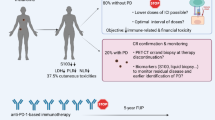
Abstract
Background
PD-1/PD-L1 immune checkpoint inhibitors (ICIs) are widely used in the treatment of metastatic malignancies. Judiciously balancing disease control (DC) against development of immune-related adverse events (irAE) remains a crucial aspect of treatment. The effect of treatment discontinuation after sustained disease control (SDC) is unknown. The purpose of this analysis was to evaluate outcomes of responders to ICI who discontinue treatment after a minimum of 12 months (SDC).
Methods
We retrospectively reviewed the database of the University of New Mexico Comprehensive Cancer Center (UNMCCC) between 2014 and 2021 and identified patients who had received ICI. Patients with metastatic solid tumors who had stopped ICI therapy after achieving SDC [stable disease, partial response, complete response (SD, PR, CR)] were selected and outcomes reviewed from their electronic health records.
Results
We identified 204 patients who were treated with ICI for various solid cancers. Forty-four patients (21.6%) met the criteria, of whom 35 with follow-up data were included in the final analysis; including 11 melanoma, 5 non-small cell lung, 4 head & neck, 8 renal, 4 urothelial, 1 anal, 1 Merkel cell carcinoma, and 1 liposarcoma. Patients were divided into two groups: those who stopped ICI due to an irAE [irAE group, n = 14, median treatment time (MTT), 16.6 mo] and those who stopped due to other reasons (eg completion of 2 years of therapy, n = 20, non-cancer related surgery, n = 1) (non-irAE group, n = 21, MTT, 23.7 mo). Among the irAE group, the most common irAE included pneumonitis, rash, transaminitis, and fatigue. As of data cutoff date, 9 of 14 (64%) patients continued to show SDC. Only 5 of 14 (36%) patients in this group experienced progression of disease (PD), with 1 of 2 patients achieving DC (median follow-up of 19.2 mo after last dose of treatment, range 3–50.2 mo). Among the non-irAE group, 13 of 21 (62%) continued to have SDC. Eight of 21 (38%) experienced PD after stopping treatment, 7 of whom received ICI rechallenge, with 2 of 7 achieving DC (median follow-up of 22.2 mo, range 3.6-54.8 mo). At a median follow-up of 21.3 mo from stopping ICI therapy (range, 3–54.8 mo), 10 patients (71%) from the irAE group and 13 (61.9%) from the non-irAE group are in DC and have not experienced PD.
Conclusions
We demonstrate that 22 (66%) patients experienced SDC, regardless of cancer type or development of irAE. After including patients who were re-challenged with ICI due to PD, 25 (71%) remain in DC. Future prospective malignancy-specific trials are warranted to evaluate optimal treatment duration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immune checkpoint inhibitors (ICIs) have demonstrated favorable response in several metastatic malignancies, including melanoma (“Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma 2022), non-small cell lung cancer (NSCLC) (Herbst et al. 2016), urothelial carcinoma (Sharma et al. 2017), and advanced renal cell carcinoma (RCC) (Tomita et al. 2017). Nivolumab, pembrolizumab, avelumab, and atezolizumab are ICIs that target the programmed cell death protein (PD-1) or ligand (PD-L1) to allow antitumor activity by T cells (Pardoll 2012). Patients who receive treatment with an ICI are generally treated for a maximum of 2 years, until progression of disease (PD), or onset of an immune-related adverse event (irAE) (Brahmer et al. 2015; Rittmeyer et al. 2017).
The optimal treatment duration for ICIs is unclear at this time, and clinicians frequently weigh the risks of developing an irAE against discontinuation of ICI after disease control (DC). During the COVID-19 pandemic, treatment duration uncertainty was further brought into question. The utility of treating patients with additional ICI therapy in stable disease (SD) was weighed against the increased exposure risk to SARS-CoV-2 (Friedlaender et al. 2020). Cancer patients, an already vulnerable patient population, were in a precarious position, especially when considering risks like the harmful synergy of COVID-19 and ICI therapy on immune hyperactivation (Bersanelli 2020).
Previous studies have explored the long-term outcomes of patients treated with ICI and subsequently discontinued, albeit with only one type of cancer evaluated. One study highlights the favorable long-term survival and durable response after ICI therapy was discontinued after a minimum of six months in NSCLC patients without disease progression (Kim et al. 2022). A recent multicenter retrospective study of twenty patients with various advanced solid malignancies demonstrated durable DC after discontinuation of ICI treatment after achieving SD, complete response (CR), or partial response (PR) (Robert et al. 2015). KEYNOTE-001 highlighted CR in patients treated with pembrolizumab for melanoma (Robert et al. 2018; Hamid et al. 2019). Additionally, the utility of rechallenging patients who experienced an irAE to evaluate PR or CR has also been explored (Simonaggio et al. 2019; Santini et al. 2018). In fact, a retrospective cohort study revealed durable responses without fatal events in patients with genitourinary cancers upon rechallenge after moderate-to-severe irAEs (Siddiqui et al. 2021).
Here, we evaluate the long-term outcomes of responders to ICI who stopped treatment after a minimum treatment duration of 12 months and sustained disease control (SDC). In addition, we study the effect of ICI rechallenge in patients that experienced PD.
Methods
Study design and patient population
Utilizing our Electronic Medical Record (EMR), we retrospectively identified patients aged 18 years or older at the University of New Mexico Comprehensive Cancer Center (UNMCCC) who received treatment with an ICI between 2014 and 2021. Data involving the following ICI agents were reviewed: nivolumab, atezolizumab, avelumab, durvalumab, and pembrolizumab. The UNM Health Sciences Center Institutional Review Board approved this study.
Inclusion criteria required patients who: received ICI for a minimum of 12 months prior to discontinuation; were evaluated at the UNMCCC by a medical provider at least once before and after receiving ICI therapy; had an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–3 or Karnofsky Performance Status (KPS) >50%; received standard of care ICI therapy for indications that were FDA-approved at the time of treatment; were diagnosed with metastatic cancer at the time of treatment initiation. Exclusion criteria screened out patients who: had an ECOG PS of 4 and KPS < 50% at the time of ICI therapy initiation; were scheduled to initiate therapy but never received treatment; were diagnosed with non-melanomatous skin cancer. The primary endpoint was overall survival (OS) in patients receiving ICI. In addition, OS was compared between irAE and non-irAE groups.
Treatment and assessments
Protected spreadsheets were generated to identify all patients who received any ICI therapy during the aforementioned time period. These lists were subsequently reviewed to filter patients that received treatment with ICI for 12 months or more. The following clinicopathological characteristics were collected: age at diagnosis, race, sex, type of cancer based on tissue diagnosis, histology, date of diagnosis and metastasis, stage at initial diagnosis, metastasis location, resection of metastasis (if applicable), start date and first line systemic treatment, ICI start and end date, reason for stopping ICI, type of adverse event (AE, if any), radiographic status at end date, last UNMCCC follow-up, radiographic status at last follow-up, death date (if applicable). Eligible patients were also screened to determine if they were rechallenged with ICI therapy due to progression of disease or patient preference to resume treatment. Online searches were performed to determine the date of death for deceased patients.
Patients were identified as having experienced an irAE based on provider documentation. irAEs included hypohysitis; fatigue; colitis; hepatic toxicity; pancreatitis, arthritis; myositis; ocular toxicity; pulmonary toxicity and renal toxicity. For comparison, patients were stratified into irAE and non-irAE groups. PD, stable disease (SD), and complete resolution (CR) were assessed using clinical judgment and, often, in combination with Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 and iRECIST criteria (Eisenhauer et al. 2009; Seymour et al. 2017). Patients underwent guideline-directed serial monitoring, predominantly in three- to four-month intervals, of their respective malignancies with computed tomography (CT) or positron emission tomography (PET) scans. Development of symptoms concerning for PD resulted in prompt repeat imaging for further evaluation. Data cutoff occurred on December 28, 2021.
Upon completion of data collection, chart review was conducted for patients in the irAE group to determine: the grade of AE based on Common Terminology Criteria for Adverse Events (CTCAE) v5.0 in respective cases and duration of these adverse effects.
Statistical analysis
Numbers of potential and eligible patients were summarized with counts and percentages, both overall and within key subgroups. One of these subgroups was defined by whether they had experienced an irAE that warranted discontinuation of ICI. These tabulations were only obtained among those with complete follow-up, defined as having been seen at UNMCCC within six months of data access. We assessed the heterogeneity in OS among cancer types with a frailty term in a Cox proportional hazards model. Kaplan-Meier survival curves were obtained, both overall and within subsets of patients defined by whether they had experienced an irAE, and median OS times in years were estimated, beginning at the one-year time point following initiation of ICI treatment. Differences in OS were further estimated between irAE groups using Cox proportional hazards models.
Results
Patients
Of the 204 patients who received at least one dose of ICI treatment at UNMCC between 2014 and 2021, 44 (21.6%) received therapy for a minimum of 12 months. The remaining 160 patients (78.4%) were treated over a shorter timeframe. Nine patients were lost to follow-up. Summary statistics were obtained for groups according to irAE among the 35 patients who were not lost to follow-up. Cancers represented in this cohort were: 11 patients with melanoma; 5 NSCLC; 3 head and neck; 8 RCC; 4 urothelial; 1 anal squamous cell carcinoma; 1 Merkel cell carcinoma; 1 neuroendocrine tumor (NET); and 1 liposarcoma. ICI MTT was 22 months, with a 21.3 month median follow-up after the last treatment dose. The reasons to discontinue treatment were patient preference upon achieving SD after MTT of 23.7 months (18 patients; 46%), irAE with SD after MTT 16.6 months (14 patients; 40%), CR (2 patients; 6%), non-cancer related surgery with SD (1 patient; 3%).
Clinical status
In the non-irAE group, patients who may have experienced an irAE but stopped ICI due to other reasons, (21 patients, 60%), reasons for stopping treatment were completion of 2 years of ICI therapy (20 patients) and non-cancer related surgery (1 patient). MTT was 23.7 months with a median follow-up of 22.2 months (range, 3.6–54.8 months). Thirteen patients (61.9%) in this group continue to remain in SDC while 8 patients (38%) have experienced PD since stopping treatment.
Five patients with RCC are in the non-irAE group, as are six melanoma patients; three patients with urothelial carcinoma; two head & neck; three NSCLC; one liposarcoma; one anal squamous cell carcinoma (Table 1). Three patients with RCC, four with melanoma, and one with urothelial carcinoma have maintained SDC. Two patients with NSCLC are in SDC, while one has PD without rechallenge.
Among the 14 patients (40%) in the irAE group, the most commonly observed irAEs were: skin related e.g. rash, bullous pemphigoid (3 patients, 21%); acute liver injury with elevated liver markers (2 patients, 14%); pneumonitis (2 patients); and fatigue/weakness (2 patients). MTT was 16.6 months with median follow-up of 19.2 months. 9 patients (64.3%) in the irAE group remain in SDC while 5 (36%) experienced PD (Fig. 1 and Table 2).
Six out of these 14 patients experienced adverse effects for longer than 3 months.
Two patients (14%) had grade 3 irAE and one of them was managed with systemic steroids and the other one with topical steroids alone. Nine patients (64.2%) had grade 2 irAE with eight of them required systemic steroids. None of them experienced life-threatening irAE.
The number of patients in the irAE group by cancer type are as follows: three RCC; five melanoma; one urothelial; two NSCLC; one head and neck; one NET; one Merkel cell carcinoma. Among RCC patients, one patient each had the outcome of: maintaining SDC, PD despite rechallenge, and SD after rechallenge. Three melanoma patients are in SDC, while two have experienced PD without rechallenge.
Thirteen patients were treated with an ICI exclusively, or as part of, their first line treatment. 10 (77%) of them have maintained SDC throughout follow-up. In comparison, 12 of 22 patients (55%) that received other forms of first line therapy (e.g. chemotherapy, tyrosine kinase inhibitor) remained in SDC at the data cutoff date.
Rechallenge: safety and efficacy
Eight patients in the non-irAE group experienced PD since stopping treatment. With the exception of one patient who declined additional treatment, the remaining seven patients were rechallenged with ICI therapy (Table 3). One patient had initially received nivolumab for RCC and was rechallenged with pembrolizumab; the remaining patients received the ICI they were originally treated with. Two of the patients that were rechallenged in this cohort once again achieved DC. With rechallenge therapy, no patient experienced irAE that warranted discontinuation of ICI until data cutoff.
Upon rechallenge, two patients with RCC (n = 8) experienced PD while another two achieved SD again. Two melanoma patients (n = 11) and two urothelial carcinoma patients (n = 4) had PD despite rechallenge.
Of the five patients in the irAE group that experienced PD, two were rechallenged with ICI therapy. Choosing not to restart treatment in the remaining three patients centered around provider judgment regarding the risk/benefit ratio. Both patients who were rechallenged had RCC as their primary cancer and received nivolumab initially. One patient continued with nivolumab and achieved subsequent DC, while the other patient was rechallenged with pembrolizumab and remains with PD. Neither patient experienced any form of irAE due to rechallenge.
Overall survival by cancer type
In this series of patients receiving ICI treatment for at least one year, median OS following the first year of ICI treatment was 5.17 years (the lower 95% Confidence Limit [CL] was 3.1 years and the upper 95% CL was not estimable) (Fig. 2). We were unable to conclude that there was significant heterogeneity in OS among cancer types (p = 0.97). Although there was an increased hazard of all-cause mortality in the irAE group (hazard ratio [95% Confidence Interval] = 1.87 [0.65–5.42]), OS did not differ significantly between groups defined by the occurrence of an irAE (p = 0.25) (Fig. 3).
Kaplan-Meier Survival Curve for patients receiving ICI treatment for at least one year. Follow-up time commenced at the one-year time point after initiation of the first round of ICI treatment. Dashed lines illustrate 95% confidence intervals, and censored observations are denoted with “+”. The median survival time for these patients was 5.17 years, with a lower CL of 3.1. The upper CL was not estimable
Kaplan-Meier Survival Curve for patients receiving ICI treatment for at least one year. Follow-up time commenced at the one-year time point after initiation of the first round of ICI treatment. Median survival was 5.17 years for the “no irAE” group, with a lower CL of 5.17 years; the upper CL was non-estimable. Median survival was 3.10 years for the “irAE” group, with a lower CL of 1.59 years; the upper CL was non-estimable
Discussion
Our study sought to understand long-term outcomes in patients who responded to ICI therapy after treatment for a minimum of 12 months and SDC. After combining outcomes of the irAE and non-irAE groups, 22 patients (63%) experienced SDC (median follow-up, 21.3 months). This result is comparable to the KEYNOTE-001 trial, in which 655 patients with advanced melanoma achieved a disease control rate [DCR (CR, PR, and SD)] of 65%13. While all patients in KEYNOTE-001 had a diagnosis of advanced melanoma, only half of the patients in our study (eleven) carried this diagnosis. In our study, 13 (61.9%) patients in the non-irAE and 9 (64.3%) patients in the irAE group remain in SDC, indicating no appreciable difference between the two groups. Patients are generally tolerating ICI therapy well and experiencing sustained responses.
Of the eight patients who experienced PD in the non-irAE group, seven (87.5%) were rechallenged with ICI therapy. In comparison, only two of the five (40%) patients in the irAE group with PD were rechallenged. Providers unsurprisingly are more likely to consider other modalities of treatment once patients experience irAEs during ICI therapy. Other factors affecting the decision to rechallenge include: patients declining additional therapy and the aggressive nature of the cancer warranting hospice treatment. Three of nine patients who were rechallenged successfully achieved DC once again. One study offers guidelines regarding the decision to rechallenge a patient with ICI therapy after irAE: avoid retreatment in patients who were hospitalized for irAE or had achieved PR or CR prior to irAE due to similar survival outcomes between patients who were retreated or had ICI therapy discontinued (Santini et al. 2018). Overall, the literature suggests to approach rechallenge with close monitoring and consideration of usefulness (Simonaggio et al. 2019; Ravi et al. 2020). Rates of irAE recurrence have been as high as 38.9% (Dolladille et al. 2020; Allouchery et al. 2020). This practice aligns with our observation of patients who experienced irAE with SD on radiographic imaging at the time of stopping treatment. Nine of these patients have remained in SDC and only one patient with PD achieved DC after being rechallenged with ICI.
Common irAEs include colitis, rash, hepatitis, and thyroiditis (Postow et al. 2018). The relationship between tumor response to immunotherapy and immune mediated toxicity can be paradoxical; in fact, some cases suggest that cutaneous adverse events may predict response to treatment (Hua et al. 2016; Sanlorenzo et al. 2015). Patients in our study received appropriate treatment with steroids based on National Comprehensive Cancer Network (NCCN) guidelines. Systemic steroids are likely to limit some anti-tumor responses and trials (NCT04305145) to evaluate for alternative treatment strategies may guide the maintenance of durable response to immunotherapy.
If shorter duration of treatment demonstrates noninferior long-term patient outcomes, patients would benefit from reduced financial and societal burdens (Marron et al. 2021). If patients can demonstrate SD after one year of treatment, those with transportation barriers or long travel times would likely experience improved quality of life without compromising care (Thomas et al. 2015). The likelihood of patients experiencing an adverse reaction, irAE or otherwise, from unnecessary medications would substantially decrease (Carroll 2017). In addition, hospital resources could be reallocated to patients who are yet to gain from the treatment potential of ICI therapy.
A highlighted strength of this study is that several cancer types were included. Most of the current literature examining the response of ICI tends to focus on a select type of cancer (e.g., melanoma, NSCLC) (Robert et al. 2015; Robert et al. 2018). The diversity in primary pathology allows for direct comparison of overall survival and response to therapy. For example, NSCLC represented the lowest median OS (43.5 months) while RCC had the highest (84.7 months). It is possible that patients with RCC have a stronger response to ICI compared to other malignant solid tumors. Additionally, two of the three patients who were successfully rechallenged with ICI therapy had an RCC primary tumor.
Limitations of our study are that it was conducted in a retrospective manner. Each cancer type cohort had a limited number of patients, hindering our ability to conduct a robust data analysis. The lack of significant heterogeneity in OS among cancer types (p = 0.97) may be attributed to the small number of patients for each cancer type. Regardless of being in the irAE or non-irAE group, none of the rechallenged patients experienced irAE. In comparison, one study highlights that 20 of 38 patients (52.6%) rechallenged with ICI experienced a recurrent or new irAE (Santini et al. 2018). Another study found that irAE recurred in 55% of rechallenged cases (Kim et al. 2022). Our differing results may be attributed to limited sample size and/or lower grade adverse events, including irAEs, less likely to be documented if they did not warrant a healthcare encounter. Additionally, in the context of both the study population and patients under routine clinical care, RECIST and iRECIST criteria are not utilized in a standardized manner to evaluate the endpoints of PD, SD, and CR. Adopting a uniform criteria-based approach, despite the clinical barriers (i.e., limited time), would allot additional credibility to the endpoints.
Malignancy-specific trials such as IMAGINE (NCT04637594), STOP-GAP (NCT02821013), PET-STOP (NCT04462406), and DANTE (NCT05085028) will be helpful in determining the appropriate length of ICI treatment with the aim of achieving DC while avoiding the harmful effects of overtreating, such as irAE. Additionally, several works have identified the impact that B cells, total tumor-infiltrating lymphocytes, and tertiary lymphoid structures have on ICI therapy (Budczies et al. 2021; Fridman et al. 2022; Helmink et al. 2020). Utilizing these predictive biomarkers may guide clinicians in determining how individuals might respond to treatment.
Conclusion
A majority of patients treated with ICI therapy continue to experience sustained disease control almost two years after stopping treatment. Some who are rechallenged after PD once again achieve disease control. Given that a shorter treatment duration may yield noninferior long term patient outcomes, reevaluating guideline recommendations for treatment length could improve patient care and reduce cost burden.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Allouchery M, Lombard T, Martin M et al (2020) Safety of immune checkpoint inhibitor rechallenge after discontinuation for grade ≥2 immune-related adverse events in patients with cancer. J Immunother Cancer 8(2):e001622. https://doi.org/10.1136/jitc-2020-001622
Bersanelli M (2020) Controversies about COVID-19 and anticancer treatment with immune checkpoint inhibitors. Immunotherapy 12(5):269–273. https://doi.org/10.2217/imt-2020-0067
Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, Antonia S et al (2015) Nivolumab versus Docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med 373(2):123–35. https://doi.org/10.1056/NEJMoa1504627
Budczies J, Kirchner M, Kluck K et al (2021) A gene expression signature associated with B cells predicts benefit from immune checkpoint blockade in lung adenocarcinoma. Oncoimmunology 10(1):1860586. https://doi.org/10.1080/2162402X.2020.1860586
Carroll AE (2017) The high costs of unnecessary care. JAMA 318(18):1748–1749. https://doi.org/10.1001/jama.2017.16193
Dolladille C, Ederhy S, Sassier M et al (2020) Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol 6(6):865–871. https://doi.org/10.1001/jamaoncol.2020.0726
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J et al (2009) New response evaluation criteria in solid tumours: Revised RECIST Guideline (Version 1.1). Eur J Cancer 45(2):228–47. https://doi.org/10.1016/j.ejca.2008.10.026
Fridman WH, Meylan M, Petitprez F, Sun CM, Italiano A, Sautès-Fridman C (2022) B cells and tertiary lymphoid structures as determinants of tumour immune contexture and clinical outcome. Nat Rev Clin Oncol 19(7):441–457. https://doi.org/10.1038/s41571-022-00619-z
Friedlaender A, Kim C, Addeo A (2020) Rethinking the optimal duration of immune checkpoint inhibitors in non-small cell lung cancer throughout the COVID-19 pandemic. Front Oncol. https://doi.org/10.3389/fonc.2020.00862
Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD et al (2019) Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol 30(4):582–88. https://doi.org/10.1093/annonc/mdz011
Helmink BA, Reddy SM, Gao J et al (2020) B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577(7791):549–555. https://doi.org/10.1038/s41586-019-1922-8
Herbst RS, Baas Paul, Kim DW, Felip E, Pérez-Gracia JL, Han JYoun, Molina J et al (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-Positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387(10027):1540–50. https://doi.org/10.1016/S0140-6736(15)01281-7
Hua C, Boussemart L, Mateus C et al (2016) Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol 152(1):45–51. https://doi.org/10.1001/jamadermatol.2015.2707
Kim H, Kim DW, Kim M et al (2022) Long-term outcomes in patients with advanced and/or metastatic non–small cell lung cancer who completed 2 years of immune checkpoint inhibitors or achieved a durable response after discontinuation without disease progression: Multicenter, real-world data (KCSG LU20-11). Cancer 128(4):778–787. https://doi.org/10.1002/cncr.33984
Marron TU, Ryan AE, Reddy SM et al (2021) Considerations for treatment duration in responders to immune checkpoint inhibitors. J Immunother Cancer 9(3):e001901. https://doi.org/10.1136/jitc-2020-001901
“Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma | NEJM.” Accessed March 4, 2022. https://www-nejm-org.libproxy.unm.edu/doi/full/https://doi.org/10.1056/nejmoa1709684.
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12(4):252–64. https://doi.org/10.1038/nrc3239
Postow MA, Sidlow R, Hellmann MD (2018) Immune-related adverse events associated with immune checkpoint blockade. N Eng J Med 378(2):158–68. https://doi.org/10.1056/NEJMra1703481
Ravi P, Mantia C, Su C et al (2020) Evaluation of the safety and efficacy of immunotherapy rechallenge in patients with renal cell carcinoma. JAMA Oncol 6(10):1606–1610. https://doi.org/10.1001/jamaoncol.2020.2169
Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM et al (2017) Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389(10066):255–65. https://doi.org/10.1016/S0140-6736(16)32517-X
Robert C, Schachter J, Long GV et al (2015) Pembrolizumab versus Ipilimumab in advanced melanoma. N Engl J Med 372:2521–32
Robert C, Ribas A, Hamid O, Daud A, Wolchok JD, Joshua AM, Hwu WJ et al (2018) Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J Clin Oncol 36(17):1668–74. https://doi.org/10.1200/JCO.2017.75.6270
Sanlorenzo M, Vujic I, Daud A et al (2015) Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol 151(11):1206–1212. https://doi.org/10.1001/jamadermatol.2015.1916
Santini FC, Rizvi H, Plodkowski AJ, Ni A, Lacouture ME, Gambarin-Gelwan M, Wilkins O et al (2018) Safety and efficacy of re-treating with immunotherapy after immune-related adverse events in patients with NSCLC. Cancer Immunol Res 6(9):1093–99. https://doi.org/10.1158/2326-6066.CIR-17-0755
Seymour L, Bogaerts J, Perrone A et al (2017) iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 18(3):e143–e152. https://doi.org/10.1016/S1470-2045(17)30074-8
Sharma P, Retz M, Siefker-Radtke A, Baron Ari, Necchi A, Bedke J, Plimack ER et al (2017) Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A Multicentre, Single-Arm, Phase 2 Trial. Lancet Oncol 18(3):312–22. https://doi.org/10.1016/S1470-2045(17)30065-7
Siddiqui BA, Gheeya JS, Goswamy R et al (2021) Durable responses in patients with genitourinary cancers following immune checkpoint therapy rechallenge after moderate-to-severe immune-related adverse events. J Immunother Cancer 9(7):e002850. https://doi.org/10.1136/jitc-2021-002850
Simonaggio A, Michot JM, Voisin AL, Le Pavec J, Collins M, Lallart A, Cengizalp G et al (2019) Evaluation of readministration of immune checkpoint inhibitors after immune-related adverse events in patients with cancer. JAMA Oncol 5(9):1310. https://doi.org/10.1001/jamaoncol.2019.1022
Thomas AA, Gallagher P, O’Céilleachair A, Pearce A, Sharp L, Molcho M (2015) Distance from treating hospital and colorectal cancer survivors’ quality of life: a gendered analysis. Support Care Cancer 23(3):741–751. https://doi.org/10.1007/s00520-014-2407-9
Tomita Y, Fukasawa S, Shinohara N, Kitamura H, Oya M, Eto M, Tanabe K et al (2017) Nivolumab versus Everolimus in advanced renal cell carcinoma: japanese subgroup analysis from the checkmate 025 study. Jpn J Clin Oncol 47(7):639–46. https://doi.org/10.1093/jjco/hyx049
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
HS: Conceptualization, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing; KRM: Conceptualization, Investigation, Resources, Writing – review & editing; VSP: Conceptualization, Data curation, Formal analysis and investigation, Writing – original draft, Writing – review & editing; EY: Conceptualization, Writing – review & editing; OG: Conceptualization, Writing – review & editing; AK: Conceptualization, Writing – review & editing; NHS: Conceptualization, Methodology, Formal analysis and investigation, Resources, Writing – original draft, Writing – review & editing, Visualization, Supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The UNM Health Sciences Center Institutional Review Board approved this study.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
The authors affirm that human research participants provided informed consent for publication of deidentified data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, H., Moturi, K.R., Pankratz, V.S. et al. Outcomes of responders to PD-1/PD-L1 inhibitors who discontinue therapy after sustained disease control. J Cancer Res Clin Oncol 149, 8673–8680 (2023). https://doi.org/10.1007/s00432-023-04812-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-04812-0