Abstract
Introduction
In RCC, systematic procedures such as surgery, chemo-radiation therapy, and application of target-based inhibitors increase the risk of several comorbidities such as chronic kidney disease, hemorrhage, and cardiac arrest that may increase the mortality rate. Even though immune-based checkpoint inhibitor therapies have an overall good response rate, it is restricted to only 30–40% of patients. Hence, an in-depth study of tumor pathophysiology in RCC is needed to identify the new therapeutic target. In RCC, persisted hypoxia is an essential phenomenon for tumor growth and progression. KCMF1 is a newly identified ubiquitin ligase whose domain interacts with destabilized proteins and reprogrammed the ubiquitin coding for lysosome-mediated degradation and autophagy under hypoxic conditions/oxidative stress and maintaining cellular homeostasis. But in RCC, the functional role of KCMF1 remains undefined to date.
Method
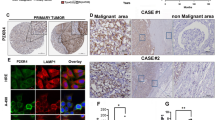
We determined KCMF1 and its associated proteins RAD6 and UBR4 expression and their co-localization using confocal microscopy in tumor and non-tumor tissues samples. Further, immunofluorescence staining was performed to determine autophagy (LC3B, p62), hypoxia-inducible factor (HIF-1A) and ion channel markers (Kv1.3, KCNN4) in RCC patients (n−10). Inductively coupled plasma mass spectrophotometry (ICPMS) was performed to estimate the concentration of potassium (K+), sodium (Na+) and Zinc (zn2+) in tumor and non-tumor cells of RCC patients (n−20). Lastly, images were analyzed using ZEN3.1, and ImageJ software.
Result and conclusion
We observed a discrepancy in the formation of ubiquitin ligase, autophagosome via KCMF1, and ionic concentration in tumor cells, which might be one of the possible factors for cancer evolution. KCMF1-associated ubiquitin ligase system could be considered as a novel therapeutic target for RCC in the future.




Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are not publicly available but are available from the corresponding author upon reasonable request.
References
Abraham GP, Cherian T, Mahadevan P, Avinash TS, George D, Manuel E (2016) Detailed study of survival of patients with renal cell carcinoma in India. Indian J Cancer 53(4):572–574
Ashton-Beaucage D, Lemieux C, Udell CM, Sahmi M, Rochette S, Therrien M (2016) The deubiquitinase USP47 Stabilizes MAPK by counteracting the function of the N-end rule ligase POE/UBR4 in drosophila. PLOS Biol 14(8):e1002539
Beilke S, Oswald F, Genze F, Wirth T, Adler G, Wagner M (2010) The zinc-finger protein KCMF1 is overexpressed during pancreatic cancer development and downregulation of KCMF1 inhibits pancreatic cancer development in mice. Oncogene 29(28):4058–4067
Capitanio U, Montorsi F (2016) Renal cancer. Lancet (london, England) 387(10021):894–906
Corn PG (2007) Role of the ubiquitin proteasome system in renal cell carcinoma. BMC Biochem 8(Suppl 1):S4
Esagian SM, Ziogas IA, Kosmidis D, Hossain MD, Tannir NM, Msaouel P (2021) Long-term survival outcomes of cytoreductive nephrectomy combined with targeted therapy for metastatic renal cell carcinoma: a systematic review and individual patient data meta-analysis. Cancers (basel) 13(4):1–20
Heo AJ et al (2021) The N-terminal cysteine is a dual sensor of oxygen and oxidative stress. Proc Natl Acad Sci 118(50):e2107993118
Hoffmann EK, Lambert IH (2014) Ion channels and transporters in the development of drug resistance in cancer cells. Philos Trans R Soc Lond B Biol Sci 369:20130109
Hong JH et al (2015) KCMF1 (potassium channel modulatory factor 1) Links RAD6 to UBR4 (ubiquitin N-recognin domain-containing E3 ligase 4) and lysosome-mediated degradation. Mol Cell Proteomics 14(3):674–685
Iyer NV et al (1998) Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev 12(2):149
“KCMF1 (potassium channel modulatory factor 1).” [Online]. Available: http://atlasgeneticsoncology.org/Genes/GC_KCMF1.html. Accessed 4 May 2019
Li Z, Stuart RO, Eraly SA, Gittes G, Beier DR, Nigam SK (2003) Debt91, a putative zinc finger protein differentially expressed during epithelial morphogenesis. Biochem Biophys Res Commun 306(3):623–628
Liu X, Zurlo G, Zhang Q (2020) The roles of cullin-2 E3 ubiquitin ligase complex in cancer. Adv Exp Med Biol 1217:173–186
Méjean A et al (2018) Sunitinib alone or after nephrectomy in metastatic renal-cell carcinoma. N Engl J Med 379(5):417–427
Nadia B, Dominique C, Alexandre C, Kevin C, Sandra T (2020) Targeting lysosome function causes selective cytotoxicity in VHL-inactivated renal cell carcinomas. Carcinogenesis 41(6):828–840
Qu L et al (2016) Exosome-transmitted lncARSR promotes sunitinib resistance in renal cancer by acting as a competing endogenous RNA. Cancer Cell 29(5):653–668
Ross K, Jones RJ (2017) Immune checkpoint inhibitors in renal cell carcinoma. Clin Sci (lond) 131(21):2627–2642
Sung H et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Swami U, Nussenzveig RH, Haaland B, Agarwal N (2019) Revisiting AJCC TNM staging for renal cell carcinoma: quest for improvement. Ann Transl Med 7(Suppl 1):S18–S18
Tan Z et al (2013) Regulation of cell proliferation and migration in gallbladder cancer by zinc finger X-chromosomal protein. Gene 528(2):261–266
Vodnala SK et al (2019) T cell stemness and dysfunction in tumors are triggered by a common mechanism. Science 363(6434):eaau0135
Yip SM et al (2018) Checkpoint inhibitors in patients with metastatic renal cell carcinoma: results from the international metastatic renal cell carcinoma database consortium. Cancer 124(18):3677–3683
Acknowledgements
We are grateful to Centralized Core Research Facility (CCRF) for providing confocal microscopy instrument facility to capture and analysis of the images using ZEN software.
Funding
This study received funding from the Council of Scientific & Industrial Research (CSIR-009/006(0480)/2018 EMR-I) (Project code C-2097).
Author information
Authors and Affiliations
Contributions
AS and AS designed the project and planned the experiments. AS performed all the experiments and generated the data. SDC helped in confocal microscopy and interpretation of the data. PS provides patient samples and clinical data. VVS and SNS contribute to the standardization of ICP-MS. AS and AS prepared the manuscript. All the authors provided critical review of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval and consent to participate
Human sample was collected and used for experiments according to AIIMS ethics Committee guidelines (India). Approved human ethics file number is IECPG-413/27.06.2019.
Consent for publication
All the authors gave their consent for publication and review of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, A., Choudhury, S.D., Singh, P. et al. KCMF1 regulates autophagy and ion channels’ function in renal cell carcinoma: a future therapeutic target. J Cancer Res Clin Oncol 149, 5617–5626 (2023). https://doi.org/10.1007/s00432-022-04507-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-04507-y