopic surgery, RFS disease-free survival; OS overall survival
Discussion
The feasibility and efficacy of VATS have been well established, making VATS to be the most prevalent MIS technic in treating NSCLC. Nowadays, RATS is also increasingly applied for NSCLC and has been accepted to be safe and oncologically effective (Jin et al. 2022; Huang et al. 2021). However, the epidemiological characteristics of NSCLC determine that the vast majority of cases occur in elderly individuals aged over 60 years. Consequently, the perioperative outcomes and long-term survival data of RATS for young NSCLC cases remain unrevealed. In this study, we retrospectively compared the short- and long-term outcomes of RATS and VATS lobectomy in NSCLC patients aged 35 years or younger based on real-world practice, suggesting that RATS accelerated postoperative recoveries, assessed increased LNs, and achieved comparable intraoperative outcomes and long-term survival when compared with VATS.
Our results showed that RATS led to a shorter postoperative hospital stay than VATS, which potentially be attributed to the flexibility of the robot arms and high-quality surgical view, which enables a more precise resection and minimizes unnecessary damage and, thus, expedites postoperative recoveries of patients. Given current clinical practice trends, increasing publications compare perioperative recoveries between RATS and VATS, but conflicting results have been reported. Recently, three studies analyzing the Premiere database observed that RATS was associated with shorter postoperative hospitalization (Reddy et al. 2018; Oh et al. 2017; Kent et al. 2023). This superiority of RATS, however, was not found in the other two publications based on public databases (Louie et al. 2016; Veluswamy et al. 2020). Furthermore, our results showed that RATS and VATS led to similar duration and volume of chest tube drainage, concordant with most previous studies. Nevertheless, Jin et al. found an increased chest tube volume with RATS, possibly due to the advantages of RATS in accessing LNs which might damage the bronchial and lymphatic vessels connected with LNs (Jin et al. 2022). Finally, no surgical-related mortality and low incidence of blood transfusion were found in the RATS and VATS groups, suggesting that both surgical approaches are safe for lobectomy in treating very young NSCLC patients.
LN retrieval is a vital part of MIS lobectomy in treating NSCLC and an essential measurement of surgical quality. Numerous previous studies have determined the capacity of the robotic-assisted surgical system in LN assessment but have produced conflicting conclusions. Three studies suggested that RATS harvested higher numbers of LNs and LN stations than VATS (Jin et al. 2022; Nelson et al. 2019; Ma et al. 2021). Nevertheless, Kneuertz et al. and Guo et al. independently found that the two procedures exhibited comparable capacity in LN exanimation (Kneuertz et al. 2019; Guo, Ma et al. 2019). Our results indicated that RATS assessed more LNs, especially N1 LNs while examining similar N2 LNs and LN stations compared to VATS. In our real-world practice, the assessment may not further be performed for LNs that are likely to be uninvolved if difficulty in dissection is estimated and sufficient LNs and LN stations have been retrieved, especially for patients with clinically early-stage disease. Given this, a more convenient process is likely to improve the willingness of thoracic surgeons to harvest more LNs. As one of the most sophisticated, complex, and expensive operational equipment in the world, da Vinci Surgical System provides many natural superiorities, including 3D, high-definitional, and tenfold magnified surgical view, and robotic arms that can rotate freely in the chest cavity and exhibit excellent maneuverability and improved dexterity over traditional 2D VATS technic (Jin et al. 2022; Pan Tian et al. 2022; Kneuertz et al. 2019). This benefit offers operators great convenience in harvesting LNs around vessels and bronchi and helps surgeons to dissect LNs that VATS does not easily assess due to limited flexibility, which may explain the increased LNs assessed by RATS. Additionally, the varied operation experiences and the learning curve in performing RATS and VATS among different thoracic surgeons may also impact LN retrieval, which was hard to be controlled in a retrospective study. Nevertheless, VATS is still the most prevalent approach in our center, and therefore most surgeons are more experienced in performing VATS than RATS. Given this, the superiority in LN dissection of RATS over VATS may largely be attributed to the advantages of the robot-assisted surgical system. The increased LN dissection could prolong the surgical duration and damage the normal mediastinal lymphatic, vascular, and neurogenic tissues, and may, thus, increase intraoperative bleeding and result in comorbidities, including recurrent laryngeal nerve injury and chylothorax (Allen et al. 2006; Zhang et al. 2023). In our study, RATS did not increase postoperative complications when harvesting more LNs compared with VATS. Given this, RATS might be especially suitable for patients with a high LN metastasis risk demanding a more complete mediastinal and pulmonary LN assessment.
In terms of long-term outcomes, our results suggested that RATS and VATS achieved comparable 5 year RFS and OS in the young. These findings are consistent with and could complement several previous studies which enrolled older cases, suggesting that RATS might be oncologically effective for all-age resectable NSCLC patients (Montagne et al. 2022; Pan et al. 2022a, b; Merritt et al. 2022). The very young NSCLC patients usually have distinctive clinic-demographic and genomic features and are associated with a higher risk of suffering from multiple pulmonary lesions than older individuals (Viñal et al. 2021). Hence, identifying the optimal extent of resection to preserve more normal lung tissue for the potential multiple lung surgeries is of critical importance for this particular group of patients. Recently, two multi-center, noninferiority, phase 3 trials, namely JCOG0802/WJOG4607L and CALGB 140503 trials, showed that sub-lobectomy was not inferior to lobectomy, and thus might be considered one of the standard treatments for patients with a peripheral stage IA NSCLC with a tumor size ≤ 2 cm (Altorki et al. 2023; Saji et al. 2022). Moreover, two independent studies also found that patients with AIS/MIA were associated with 100% RFS during the follow-up for at least 5 years, regardless of the surgical method (lobectomy, segmentectomy, or wedge resection) (Yotsukura et al. 2021; Zhang et al. 2022). These crucial discoveries are expected to promote the prevalence of sub-lobectomy in treating early-stage NSCLC. In our study, most young patients had early-stage disease and met the criteria for sub-lobar resection proposed by the trials mentioned above. Therefore, further comparison of surgical-related outcomes and oncological efficacies of RATS versus VATS for sub-lobectomy in young NSCLC cases is essential to expand the scope of the application of RATS.
In the present study, most young NSCLC patients were associated with early-stage disease and achieved excellent 5-year survival outcomes. However, many previous publications have found distinct clinic-epidemiological features, indicating that NSCLC in the young may represent a more aggressive tumor, and most young cases had the late-stage (III–IV) disease at the first diagnosis and were associated with a poor prognosis (Duan et al. 2013; Subramanian et al. 2010; Galvez-Nino et al. 2020). Nevertheless, most included cases in these studies were diagnosed before 2016, and early-stage NSCLC is increasingly prevalent nowadays with the increased implementation of thin-section thoracic CT and development in diagnostic modalities. Meanwhile, more attention has been paid to physical health, and routine medical examination is becoming increasingly popular among young adults in recent years. Our study mostly identified young NSCLC patients undergoing surgery after 2016 and, thus, included more early-stage cases. Moreover, the present study excluded patients with stage T4/N3 disease or intra-pulmonary or distant metastasis, and therefore the vast majority of patients with advanced disease were excluded.
To the best of our knowledge, the present study is the first retrospective analysis comparing perioperative outcomes and long-term survival of RATS versus VATS lobectomy specified for very young NSCLC patients aged ≤ 35 years based on real-world practice. However, we have also acknowledged some limitations of the present study. The retrospective analysis usually leads to undiscovered patient selection bias and unbalanced case distribution. The massive difference in the size of included patients resulted in excluding many patients in the VATS group, and the potential selection bias may still exist despite PSM having been applied in the present study to balance the key confounding factors of patients. Moreover, the single-center analysis property of the present study limited the size of the case sample and weakened its representative, though the patient data were identified from one of the most famous and highest-volume medical centers in China. Therefore, further multi-center prospective research is necessary to validate the findings of our analysis.
Conclusions
In conclusion, RATS exhibited superiorities over VATS in shorter postoperative hospital stay and more assessed LNs than VATS, and the two surgical approaches achieved similar long-term outcomes in treating NSCLC patients aged 35 years or younger.
Data availability
The data generated and analyzed during the present study are available from the corresponding authors, Prof. Huang J and Luo QQ, upon reasonable request.
References
Allen MS, Darling GE, Pechet TT, Mitchell JD, HerndonLandreneau JERJ, Inculet RI, Jones DR, Meyers BF, Harpole DH, Putnam JB Jr, Rusch VW (2006) Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg 81(3):1013–1019. https://doi.org/10.1016/j.athoracsur.2005.06.066
Altorki N, Wang X, Kozono D, Watt C, Landrenau R, Wigle D, Port J, Jones DR, Conti M, Ashrafi AS, Liberman M, Yasufuku K, Yang S, Mitchell JD, Pass H, Keenan R, Bauer T, Miller D, Kohman LJ, Stinchcombe TE, Vokes E (2023) Lobar or sublobar resection for peripheral stage ia non-small-cell lung cancer. N Engl J Med 388(6):489–498. https://doi.org/10.1056/NEJMoa2212083
Bendixen M, Jørgensen OD, Kronborg C, Andersen C, Licht PB (2016) Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 17(6):836–844. https://doi.org/10.1016/s1470-2045(16)00173-x
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213. https://doi.org/10.1097/01.sla.0000133083.54934.ae
Duan L, You Q, Chen X, Wang H, Zhang H, Xie D, Xu X, Jiang G (2013) Outcome and prognosis for patients younger than thirty with primary lung cancer. Minerva Chir 68(2):175–182
Galvez-Nino M, Ruiz R, Pinto JA, Roque K, Mantilla R, Raez LE, Mas L (2020) Lung cancer in the young. Lung 198(1):195–200. https://doi.org/10.1007/s00408-019-00294-5
Garrana SH, Dagogo-Jack I, Cobb R, Kuo AH, Mendoza DP, Zhang EW, Heeger A, Sequist LV, Digumarthy SR (2021) Clinical and imaging features of non-small-cell lung cancer in young patients. Clin Lung Cancer 22(1):23–31. https://doi.org/10.1016/j.cllc.2020.10.012
Guo F, Ma D, Li S (2019) "Compare the prognosis of Da Vinci robot-assisted thoracic surgery (RATS) with video-assisted thoracic surgery (VATS) for non-small cell lung cancer: a meta-analysis. Med (baltimore). 98(39):e17089. https://doi.org/10.1097/md.0000000000017089
Hou Y, Hu Y, Song W, Zhang J, Luo Q, Zhou Q (2022) Surgical site infection following minimally invasive lobectomy: Is robotic surgery superior? Cancer Med 11(11):2233–2243. https://doi.org/10.1002/cam4.4609
Huang J, Li J, Li H, Lin H, Lu P, Luo Q (2018) Continuous 389 cases of Da Vinci robot-assisted thoracoscopic lobectomy in treatment of non-small cell lung cancer: experience in Shanghai Chest Hospital. J Thorac Dis 10(6):3776–3782. https://doi.org/10.21037/jtd.2018.06.80
Huang J, Tian Y, Zhou QJ, Ning JW, Gu ZN, Lu PJ, Li JT, Lin H, Chen TX, Yang YH, Kim MP, Merritt RE, Ghisalberti M, Jiang L, Luo QQ (2021) Comparison of perioperative outcomes of robotic-assisted versus video-assisted thoracoscopic right upper lobectomy in non-small cell lung cancer. Transl Lung Cancer Res 10(12):4549–4557. https://doi.org/10.21037/tlcr-21-960
Jin R, Zheng Y, Yuan Y, Han D, Cao Y, Zhang Y, Li C, Xiang J, Zhang Z, Niu Z, Lerut T, Lin J, Abbas AE, Pardolesi A, Suda T, Amore D, Schraag S, Aigner C, Li J, Che J, Hang J, Ren J, Zhu L, Li H (2022) Robotic-assisted versus video-assisted thoracoscopic lobectomy: short-term results of a randomized clinical trial (RVlob Trial). Ann Surg 275(2):295–302. https://doi.org/10.1097/sla.0000000000004922
Kent MS, Hartwig MG, Vallières E, Abbas AE, Cerfolio RJ, Dylewski MR, Fabian T, Herrera LJ, Jett KG, Lazzaro RS, Meyers B, Mitzman BA, Reddy RM, Reed MF, Rice DC, Ross P, Sarkaria IS, Schumacher LY, Tisol WB, Wigle DA, Zervos M (2023) Pulmonary open, robotic, and thoracoscopic lobectomy (PORTaL) Study: An Analysis of 5721 cases. Ann Surg 277(3):528–533. https://doi.org/10.1097/sla.0000000000005115
Kneuertz PJ, Cheufou DH, D’Souza DM, Mardanzai K, Abdel-Rasoul M, Theegarten D, Moffatt-Bruce SD, Aigner C, Merritt RE (2019) Propensity-score adjusted comparison of pathologic nodal upstaging by robotic, video-assisted thoracoscopic, and open lobectomy for non-small cell lung cancer. J Thorac Cardiovasc Surg 158(5):1457-1466.e2. https://doi.org/10.1016/j.jtcvs.2019.06.113
Landwehr MS, Watson SE, Macpherson CF, Novak KA, Johnson RH (2016) The cost of cancer: a retrospective analysis of the financial impact of cancer on young adults. Cancer Med 5(5):863–870. https://doi.org/10.1002/cam4.657
Louie BE, Wilson JL, Kim S, Cerfolio RJ, Park BJ, Farivar AS, Vallières E, Aye RW, Burfeind WR Jr, Block MI (2016) Comparison of video-assisted thoracoscopic surgery and robotic approaches for clinical stage i and stage ii non-small cell lung cancer using the society of thoracic surgeons database. Ann Thorac Surg 102(3):917–924. https://doi.org/10.1016/j.athoracsur.2016.03.032
Ma J, Li X, Zhao S, Wang J, Zhang W, Sun G (2021) Robot-assisted thoracic surgery versus video-assisted thoracic surgery for lung lobectomy or segmentectomy in patients with non-small cell lung cancer: a meta-analysis. BMC Cancer 21(1):498. https://doi.org/10.1186/s12885-021-08241-5
Merritt RE, Abdel-Rasoul M, D’Souza DM, Kneuertz PJ (2022) Comparison of the long-term oncologic outcomes of robotic-assisted and video-assisted thoracoscopic lobectomy for resectable non-small cell lung carcinoma. J Robot Surg 16(6):1281–1288. https://doi.org/10.1007/s11701-022-01368-y
Montagne F, Chaari Z, Bottet B, Sarsam M, Mbadinga F, Selim J, Guisier F, Gillibert A, Baste JM (2022) Long-term survival following minimally invasive lung cancer surgery: comparing robotic-assisted and video-assisted surgery. Cancers (basel). https://doi.org/10.3390/cancers14112611
Nelson DB, Mehran RJ, Mitchell KG, Rajaram R, Correa AM, Bassett RL Jr, Antonoff MB, Hofstetter WL, Roth JA, Sepesi B, Swisher SG, Walsh GL, Vaporciyan AA, Rice DC (2019) Robotic-assisted lobectomy for non-small cell lung cancer: a comprehensive institutional experience. Ann Thorac Surg 108(2):370–376. https://doi.org/10.1016/j.athoracsur.2019.03.051
Oh DS, Reddy RM, Gorrepati ML, Mehendale S, Reed MF (2017) Robotic-assisted, video-assisted thoracoscopic and open lobectomy: propensity-matched analysis of recent premier data. Ann Thorac Surg 104(5):1733–1740. https://doi.org/10.1016/j.athoracsur.2017.06.020
Pan H, Gu Z, Tian Y, Jiang L, Zhu H, Ning J, Huang J, Luo Q (2022a) Propensity score-matched comparison of robotic- and video-assisted thoracoscopic surgery, and open lobectomy for non-small cell lung cancer patients aged 75 years or older. Front Oncol 12:1009298. https://doi.org/10.3389/fonc.2022.1009298
Pan H, Tian Y, Wang H, Jiang L, Gu Z, Zhu H, Ning J, Huang J, Luo Q (2022b) Perioperative and oncological outcomes of robotic-assisted, video-assisted thoracoscopic and open lobectomy for patients with n1-metastatic non-small cell lung cancer: a propensity score-matched study. Cancers (basel). https://doi.org/10.3390/cancers14215249
Reddy RM, Gorrepati ML, Oh DS, Mehendale S, Reed MF (2018) Robotic-assisted versus thoracoscopic lobectomy outcomes from high-volume thoracic surgeons. Ann Thorac Surg 106(3):902–908. https://doi.org/10.1016/j.athoracsur.2018.03.048
Sacher AG, Dahlberg SE, Heng J, Mach S, Jänne PA, Oxnard GR (2016) Association between younger age and targetable genomic alterations and prognosis in non-small-cell lung cancer. JAMA Oncol 2(3):313–320. https://doi.org/10.1001/jamaoncol.2015.4482
Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K, Aoki T, Okami J, Yoshino I, Ito H, Okumura N, Yamaguchi M, Ikeda N, Wakabayashi M, Nakamura K, Fukuda H, Nakamura S, Mitsudomi T, Watanabe SI, Asamura H (2022) Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 399(10335):1607–1617. https://doi.org/10.1016/s0140-6736(21)02333-3
Siegel RL, Miller KD, Wagle NS, Jemal A (2023) Cancer statistics, 2023. CA Cancer J Clin 73(1):17–48. https://doi.org/10.3322/caac.21763
Subramanian J, Morgensztern D, Goodgame B, Baggstrom MQ, Gao F, Piccirillo J, Govindan R (2010) Distinctive characteristics of non-small cell lung cancer (NSCLC) in the young: a surveillance, epidemiology, and end results (SEER) analysis. J Thorac Oncol 5(1):23–28. https://doi.org/10.1097/JTO.0b013e3181c41e8d
Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS (2021) Lung cancer. Lancet 398(10299):535–554. https://doi.org/10.1016/s0140-6736(21)00312-3
Veluswamy RR, Whittaker Brown SA, Mhango G, Sigel K, Nicastri DG, Smith CB, Bonomi M, Galsky MD, Taioli E, Neugut AI, Wisnivesky JP (2020) Comparative effectiveness of robotic-assisted surgery for resectable lung cancer in older patients. Chest 157(5):1313–1321. https://doi.org/10.1016/j.chest.2019.09.017
Veronesi G, Novellis P, Voulaz E, Alloisio M (2016) Robot-assisted surgery for lung cancer: State of the art and perspectives. Lung Cancer 101:28–34. https://doi.org/10.1016/j.lungcan.2016.09.004
Viñal D, Martínez D, Higuera O, de Castro J (2021) "Genomic profiling in non-small-cell lung cancer in young patients A systematic review. ESMO Open 6(1):100045. https://doi.org/10.1016/j.esmoop.2020.100045
Yotsukura M, Asamura H, Motoi N, Kashima J, Yoshida Y, Nakagawa K, Shiraishi K, Kohno T, Yatabe Y, Watanabe SI (2021) Long-term prognosis of patients with resected adenocarcinoma in situ and minimally invasive adenocarcinoma of the lung. J Thorac Oncol 16(8):1312–1320. https://doi.org/10.1016/j.jtho.2021.04.007
Zhang Y, Ma X, Shen X, Wang S, Li Y, Hu H, Chen H (2022) Surgery for pre- and minimally invasive lung adenocarcinoma. J Thorac Cardiovasc Surg 163(2):456–464. https://doi.org/10.1016/j.jtcvs.2020.11.151
Zhang Y, Deng C, Zheng Q, Qian B, Ma J, Zhang C, Jin Y, Shen X, Zang Y, Guo Y, Fu F, Li H, Zheng S, Wu H, Huang Q, Wang S, Liu Q, Ye T, Sun Y, Zhang Y, Xiang J, Hu H, Li Y, Chen H (2023) Selective mediastinal lymph node dissection strategy for clinical t1n0 invasive lung cancer: a prospective, multicenter, clinical trial. J Thorac Oncol. https://doi.org/10.1016/j.jtho.2023.02.010
Funding
This work was supported by National Nature Science Foundation (Grant No. 81772482), the Joint Clinical Research Centre of Institute of Medical Robotics@SJTU-Shanghai Chest Hospital (IMR-XKH202104), and the Shanghai Hospital Development Center (Grant No. SHDC12016113).
Author information
Authors and Affiliations
Contributions
PHB, ZJQ, and TY contributed equally to this work as the co-first authors. Conceptualization and design: HJ, LQQ; Methodology: PHB, ZJQ, TY, JWQ; Resources: TY, GZN, NJW, ZHD, JL; Formal analysis and investigation: PHB, ZJQ, TY, ZHD, JWQ; Writing—original draft preparation: PHB, ZJQ, TY; Writing—review and editing: JL, HJ, LQQ; Funding acquisition: LQQ; Supervision: HJ, LQQ.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was conducted following the Declaration of Helsinki (as revised in 2013) and was approved by the Institutional Review Board of Shanghai Chest Hospital, Shanghai Jiao Tong University School of Medicine (approval No. KS1735). The written informed consent was waived due to the retrospective nature of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 146915 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pan, H., Zhang, J., Tian, Y. et al. Short- and long-term outcomes of robotic-assisted versus video-assisted thoracoscopic lobectomy in non-small cell lung cancer patients aged 35 years or younger: a real-world study with propensity score-matched analysis. J Cancer Res Clin Oncol 149, 9947–9958 (2023). https://doi.org/10.1007/s00432-023-04933-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-04933-6