Abstract
Purpose
Achieving complete response (CR) after first-line chemotherapy in gastric DLBCL patients often results in longer disease-free survival. We explored whether a model based on imaging features combined with clinicopathological factors could assess the CR to chemotherapy in patients with gastric DLBCL.
Methods
Univariate (P < 0.10) and multivariate (P < 0.05) analyses were used to identify factors associated with a CR to treatment. As a result, a system was developed to evaluate whether gastric DLBCL patients had a CR to chemotherapy. Evidence was found to support the model's ability to predict outcomes and demonstrate clinical value.
Results
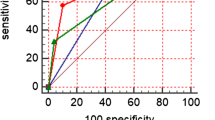
We retrospectively analysed 108 people who had been diagnosed gastric DLBCL; 53 were in CR. Patients were divided at random into a 5:4 training/testing dataset split. β2 microglobulin before and after chemotherapy and lesion length after chemotherapy were independent predictors of the CR of gastric DLBCL patients after chemotherapy. These factors were used in the predictive model construction. In the training dataset, the area under the curve (AUC) of the model was 0.929, the specificity was 0.806, and the sensitivity was 0.862. In the testing dataset, the model had an AUC of 0.957, specificity of 0.792, and sensitivity of 0.958. The AUC did not differ significantly between the training and testing dates (P > 0.05).
Conclusion
A model constructed using imaging features combined with clinicopathological factors could effectively evaluate the CR to chemotherapy in gastric DLBCL patients. The predictive model can facilitate the monitoring of patients and be used to adjust individualised treatment plans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
DLBCL is the most prevalent kind of non-Hodgkin lymphoma (NHL), which is a category of diseases with high heterogeneity. DLBCL accounts for approximately 30–40% of NHL cases (Vaidya and Witzig 2014). About a quarter of all instances of DLBCL are diagnosed at an early stage, known as limited-stage lymphoma, and are classified as stage I or II. 75% of DLBCL cases are diagnosed at more advanced stages (stages III and IV) (Cottereau et al. 2021). Chemotherapy is the main treatment modality for NHL, and the standard regimen is a combination of rituximab and anthracycline, such as R-CHOP (rituximab, cyclophosphamide, hydroxydaunorubicin hydrochloride, Oncovin® [vincristine], and prednisone) (Maurer et al. 2016; Hu et al. 2019). However, the treatment response varies widely, ranging from no tumour regression to complete response (CR). The 5-year survival rate associated with R-CHOP treatment is approximately 50–55%. In addition, 40–50% of patients develop relapsed or refractory DLBCL (Fan et al. 2021). Achieving complete response after the first course of induction chemotherapy is critical, as it often results in long-term progression-free survival (Zhang et al. 2015; Hawkes et al. 2018).
Imaging plays an important role in assessing the response to treatment in patients with gastric DLBCL (Kwee et al. 2008). In a previous study, follow-up was performed according to the National Comprehensive Cancer Network guidelines in which computed tomography (CT) is recommended every 6 months for 2 years after treatment completion. However, many patients underwent more scans than recommended by the National Comprehensive Cancer Network (Abel et al. 2012). Studies have also suggested that positron emission tomography (PET) should be performed 6–8 weeks after chemoimmunotherapy (Zelenetz et al. 2013). With growing evidence supporting the central role of PET–CT in NHL staging and response assessment (Gómez León et al. 2017; Wu et al. 2014), a five-point scale has been developed to visually assess varying degrees of response in the middle and at the end of treatment; however, there was a wide range of subjective differences in people used the five-point scale to assess different levels of response at the middle and end of treatment (Thompson et al. 2014). Therefore, visual assessment is still challenging, and a method is urgently needed to help clinicians accurately assess the post-treatment response of patients with gastric DLBCL.
To date, no study has evaluated the CR after treatment in gastric DLBCL patients using a predictive model. Therefore, the goals of this research were to conduct a screening for characteristics associated with CR after treatment in gastric DLBCL patients, develop a predictive model, and create a nomogram, and verify the accuracy and clinical validity of the model.
Materials and methods
Patients and reference standard
We retrospectively analysed all patients with pathologically confirmed gastric DLBCL between January 2017 and October 2022. Finally, 108 patients (aged 25–78 years; mean age: 52.5 years) were included. The inclusion criteria were as follows: (1) histopathologically diagnosed gastric DLBCL for the first time; (2) complete clinical and pathological information and auxiliary examination results; and (3) acceptance of a complete treatment cycle in our hospital. The exclusion criteria were (1) poor image quality owing to various reasons and (2) local or systemic antitumour therapy before chemotherapy.
We used a random 5:4 split to separate the patients into our training and testing datasets. The model was developed using the training dataset, and its accuracy was checked using the testing dataset. Our institution's ethics committee green-lighted this retrospective study without requiring participants to give their permission.
Treatment and follow-up
All patients with gastric DLBCL received standard treatment within 1 week after CT, and the treatment plan was six cycles of R-CHOP alone. The response to treatment was determined based on post-treatment physical condition and information gathered from the PET–CT scan results. The patients' electronic medical record (EMR) was mined for information on their clinical and pathological details (Table 1). Routine blood tests for lactate dehydrogenase (LDH) and β2 microglobulin were performed within 1 week before chemotherapy and within 1 week of the first cycle of chemotherapy. Normal levels of LDH and β2 microglobulin at our hospital are 120–246 U/L and 0–3.0 mg/L, respectively. Before treatment, the lesion length and longest diameter of the largest lymph node were simultaneously measured by two radiologists on the venous phase images, and the average value of the two measurements was calculated. In addition, the longest diameter of the lesion after treatment and the longest diameter of the largest lymph node were quantified on the PET–CT scans simultaneously by two radiologists, and the average of the two measurements was calculated. The Ann Arbor Staging System was used to stage all of the included patients (Carbone et al. 1971) using the first contrast-enhanced CT examination. The study process is shown in Fig. 1.
Clinical feature selection
Clinical variables relevant to the CR after treatment in gastric DLBCL patients include sex, pre-chemotherapy β2 microglobulin, post-chemotherapy β2 microglobulin, pre-chemotherapy LDH, post-chemotherapy LDH, pre-chemotherapy lesion length, post-chemotherapy lesion length, pre-chemotherapy longest lymph-node diameter, post-chemotherapy longest lymph-node diameter, tumour location, and stage. We first used univariate logistic regression analysis to identify candidate clinical factors related to gastric DLBCL response after treatment (P < 0.10) and then used multivariate logistic regression analysis to determine the independent predictors related to CR after treatment in gastric DLBCL patients (P < 0.05) for model development.
Model building and verification
We developed a nomogram (Kattan and Marasco 2010) including clinical features to provide clinicians with a quantitative tool to assess the CR of gastric DLBCL patients after chemotherapy. A testing dataset was used to validate the model's performance. Receiver-operating characteristic (ROC) curves were used to measure the ability of the model to evaluate the response after gastric DLBCL treatment. The nomogram's clinical usefulness was evaluated with the help of decision curve analysis [17], which quantified the net benefit at varying levels of certainty. Calibration curves (Li et al. 2021) were used to reflect the agreement between the predicted model and the observations.
Statistical analyses
For this retrospective study, we employed the Kruskal–Wallis test for continuous and ordinal variables and either the chi-squared or Fisher's exact test for categorical data. The model's discrimination, sensitivity, and specificity for the occurrence of events were measured using the ROC and AUC curves. A significance level of P < 0.050 was used. The nomogram and model assessment were carried out in R language (version 4.1.1; R Foundation for Statistical Computing, Vienna, Austria). The data were analysed statistically by use of SPSS (version 17.0; SPSS Inc., Chicago, IL). Statspackage (version 4.1.1) in R software was used for single and multi-factor logistic regression analysis. pROCpackage (version 1.18.0) was used for ROC curve. ModelGood (version 1.0.9) was used to draw calibration curve. Plot decision curves were plotted using ggDCA (version 1.2).
Results
Clinical and pathological characteristics
The clinical and pathological features of the 108 patients are shown in Table 1. CR was observed in 53 patients (49.07%). There was no significant difference in sex, LDH level before chemotherapy, or tumour location between the CR and non-CR groups (P > 0.05). However, β2 microglobulin before and after chemotherapy, LDH after chemotherapy, lesion length before and after chemotherapy, longest diameter of the lymph node before and after chemotherapy, and stage differed significantly between the two groups (P < 0.05). There were no significant differences (P < 0.05) in clinical and pathological features between the testing and training datasets (Table 2).
Clinical feature selection
Univariate analysis (P < 0.10) showed that β2 microglobulin before and after chemotherapy, LDH after chemotherapy, lesion length after chemotherapy, longest diameter of the lymph node before and after chemotherapy, and stage were significantly associated with treatment response after chemotherapy in patients with gastric DLBCL. All seven significant variables were included in the multivariate analysis, and β2 microglobulin before chemotherapy and lesion length after chemotherapy were statistically significant (Table 3). Although β2 microglobulin levels after chemotherapy were not statistically significant after multivariate logistic regression analysis, according to the literature (Wang et al. 2015), we believe that β2 microglobulin after chemotherapy is an independent predictor of the CR after treatment in gastric DLBCL patients. Therefore, β2 microglobulin before chemotherapy, β2 microglobulin after chemotherapy, and lesion length after chemotherapy were selected as independent predictors of the CR after treatment in gastric DLBCL patients.
The new nomogram
Based on these three independent prognostic factors, a model and nomogram for evaluating the CR after treatment in gastric DLBCL patients were constructed (Fig. 2). The probability of each predicted value can be converted into a score based on the scale at the top of the chart. The corresponding predicted probabilities were then summed. The bottom of the series shows the model-assessed probability of CR after chemotherapy in patients with gastric DLBCL, and the ROC curve (Fig. 3) shows the discriminative performance of the predictive model. In the training dataset, the model achieved an AUC of 0.929, a specificity of 0.806, and a sensitivity of 0.862 (Fig. 3a). In the testing dataset, the model had an AUC of 0.957, a specificity of 0.792, and a sensitivity of 0.958 (Fig. 3b). There was no statistically significant difference in the AUC between the training and testing groups (P > 0.05), indicating that the prediction model has a high discriminative ability. The calibration curve (Fig. 4a) showed good agreement between the predicted and actual probabilities in the testing dataset, indicating a high degree of calibration for the predictive model (P > 0.05). Decision curve analysis of the testing dataset (Fig. 4b) showed that the model had high clinical utility when the threshold probability was 0.033–0.879.
Discussion
Following chemotherapy in gastric DLBCL, treatment responses vary widely, ranging from no tumour regression to complete remission (Yin et al. 2014). The standard of complete remission is based on the new consensus reached by the International Conference of the Imaging of Malignant Lymphoma Working Group published by the Journal of Clinical Oncology, which recommends the use of a visual judgement method to report the results of PET–CT using a five-point scale [(Deauville) standard], combined with the patients’ expected prognosis, clinical manifestations, and other response indicators for joint interpretation (Barrington et al. 2014). The assessment of CR after gastric DLBCL treatment largely depends on radiologists’ experience; therefore, the assessment of CR after treatment in patients with gastric DLBCL remains challenging. In this retrospective study, we screened for independent factors associated with CR after chemotherapy in patients with gastric DLBCL and developed and validated a model for individualised noninvasive assessment of CR. To the best of our knowledge, no study has been conducted on the evaluation of CR after gastric DLBCL treatment using nomograms. Our study has important implications for the treatment and prognosis of patients with gastric DLBCL as the nomogram could be used to monitor patients and personalise their treatment.
Univariate and multivariate analyses revealed that β2 microglobulin levels before chemotherapy and lesion length after chemotherapy were independent predictors of CR in patients with gastric DLBCL. Wilder et al. (2001) demonstrated that bulky disease was a poor prognostic factor in patients with aggressive lymphoma treated with cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy, with or without radiation, which is consistent with our study findings. Fang et al. (2019) showed that an abnormally elevated β2 microglobulin level is an independent factor affecting the prognosis of patients with gastric DLBCL; however, in our study, the P value of β2 microglobulin after chemotherapy was more than 0.05, which may be related to the small number of patients included. Therefore, we included post-chemotherapy β2 microglobulin levels in the predictive model.
Kim et al. (2007) used absolute lymphocyte counts to predict the response of gastric DLBCL to chemotherapy; however, their study considered haematological features and did not include imaging features. Studies (El-Galaly et al. 2014; Yang et al. 2011) have confirmed that imaging examinations play an important role in the assessment of response in patients with gastric DLBCL after treatment. Yoon et al. (2016) explored the utility of endoscopy during and after DLBCL treatment. They suggested that in addition to radiological examinations, endoscopy and biopsy should also be performed; however, the number of patients included in their study was small, only 45 cases; thus, there may be selection bias. Moreover, endoscopy and biopsy are invasive examinations, which increases the financial burden on patients. Wu et al. (2013) examined several magnetic resonance imaging sequences for the detection of lesions and the assessment of treatment response in gastric DLBCL patients. The diagnostic precision and prognosis utility of magnetic resonance imaging may be enhanced by new complementary techniques, such as diffusion-weighted imaging (DWI) with apparent diffusion coefficient; however, DWI has some drawbacks as well. DWI is artefact-sensitive, and DWI based on echo-planar imaging is susceptible to image distortion. In addition, the study by Wu et al. included a small sample of only 18 patients with histologically confirmed gastric DLBCL and lacked independent validation. Trotman et al. (2011) proposed that PET–CT is more accurate in evaluating the end of treatment, but after treatment, an inflammatory reaction will occur around the lesion, which will increase the local uptake of contrast agent in the lesion and interfere with the doctors’ assessment of complete remission. The main focus of the current study was to build a model that integrates clinical features and indirect radiation features to quantitatively evaluate the CR of patients with gastric DLBCL after chemotherapy, eliminating the influence of subjective factors. In the training dataset, the AUC of the model was 0.929, specificity was 0.806, and sensitivity was 0.862. In the testing dataset, the AUC of the model was 0.957, specificity was 0.792, and sensitivity was 0.958. The model has good evaluation ability, and its advantages are as follows. First, our model is simple and convenient and includes quantitative features. Second, in the PET–CT images, we used the length of the lesion to represent its size, and two doctors reassessed the lesion length. Although this assessment method has measurement errors, including lesion length is necessary, because studies (Pfreundschuh et al. 2008) have shown that lesion size is one of the risk factors affecting the prognosis of patients with gastric DLBCL. Finally, we quantified the predictive model using a visualisation and interpretation tool, the nomogram, which is an easy-to-use personalised decision-making tool that helps clinicians accurately assess the response of patients with gastric DLBCL to chemotherapy and facilitates personalised precision medicine. Accordingly, we believe that the proposed model is reliable and stable and can help radiologists to make more accurate diagnoses.
This study had several limitations. First, a small number of quantitative features were extracted from the PET–CT images, and the model only considered the length of the lesion. Therefore, selection bias may have occurred in our analysis. Second, the number of patients we included is small, and most of them are concentrated in phase I and phase II. In future studies, we will further expand the number of patients; third, this was a single-centre study, and prospective multicentre experimental studies are needed to validate the model experimentally. In the future, we will consider using radiomics to evaluate the response of patients with gastric DLBCL after chemotherapy and explore more clinical risk factors related to the prognosis of these patients, such as lesion volume and metabolic tumour burden.
Conclusion
A model constructed using indirect imaging features combined with clinicopathological factors could effectively evaluate the response of patients with gastric DLBCL after chemotherapy. Our findings are of great significance as the model could be used to facilitate careful and regular patient monitoring, accurately evaluate the treatment response, and adjust the individualised treatment plans.
Data availability
The data that support the findings of this study were available upon request from the corresponding author. The data were not publicly available due to privacy or ethical restrictions.
References
Abel GA, Vanderplas A, Rodriguez MA, Crosby AL, Czuczman MS, Niland JC, Gordon LI, Millenson M, Zelenetz AD, Friedberg JW, LaCasce AS (2012) High rates of surveillance imaging for treated diffuse large B-cell lymphoma: findings from a large national database. Leuk Lymphoma 53(6):1113–1116. https://doi.org/10.3109/10428194.2011.639882
Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Müeller SP, Schwartz LH, Zucca E, Fisher RI, Trotman J, Hoekstra OS, Hicks RJ, O’Doherty MJ, Hustinx R, Biggi A, Cheson BD (2014) Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol 32(27):3048–3058
Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M (1971) Report of the committee on Hodgkin’s disease staging classification. Cancer Res 31(11):1860–1861
Cottereau A-S, Meignan M, Nioche C, Clerc J, Chartier L, Vercellino L, Casasnovas O, Thieblemont C, Buvat I (2021) New approaches in characterization of lesions dissemination in DLBCL patients on baseline PET/CT. Cancers (basel). https://doi.org/10.3390/cancers13163998
El-Galaly TC, Mylam KJ, Bøgsted M, Brown P, Rossing M, Gang AO, Haglund A, Arboe B, Clausen MR, Jensen P, Pedersen M, Bukh A, Jensen BA, Poulsen CB, d’Amore F, Hutchings M (2014) Role of routine imaging in detecting recurrent lymphoma: a review of 258 patients with relapsed aggressive non-Hodgkin and Hodgkin lymphoma. Am J Hematol 89(6):575–580. https://doi.org/10.1002/ajh.23688
Fan L, Lin Q, Huang X, Fu D, Huang H (2021) Prognostic significance of pretreatment serum free fatty acid in patients with diffuse large B cell lymphoma in the rituximab era: a retrospective analysis. BMC Cancer 21(1):1255. https://doi.org/10.1186/s12885-021-08963-6
Fang S, Zhao S-S, Zhu C-Y, Yang N, Wang F-Y, Wang L-L, Huang W-R, Gao C-J (2019) Analysis of clinical pathological features and prognosis in young patients with diffuse large B cell lymphoma. Zhongguo Shi Yan Xue Ye Xue Za Zhi 27(3):802–808. https://doi.org/10.19746/j.cnki.issn.1009-2137.2019.03.026
Gómez León N, Delgado-Bolton RC, Del Campo-Del-Val L, Cabezas B, Arranz R, García M, Cannata J, González Ortega S, Pérez Sáez MÁ, López-Botet B, Rodríguez-Vigil B, Mateo M, Colletti PM, Rubello D, Carreras JL (2017) Multicenter comparison of contrast-enhanced FDG PET/CT and 64-slice multi-detector-row CT for initial staging and response evaluation at the end of treatment in patients with lymphoma. Clin Nucl Med 42(8):595–602. https://doi.org/10.1097/RLU.0000000000001718
Hawkes EA, Loh Z, Estacio O, Chong G, Ha FJ, Gilbertson M, Grigg A (2018) Routine blood investigations have limited utility in surveillance of aggressive lymphoma in asymptomatic patients in complete remission. Br J Cancer 119(5):546–550. https://doi.org/10.1038/s41416-018-0183-x
Hu Y, Zhao Y, Shi C, Ren P, Wei B, Guo Y, Ma J (2019) A circular RNA from inhibits the proliferation of diffuse large B cell lymphoma by inactivating Wnt/β-catenin signaling via interacting with TET1 and miR-888. Aging (albany NY) 11(19):8068–8084. https://doi.org/10.18632/aging.102122
Kattan MW, Marasco J (2010) What is a real nomogram? Semin Oncol 37(1):23–26. https://doi.org/10.1053/j.seminoncol.2009.12.003
Kim DH, Baek JH, Chae YS, Kim YK, Kim HJ, Park YH, Song HS, Chung JS, Hyun MS, Sohn SK (2007) Absolute lymphocyte counts predicts response to chemotherapy and survival in diffuse large B cell lymphoma. Leukemia 21(10):2227–2230
Kwee TC, Kwee RM, Nievelstein RAJ (2008) Imaging in staging of malignant lymphoma: a systematic review. Blood 111(2):504–516
Li W, Dong S, Wang B, Wang H, Xu C, Zhang K, Li W, Hu Z, Li X, Liu Q, Wu R, Yin C (2021) The construction and development of a clinical prediction model to assess lymph node metastases in osteosarcoma. Front Public Health 9:813625. https://doi.org/10.3389/fpubh.2021.813625
Maurer MJ, Jais J-P, Ghesquières H, Witzig TE, Hong F, Haioun C, Thompson CA, Thieblemont C, Micallef IN, Porrata LF, Ribrag V, Nowakowski GS, Casasnovas O, Bologna S, Morschhauser F, Morrison VA, Peterson BA, Macon WR, Copie-Bergman C, Feldman AL, Syrbu SI, Kurtin PJ, Gascoyne RD, Li H, Allmer C, Kahl BS, Ansell SM, Slager SL, Link BK, Salles G, Habermann TM, Tilly H, Cerhan JR (2016) Personalized risk prediction for event-free survival at 24 months in patients with diffuse large B cell lymphoma. Am J Hematol 91(2):179–184. https://doi.org/10.1002/ajh.24223
Pfreundschuh M, Ho AD, Cavallin-Stahl E, Wolf M, Pettengell R, Vasova I, Belch A, Walewski J, Zinzani P-L, Mingrone W, Kvaloy S, Shpilberg O, Jaeger U, Hansen M, Corrado C, Scheliga A, Loeffler M, Kuhnt E (2008) Prognostic significance of maximum tumour (bulk) diameter in young patients with good-prognosis diffuse large-B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: an exploratory analysis of the MabThera International Trial Group (MInT) study. Lancet Oncol 9(5):435–444. https://doi.org/10.1016/S1470-2045(08)70078-0
Thompson CA, Ghesquieres H, Maurer MJ, Cerhan JR, Biron P, Ansell SM, Chassagne-Clément C, Inwards DJ, Gargi T, Johnston PB, Nicolas-Virelizier E, Macon WR, Peix M, Micallef IN, Sebban C, Nowakowski GS, Porrata LF, Weiner GJ, Witzig TE, Habermann TM, Link BK (2014) Utility of routine post-therapy surveillance imaging in diffuse large B cell lymphoma. J Clin Oncol 32(31):3506–3512. https://doi.org/10.1200/JCO.2014.55.7561
Trotman J, Fournier M, Lamy T, Seymour JF, Sonet A, Janikova A, Shpilberg O, Gyan E, Tilly H, Estell J, Forsyth C, Decaudin D, Fabiani B, Gabarre J, Salles B, Van Den Neste E, Canioni D, Garin E, Fulham M, Vander Borght T, Salles G (2011) Positron emission tomography-computed tomography (PET–CT) after induction therapy is highly predictive of patient outcome in follicular lymphoma: analysis of PET–CT in a subset of PRIMA trial participants. J Clin Oncol 29(23):3194–3200. https://doi.org/10.1200/JCO.2011.35.0736
Vaidya R, Witzig TE (2014) Prognostic factors for diffuse large B cell lymphoma in the R(X)CHOP era. Ann Oncol 25(11):2124–2133. https://doi.org/10.1093/annonc/mdu109
Wang X-L, Wang X-L, He S, Zhai H-L (2015) Association of β2-microglobulin with the prognosis of non-Hodgkin’s lymphoma: a meta analysis. Int J Clin Exp Med 8(3):3992–3999
Wilder RB, Rodriguez MA, Ha CS, Pro B, Hess MA, Cabanillas F, Cox JD (2001) Bulky disease is an adverse prognostic factor in patients treated with chemotherapy comprised of cyclophosphamide, doxorubicin, vincristine, and prednisone with or without radiotherapy for aggressive lymphoma. Cancer 91(12):2440–2446
Wu X, Nerisho S, Dastidar P, Ryymin P, Järvenpää R, Pertovaara H, Eskola H, Kellokumpu-Lehtinen P-L (2013) Comparison of different MRI sequences in lesion detection and early response evaluation of diffuse large B-cell lymphoma – a whole-body MRI and diffusion-weighted imaging study. NMR Biomed 26(9):1186–1194. https://doi.org/10.1002/nbm.2933
Wu X, Pertovaara H, Korkola P, Vornanen M, Järvenpää R, Dastidar P, Eskola H, Kellokumpu-Lehtinen P-L (2014) Early interim PET/CT predicts post-treatment response in diffuse large B cell lymphoma. Acta Oncol 53(8):1093–1099. https://doi.org/10.3109/0284186X.2014.927074
Yang D-H, Min J-J, Song H-C, Jeong YY, Chung W-K, Bae S-Y, Ahn J-S, Kim Y-K, Bom H-S, Chung I-J, Kim H-J, Lee J-J (2011) Prognostic significance of interim 18F-FDG PET/CT after three or four cycles of R-CHOP chemotherapy in the treatment of diffuse large B cell lymphoma. Eur J Cancer (oxf, Engl) 47(9):1312–1318. https://doi.org/10.1016/j.ejca.2010.12.027
Yin Q, Chen L, Li Q, Mi R, Li Y, Wei X, Song Y (2014) Changes of T lymphocyte subpopulation and differential expression pattern of the T-bet and GATA-3 genes in diffuse large B cell lymphoma patients after chemotherapy. Cancer Cell Int 14:85. https://doi.org/10.1186/s12935-014-0085-9
Yoon SB, Lee IS, Lee HN, Kim E, Kim W, Lee HH, Lee B-I, Choi M-G, Jung SE, Choi BO, Park GS, Cho S-G (2016) Role of follow-up endoscopic examination in treatment response assessment for patients with gastric diffuse large B cell lymphoma. Scand J Gastroenterol 51(9):1111–1117. https://doi.org/10.1080/00365521.2016.1177854
Zelenetz AD, Wierda WG, Abramson JS, Advani RH, Andreadis CB, Bartlett N, Bellam N, Byrd JC, Czuczman MS, Fayad LE, Glenn MJ, Gockerman JP, Gordon LI, Harris NL, Hoppe RT, Horwitz SM, Kelsey CR, Kim YH, Krivacic S, LaCasce AS, Nademanee A, Porcu P, Press O, Pro B, Reddy N, Sokol L, Swinnen L, Tsien C, Vose JM, Yahalom J, Zafar N, Dwyer MA, Naganuma M (2013) Non-Hodgkin’s lymphomas, version 1.2013. J Natl Compr Canc Netw 11(3):257–273
Zhang X, Fan W, Hu Y-Y, Li Z-M, Xia Z-J, Lin X-P, Zhang Y-R, Liang P-Y, Li Y-H (2015) Qualitative visual trichotomous assessment improves the value of fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography in predicting the prognosis of diffuse large B cell lymphoma. Chin J Cancer 34(6):264–271. https://doi.org/10.1186/s40880-015-0021-y
Funding
The authors declare that there are no funds and grants.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by PW, KC, JW, NS, and ZN. The first draft of the manuscript was written by PW. WM provided clinical expertise and guidance for study design and oversaw the project. And all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The study was reviewed and approved by the Ethics Committee of the Cancer Hospital Affiliated to Harbin Medical University, and was in line with the purpose of the Declaration of Helsinki. All patients waived the requirement for informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, P., Chen, K., Wang, J. et al. A new nomogram for assessing complete response (CR) in gastric diffuse large B-cell lymphoma (DLBCL) patients after chemotherapy. J Cancer Res Clin Oncol 149, 9757–9765 (2023). https://doi.org/10.1007/s00432-023-04862-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-04862-4