Abstract
Purpose
The efficacy and safety of nimotuzumab (NTZ) added to concurrent chemoradiotherapy (CCRT) were investigated in patients with stage III–IVa nasopharyngeal carcinoma (NPC).
Methods
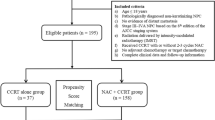
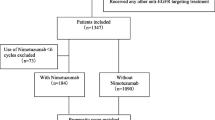
Patients with stage III–IVa NPC treated with CCRT, with or without NTZ, were screened between January 2015 and December 2017. We compared patients’ overall survival (OS), progression-free survival (PFS), locoregional recurrence-free survival (LRFS), and distant metastasis-free survival (DMFS) between different therapeutic regimens. Propensity score matching (PSM) was applied to reduce the selection bias. Nomogram models were developed to predict the survival of CCRT with or without NTZ.
Results
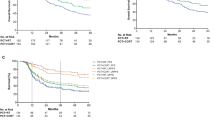
Four hundred and twenty-six patients were included after PSM, with 213 patients in each regimen. Compared with NPC patients receiving CCRT alone, patients who received NTZ plus CCRT treatment had significantly better OS (5 year OS, 76.1 vs. 72.3%, P = 0.004), PFS (5 year PFS, 73.2 vs. 69.0%, P = 0.002), and LRFS (5 year LRFS, 73.2 vs. 69.0%, P = 0.028). A multivariate Cox regression analysis demonstrated that, compared with receiving CCRT alone, NTZ plus CCRT was an independently positive factor for OS, PFS, and LRFS. No significant difference was observed in the major toxicities between the two treatments (all P > 0.05). In addition, the nomogram presented good accuracy for predicting the prognosis of NPC patients.
Conclusion
CCRT combined with NTZ presented favorable clinical outcomes for stage III–IVa NPC patients with good tolerance and similar toxicity compared to CCRT alone. A prospective, randomized clinical trial is essential to validate the current findings.










Similar content being viewed by others
Data availability
The datasets for the current study are available on request to the corresponding authors.
Abbreviations
- CCRT:
-
Concurrent chemoradiotherapy
- NTZ:
-
Nimotuzumab
- NPC:
-
Nasopharyngeal carcinoma
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- LRFS:
-
Locoregional recurrence-free survival
- DMFS:
-
Distant metastasis-free survival
- PSM:
-
Propensity score matching
- CSCO:
-
Chinese Society of Clinical Oncology
- IMRT:
-
Intensity-modulated radiotherapy
- EGFR:
-
Epidermal growth factor receptor
- CTX:
-
Cetuximab
- CT:
-
Computed tomography
- GTV:
-
Gross tumor volume
- GTVnx:
-
Gross tumor volume of nasopharynx
- GTVnd:
-
Gross tumor volume of cervical lymph node
- CTV:
-
Clinical tumor volume
- BMI:
-
Body mass index
- LDH:
-
Lactate dehydrogenase
- CRP:
-
C reactive protein
- C-index:
-
Concordance index
- PFS:
-
Progression-free survival
- IC:
-
Induction chemotherapy
References
Brigette BYM, Edwin PH, Anthony TCC (2008) Systemic approach to improving treatment outcome in nasopharyngeal carcinoma: current and future directions. Cancer Sci 99:1311–1318. https://doi.org/10.1111/j.1349-7006.2008.00836.x
Cristina M, Ernest M, Kathryn A, Josefa L, William H, Rolando P (1997) Humanization of a mouse monoclonal antibody that blocks the epidermal growth factor receptor: recovery of antagonistic activity. Immunotechnology 3:71–81
Crombet-Ramos T, Rak J, Perez R, Viloria-Petit A (2002) Antiproliferative, antiangiogenic and proapoptotic activity of h-R3: a humanized anti-EGFR antibody. Int J Cancer 101:567–575. https://doi.org/10.1002/ijc.10647
Dong-Seok H, Yun-Suhk S, Seong-Ho K, Hyuk-Joon L, Yunhee C, Susumu A, Takeshi S, Byung-Joo P, Woo-Ho K, Yang H-K (2012) Nomogram predicting long-term survival after d2 gastrectomy for gastric cancer. J Clin Oncol 30:3834–3840. https://doi.org/10.1200/JCO.2012.41.8343
Dorsey K, Agulnik M (2013) Promising new molecular targeted therapies in head and neck cancer. Drugs 73:315–325. https://doi.org/10.1007/s40265-013-0025-3
Fangzheng W, Chuner J, Zhiming Y, Tongxin L, Fengqin Y, Lei W, Bin L, Fujun H, Ming C, Weifeng Q, Zhenfu F (2018a) Long-term use of nimotuzumab in combination with intensity-modulated radiotherapy and chemotherapy in the treatment of locoregionally advanced nasopharyngeal carcinoma: experience of a single institution. Oncol Res Featuring Preclin Clin Cancer Ther 26:277–287. https://doi.org/10.3727/096504017x15079846743590
Fangzheng W, Quanquan S, Chuner J, Tongxin L, Aizawa R, Sakamoto M, Yuezhen W, Zhenfu F, Chen M (2018b) Additional induction chemotherapy to concurrent chemotherapy and intensity-modulated radiotherapy with or without nimotuzumab in first-line treatment for locoregionally advanced nasopharyngeal carcinoma: a propensity score matched analysis. J Cancer 9:594–603. https://doi.org/10.7150/jca.20461
Fortunato C, Giampaolo T (2001) A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res 7:2958–2970
Jian Ji P, Wai Tong N, Jing Feng Z, Lucy L. K. C, Brian O’s, Shao Jun L, Henry C. K. S, Yun Bin C, Horace C.W. C, Qiao Juan G, Wai Kuen K, You Ping X, Xu W, Quynh Thu L, Christine M. G, A. Dimitrios C, Randal S. W, Jatin P. S,Anne W. M. L (2016) Proposal for the 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer 122:546–558. https://doi.org/10.1002/cncr.29795.
Jian-Feng H, Fu-Zheng Z, Qin-Zhou Z, Le-Yuan Z, Bo Y, Jian-Jun C, Jia-Hua Y, Hao-Wen Z, Xiao-Peng Y, Guo-Mei T, Fen-Ju L, Charlie MC-M (2017) Induction chemotherapy followed by concurrent chemoradiation and nimotuzumab for locoregionally advanced nasopharyngeal carcinoma: preliminary results from a phase II clinical trial. Oncotarget 8:2754–2465. https://doi.org/10.18632/oncotarget.13899
Jianpei L, Shulin C, Songguo P, Yijun L, Shan X, Xia H, Chen H (2018) Prognostic nomogram for patients with Nasopharyngeal Carcinoma incorporating hematological biomarkers and clinical characteristics. Int J of Biol Sci 14:549–556. https://doi.org/10.7150/ijbs.24374
Jian-Yong S, Yu Z, Yu-Hong L, Hong-Yi G, Hui-Xia F, Qiu-Liang W, Nian-Ji C, Gang C, Bin H, Li-Fu H, Ingemar E, Yi-Xin Z (2004) Comparison of Epstein-Barr virus DNA level in plasma, peripheral blood cell and tumor tissue in nasopharyngeal carcinoma. Anticancer Res 24:4059–4066
Ji-Jin Y, Guan-Qun Z, Si-Yang W, Lu-Lu Z, Tian-Sheng G, Ying-Lin P, Wayne RL, Ying S, Wang-Jian Z, Fan Z (2018) Comparing treatment outcomes of concurrent chemoradiotherapy with or without nimotuzumab in patients with locoregionally advanced nasopharyngeal carcinoma. Cancer Biol Ther 19:1102–1107. https://doi.org/10.1080/15384047.2018.1491501
Karakiewicz PI, Briganti A, Chun FK, Trinh QD, Perrotte P, Ficarra V, Cindolo L, De La Taille A, Tostain J, Mulders PF, Salomon L, Zigeuner R, Prayer-Galetti T, Chautard D, Valeri A, Lechevallier E, Descotes JL, Lang H, Mejean A, Patard JJ (2007) Multi-institutional validation of a new renal cancer-specific survival nomogram. J Clin Oncol 25:1316–1322. https://doi.org/10.1200/JCO.2006.06.1218
Kattan MW, Scardino PT (2007) Evidence for the usefulness of nomograms. Nat Clin Pract Urol 4:638–639. https://doi.org/10.1038/ncpuro0968
Lee AW, Sze WM, Au JS, Leung SF, Leung TW, Chua DT, Zee BC, Law SC, Teo PM, Tung SY, Kwong DL, Lau WH (2005) Treatment results for nasopharyngeal carcinoma in the modern era: the Hong Kong experience. Int J Radiat Oncol Biol Phys 61:1107–1116. https://doi.org/10.1016/j.ijrobp.2004.07.702
Lei C, Chao-Su H, Xiao-Zhong Chen, Guo-Qing H, Zhi-Bin C, Yan S, Wei-Xiong L, Yuan-Yuan C, Fang-Yun X, Shao-Bo L, Yong C, Ting-Ting X, Bin L, Guo-Xian L, Si-Yang W, Bao-Min Z, Ying G, Ying S, Yan-Ping M, Ling-Long T, Yu-Ming C, Meng-Zhong L,M J(2012) Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. The Lancet Oncology 13:163-171. https://doi.org/10.1016/s1470-2045(11)70320-5
Li HM, Li P, Qian YJ, Wu X, Xie L, Wang F, Zhang H, Liu L (2016) A retrospective paired study: efficacy and toxicity of nimotuzumab versus cisplatin concurrent with radiotherapy in nasopharyngeal carcinoma. BMC Cancer. https://doi.org/10.1186/s12885-016-2974-x
Ling-Long T, Lei C, Li T, Yu-Pei C, Yan-Ping M, Ai-Hua L, Li L, Zi-Xian W, Ying S, Jun M, Rui G(2017) Validation of the 8th Edition of the UICC/AJCC Staging System for Nasopharyngeal Carcinoma From Endemic Areas in the Intensity-Modulated Radiotherapy Era. J Natl Compr Canc Netw 15:913–919. https://doi.org/10.6004/jnccn.2017.0121.
Lin-Quan T, Chao-Feng L, Jing L, Wen-Hui C, Qiu-Yan C, Lian-Xiong Y, Xiao-Ping L, Yun H, Yun-Xiu-Xiu X, Dong-Peng H, Shi-Hua W, Yu-Tuan P, Lu Z, Shan-Shan G, Li-Ting L, Ling G, Yi-Shan W, Dong-Hua L, Pei-Yu H, Hao-Yuan M, Yan-Qun X, Rui S, Ming-Yuan C, Yi-Jun H, Xing L, Lin W, Chong Z, Ka-Jia C, Chao-Nan Q, Xiang G, Yi-Xin Z, Hai-Qiang M, Mu-Sheng Z (2015) Establishment and validation of prognostic nomograms for endemic nasopharyngeal carcinoma. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djv291
Mariani L, Miceli R, Kattan MW, Brennan MF, Colecchia M, Fiore M, Casali PG, Gronchi A (2005) Validation and adaptation of a nomogram for predicting the survival of patients with extremity soft tissue sarcoma using a three-grade system. Cancer 103:402–408. https://doi.org/10.1002/cncr.20778
Mei L, Rui Y, You-Ping L, Yi-Nuan Z, Hao-Jiong Z, Xiong Z, Qi Y, Li C-F, Yi-Jun H, Tao Y, Jing-Yu C, Ji-Bin L, Hao-Yuan M, Ling G, Ai-Hua L, Ying S, Chao-Nan Q, Jun M, Hai-Qiang M, Ming-Yuan C (2018) Beneficial effects of anti-EGFR agents, Cetuximab or Nimotuzumab, in combination with concurrent chemoradiotherapy in advanced nasopharyngeal carcinoma. Oral Oncol 80:1–8. https://doi.org/10.1016/j.oraloncology.2018.03.002
Meng-Xia Z, Jing L, Guo-Ping XZ, Jun-Jie X, Rou J, Rui Y, Yi-Jun H, Ying S, Jun M, Ming-Huang H, Ming-Yuan C (2015) Intensity-modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two-dimensional radiotherapy: a 10-year experience with a large cohort and long follow-up. Eur J Cancer 51:2587–2595. https://doi.org/10.1016/j.ejca.2015.08.006
Patil VM, Noronha V, Joshi A, Agarwal J, Ghosh-Laskar S, Budrukkar A, Murthy V, Gupta T, Mahimkar M, Juvekar S, Arya S, Mahajan A, Agarwal A, Purandare N, Rangarajan V, Balaji A, Chaudhari SV, Banavali S, Kannan S, Bhattacharjee A, D’cruz AK, Chaturvedi P, Pai PS, Chaukar D, Pantvaidya G, Nair D, Nair S, Deshmukh A, Thiagarajan S, Mathrudev V, Manjrekar A, Dhumal S, Maske K, Bhelekar AS, Nawale K, Chandrasekharan A, Pande N, Goel A, Talreja V, Simha V, Srinivas S, Swami R, Vallathol DH, Dsouza H, Shrirangwar S, Turkar S, Abraham G, Thanky AH, Patel U, Pandey MK, Prabhash K (2019) A randomized phase 3 trial comparing nimotuzumab plus cisplatin chemoradiotherapy versus cisplatin chemoradiotherapy alone in locally advanced head and neck cancer. Cancer 125:3184–3197. https://doi.org/10.1002/cncr.32179
Prenen H, Vecchione L, Van Cutsem E (2013) Role of targeted agents in metastatic colorectal cancer. Target Oncol 8:83–96. https://doi.org/10.1007/s11523-013-0281-x
Reddy BKM, Lokesh V, Vidyasagar MS, Shenoy K, Babu KG, Shenoy A, Naveen T, Joseph B, Bonanthaya R, Nanjundappa BPP, Loknatha SJ, Prasad K, Tanvir Pasha CR (2014) Nimotuzumab provides survival benefit to patients with inoperable advanced squamous cell carcinoma of the head and neck: a randomized, open-label, phase IIb, 5-year study in Indian patients. Oral Oncol 50:498–505. https://doi.org/10.1016/j.oraloncology.2013.11.008
Robert LC, David LR, Marisa Dolled F (2004) X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 10:7252–7259. https://doi.org/10.1158/1078-0432.CCR-04-0713
Rui Y, Rui S, Yi-Jun H, Chao-Feng L, Ji-Bin L, Xiong Z, Qi Y, You-Ping L, Yi-Nuan Z, Tao Y, Jing-Yu C, Meng-Xia Z, Rou J, Hao-Yuan M, Ling G, Ka-Jia C, Ai-Hua L, Chao-Nan Q, Ying S, Jun M, Ming-Yuan C (2017a) Cetuximab or nimotuzumab plus intensity-modulated radiotherapy versus cisplatin plus intensity-modulated radiotherapy for stage II–IVb nasopharyngeal carcinoma. Int J Cancer 141:1265–1276. https://doi.org/10.1002/ijc.30819
Rui Y, Yi-Jun H, You-Ping L, Qi Y, Yi-Nuan Z, Ji-Bin L, Chao-Feng L, Xiong Z, Tao Y, Jing-Yu C, Meng-Xia Z, Rou J, Rui S, Hao-Yuan M, Ling G, Ka-Jia C, Ai-Hua L, Ying S, Chao-Nan Q, Jun M, Ming-Yuan C (2017b) Concurrent chemoradiotherapy with or without anti-EGFR-targeted treatment for stage II-IVb nasopharyngeal carcinoma: retrospective analysis with a large cohort and long follow-up. Theranostics 7:2314–2324. https://doi.org/10.7150/thno.19710
Talavera A, Friemann R, Gomez-Puerta S, Martinez-Fleites C, Garrido G, Rabasa A, Lopez-Requena A, Pupo A, Johansen RF, Sanchez O, Krengel U, Moreno E (2009) Nimotuzumab, an antitumor antibody that targets the epidermal growth factor receptor, blocks ligand binding while permitting the active receptor conformation. Cancer Res 69:5851–5859. https://doi.org/10.1158/0008-5472.CAN-08-4518
Tania C, Leonel T, Elia N, Mauricio C, Marı´a E. S, Alejandro P, Olga T, Normando I, Franz T, Rolando P, L AN (2003) Pharmacological evaluation of humanized anti-epidermal growth factor receptor, monoclonal antibody h-R3, in patients with advanced epithelial-derived cancer. J Immunother 26:139-148. https://doi.org/10.1097/00002371-200303000-00006
Wang-Zhong L, Shu-Hui L, Guo-Ying L, Hu L, Xiang G, Xing L, Kui-Yuan L, Meng-Yun Q, Xi C, Zg S, Chang-Qing X, Wei-Xiong X, Xiang Y-Q (2021) Development of a prognostic model to identify the suitable definitive radiation therapy candidates in de novo metastatic nasopharyngeal carcinoma: a real-world study. Int J Radiat Oncol Biol Phys 109:120–130. https://doi.org/10.1016/j.ijrobp.2020.08.045
Wei-Xiong X, Hu L, Xing L, Lin W, Chao-Nan Q, Yan-Fang Y, Liang-Ru K, Wen-Ze Q, Ya-Hui Y, Xin-Jun H, Guo-Ying L, Chong Z, Yan-Qun X, Xiang G (2017) Combining cetuximab with chemoradiotherapy in patients with locally advanced nasopharyngeal carcinoma: a propensity score analysis. Oral Oncol 67:167–174. https://doi.org/10.1016/j.oraloncology.2017.02.026
William ML, Patel SG, Brian O’sullivan, Margaret S. Brandwein, John A. Ridge M, Jocelyn C. Migliacci, Ashley M. Loomis,Shah JP (2017) Head and neck cancers-major changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA Cancer J Clin 67:122–137.
Xia H, Jianhua X, Wenjie G, Xuesong J, Xue W, Dan Z (2013) Cetuximab in combination with chemoradiation after induction chemotherapy of locoregionally advanced nasopharyngeal carcinoma: preliminary results. Future Oncol 9:1459–1467. https://doi.org/10.2217/fon.13.151
Xu T, Liu Y, Dou S, Li F, Guan X, Zhu G (2015) Weekly cetuximab concurrent with IMRT aggravated radiation-induced oral mucositis in locally advanced nasopharyngeal carcinoma: Results of a randomized phase II study. Oral Oncol 51:875–879. https://doi.org/10.1016/j.oraloncology.2015.06.008
Xue-Song S, Yu-Jing L, Xiao-Yun L, Sai-Lan L, Qiu-Yan C, Lin-Quan T, Hai-Qiang M (2019) Palliative chemotherapy with or without anti-EGFR therapy for de novo metastatic nasopharyngeal carcinoma: a propensity score-matching study. Drug Des Dev Ther 13:3207–3216. https://doi.org/10.2147/dddt.S215190
Yan H, Cao X, Wang J (2017) Application of intensity-modulated radiation therapy in the treatment of nasopharyngeal carcinoma. Oncol Lett 14:7773–7776. https://doi.org/10.3892/ol.2017.7186
Yang L, Qiu-Yan C, Lin-Quan T, Li-Ting L, Shan-Shan G, Ling G, Hao-Yuan M, Ming-Yuan C, Xiang G, Ka-Jia C, Chao-Nan Q, Mu-Shen Z, Jin-Xin B, Jian-Yong S, Ying S, Jing T, Shuai C, Jun M, Chong Z, Hai-Qiang M (2017) Concurrent chemoradiotherapy with or without cetuximab for stage II to IVb nasopharyngeal carcinoma: a case–control study. BMC Cancer. https://doi.org/10.1186/s12885-017-3552-6
Yewale C, Baradia D, Vhora I, Patil S, Misra A (2013) Epidermal growth factor receptor targeting in cancer: a review of trends and strategies. Biomaterials 34:8690–8707. https://doi.org/10.1016/j.biomaterials.2013.07.100
Ying S, Wen-Fei L, Nian-Yong C, Ning Z, Guo-Qing H, Fang-Yun X, Yan S, Xiao-Zhong C, Jin-Gao L, Xiao-Dong Z, Chao-Su H, Xiang-Ying X, Yuan-Yuan C, Wei-Han H, Ling G, Hao-Yuan M, Lei C, Yan-Ping M, Rui S, Ping A, Shao-Bo L, Guo-Xian L, Bao-Min Z, Xing-Lai F, Xiao-Chang G, Ling L, Chun-Ying S, Jian-Yu X, Ying G, Yu-Ming C, Fan Z, Li L, Ling-Long T, Meng-Zhong L, M J, (2016) Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol 17:1509–1520. https://doi.org/10.1016/s1470-2045(16)30410-7
Yizhou W, Jun L, Yong X, Renyan G, Kui W, Zhenlin Y, Xuying W, Guanghua L, Dong W, Lehua S, Wanyee L, Mengchao W, Shen F (2013) Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 31:1188–1195. https://doi.org/10.1200/JCO.2012.41.5984
Yuan Z, Wen-Fei L, Xu L, Lei C, Rui S, Ying S, Qing L, Jun M (2018) Nomogram to predict the benefit of additional induction chemotherapy to concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: analysis of a multicenter, phase III randomized trial. Radiother Oncol 129:18–22. https://doi.org/10.1016/j.radonc.2017.12.002
Yu-Pei C, Nofisat I, Melvin L. K. C, A. Dimitrios C, Robert H, Shao-Hui H, Joseph T. S. W, Alexander C. Whitley, Jun-Lin Y, Sue S. Y, Anthony T. C. C, Chao-Su H, Jin-Yi L, Quynhthu L, Anne W. M. L, Nancy L, Jinching L, Brigette M, Thomas J. M, Jatin S, Ying S,Jun M(2021) Chemotherapy in Combination With Radiotherapy for Definitive-Intent Treatment of Stage II-IVA Nasopharyngeal Carcinoma: CSCO and ASCO Guideline. Journal of Clinical Oncology 39:840-859. https://doi.org/10.1200/JCO.20
Zhi-Gang L, Yu Z, Jiao T, Yu-Juan Z, Wen-Juan Y, Yan-Fang Q, Hui W (2016) Nimotuzumab combined with concurrent chemoradiotherapy in locally advanced nasopharyngeal carcinoma: a retrospective analysis. Oncotarget. https://doi.org/10.18632/oncotarget.8225
Zhi-Qiang W, Qi M, Ji-Bin L, Rui Y, You-Ping L, Rui S, Guang-Yuan H, Ming-Yuan C, Yi-Jun H (2019) The long-term survival of patients with III–IVb stage nasopharyngeal carcinoma treated with IMRT with or without Nimotuzumab: a propensity score-matched analysis. BMC Cancer. https://doi.org/10.1186/s12885-019-6156-5
Acknowledgements
We are grateful to our patients and staff involved in the patient care for making this work possible. We kindly thank the editor and reviewers for their careful review and valuable comments, which have helped significantly improve the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (grant numbers 81872375 and 82172863) and the Natural Science Foundation of Guangdong Province (grant numbers 2021A1515010118).
Author information
Authors and Affiliations
Contributions
ZC developed the study concepts and design. CL and YH participated in the data acquisition. DC and WQ participated in the data analysis and interpretation. ZC, JZ and ZZ participated in the manuscript writing and editing. YX, XG and XL participated in the manuscript review. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Conflict of interest
Conflict of interest relevant to this article was not reported.
Ethical approval
This retrospective study was approved by the Clinical Research Committee of Sun Yat-sen University Cancer Center (B2021-366-01).
Consent to participate
Written informed consent was obtained from each patient for the publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cai, Z., Chen, D., Qiu, W. et al. Concurrent chemoradiotherapy combined with nimotuzumab in stage III–IVa nasopharyngeal carcinoma: a retrospective analysis. J Cancer Res Clin Oncol 149, 2327–2344 (2023). https://doi.org/10.1007/s00432-022-04355-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-04355-w