Abstract
Purpose
The COVID-19 pandemic changed diagnostic and treatment pathways in oncology. We compared the safety and efficacy of pembrolizumab amongst advanced nonsmall cell lung cancer (NSCLC) patients with a PD-L1 tumor proportion score (TPS) ≥ 50% before and during the pandemic.
Methods
Advanced NSCLC patients initiating pembrolizumab between June 2015 and December 2019 (“pre-pandemic cohort”) and between March 2020 and March 2021 (“pandemic cohort”) at BC Cancer were identified retrospectively. Multivariable logistic regression evaluated risk factors for immune-related adverse events (irAE) ≥ grade 3 at the 6 week, 3 month, and 6 month landmarks. Cox regression models of overall survival (OS) were constructed.
Results
The study population comprised 417 patients in the pre-pandemic cohort and 111 patients in the pandemic cohort. Between March and May 2020, 48% fewer advanced NSCLC cases with PD-L1 TPS ≥ 50% were diagnosed compared to similar intervals in 2018–2019. Telemedicine assessment [new patient consultations (p < 0.001) and follow-up (p < 0.001)] and extended interval pembrolizumab dosing (p < 0.001) were more common in the pandemic cohort. Patients initiating pembrolizumab after February 2020 (vs. before January 2020) experienced similar odds of developing severe irAE. 2/111 (1.8%) patients receiving pembrolizumab during the pandemic tested positive for COVID-19. On multivariable analysis, no association between pembrolizumab treatment period (before vs. during the COVID-19 pandemic) and OS was observed (p = 0.18).
Conclusion
Significant changes in healthcare delivery in response to the pandemic did not result in increased high grade toxicity or lower survival outcomes in patients with advanced NSCLC treated with pembrolizumab.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pembrolizumab, a monoclonal antibody checkpoint inhibitor directed against programmed death receptor 1 (PD-1), is commonly prescribed as a first line treatment for advanced nonsmall cell lung cancer (NSCLC) patients whose tumors have a programmed death ligand 1 (PD-L1) tumor proportion score (TPS) ≥ 50% and no actionable mutations. KEYNOTE-024 demonstrated superior overall survival (OS) of pembrolizumab monotherapy administered every 3 weeks for up to 35 cycles compared to standard platinum doublet chemotherapy in this patient population (Reck et al. 2016). Importantly, pembrolizumab was well tolerated with a low incidence of severe immune-related adverse events (irAE, 9.7%) and need for treatment discontinuation (7.1%).
Coronavirus disease 2019 (COVID-19), the illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has exerted an unprecedented effect on society and health care services. While COVID-19 causes limited symptoms in some individuals, others may develop more severe sequelae including respiratory failure, cytokine release syndrome, multi-organ failure, and death (Curigliano et al. 2020). Patients with cancer are at risk of both contracting SARS-CoV-2 and developing severe sequelae due to frequent contacts with the healthcare system, immunocompromised state, and presence of comorbidities (Passaro et al. 2021). Individuals with thoracic malignancies are an especially vulnerable population with high risks of hospitalization and death due to COVID-19 (Garassino et al. 2020).
British Columbia (BC) Cancer provides publicly funded oncologic care for the 5.2 million residents of the province of British Columbia, Canada. At the onset of the pandemic, BC Cancer issued clinical management guidelines to limit COVID-19 dissemination amongst patients with cancer and to prioritize healthcare services (BC Cancer 2020). For example, in-person appointments were deferred in favor of telemedicine (defined as the delivery of health care services using communication technologies where distance is a critical factor) and extended interval immunotherapy dosing became available (Pan American Health Organization 2016). In anticipation of a surge in critically ill patients with COVID-19 province-wide requiring healthcare resources, it was recommended that all non-urgent imaging and pathology testing be postponed. In the context of aggressive malignancies such as NSCLC, these policies have challenged clinicians to maintain high standards of cancer care while minimizing direct contact with patients.
Mitigation strategies during the pandemic have changed diagnostic and treatment pathways in oncology, but the impact of these changes on the toxicity and efficacy of immune checkpoint inhibitors for advanced NSCLC remains unclear. For instance, a shift from in-person to telemedicine assessments could impede early recognition of irAE resulting in more severe immune toxicity. In this multicenter retrospective cohort study of patients receiving first-line pembrolizumab for advanced NSCLC, we compared safety and survival outcomes before and during the COVID-19 pandemic.
Methods
Study design and setting
All lung cancer biomarker testing in British Columbia is completed centrally at the BC Cancer pathology lab. We conducted this retrospective cohort study through a search of the pathology database for all advanced NSCLC tumors (stage IV, 7th edition Union for International Cancer Control TNM classification or stage IIIB or recurrent nonresectable disease not amenable to curative intent radiotherapy) with PD-L1 TPS ≥ 50% between June 2015 and March 2021. Assay methods have been previously described (Ksienski et al. 2021). Prospective data were captured until December 2021.
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the University of British Columbia Research Ethics Board (Date July 2021/No H21-02241), which considered the study exempt from obtaining patient informed consent on the basis of its code of regulations. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Patient selection
Patients treated with pembrolizumab were divided into two groups according to date of diagnosis of advanced NSCLC:
-
A “pre-pandemic” cohort including all advanced NSCLC patients with PD-L1 TPS ≥ 50% who received pembrolizumab as a first treatment for advanced NSCLC any time between June 2015 and December 2019. Individuals diagnosed with advanced NSCLC between June 2015 and December 2019 who were treated with pembrolizumab on or after January 1, 2020, were excluded.
-
A “pandemic” cohort including all patient diagnosed with advanced NSCLC between March 11, 2020 (date the World Health Organization declared COVID-19 a pandemic), and March 2021 whose tumors demonstrated PD-L1 TPS ≥ 50% and received first-line pembrolizumab.
We excluded patients treated with pembrolizumab as second line or greater therapy or as part of a clinical trial. In addition, since combination chemotherapy and pembrolizumab can be given to a more heterogeneous population (any PD-L1 TPS), we only included patients receiving pembrolizumab monotherapy as all such tumors demonstrated a PD-L1 TPS ≥ 50%. Pembrolizumab was administered at either 2 mg/kg intravenous every 3 weeks or, stating April 1, 2020, 4 mg/kg intravenous every 6 weeks.
Outcomes
The primary endpoints were the incidence of irAE grade ≥ 3 and survival outcomes (overall survival, OS, and progression free survival, PFS) in the pre-pandemic and pandemic cohorts. High grade irAE were studied as they are always clinically relevant and are subject to less interobserver variation than grade 1 or 2 irAE (Hsiehchen et al. 2019). OS was defined as the time from first pembrolizumab treatment until the date of death from any cause. PFS was measured from pembrolizumab start to date of physician determined progression or death. Patients not experiencing an event of interest were censored at last follow-up.
Secondary outcomes were rates of newly diagnosed advanced NSCLC cases with high PD-L1 expression, time from diagnosis of advanced NSCLC to pembrolizumab initiation, and treatment modifications due to the pandemic in the two cohorts.
Data extraction
Information extracted from chart review included: (1) the mode of consultation (in-person vs. telemedicine) for the new patient appointment and first 3 months of follow-up after starting pembrolizumab, (2) clinical characteristics: age, gender, Charlson Comorbidity Index (advanced NSCLC not included as a comorbidity), Eastern Cooperative Oncology Group (ECOG) performance status, and smoking status, (3) tumor-level covariates: histology, PD-L1 TPS, and presence of oncogenic driver mutations, (4) the ratio of serum absolute neutrophil count to absolute lymphocyte count prior to the first pembrolizumab infusion were used to calculate the neutrophil to lymphocyte (NLR) ratio; a cut-off of 6.4 was chosen as values below this level have a positive prognostic implication (Ksienski et al. 2021) and (5) any modification to patient care specifically resulting from the COVID-19 pandemic.
Regarding safety data, identification of irAE was based on the treating healthcare practitioner’s assessment while grade was assigned by the abstractor using Common Terminology Criteria for Adverse Events version 5 (U.S. Department of Health and Human Services 2017). SARS-CoV-2 infection confirmed by reverse transcriptase polymerase chain reaction, rapid antigen test, or serology at any point during follow-up was also recorded.
Statistical analysis
Baseline demographic and clinical characteristics were summarized using descriptive statistics and compared with two sample Wilcox tests (continuous variables) and Pearson chi-square tests of homogeneity (categorical variables) risk ratios were calculated using unconditional maximum likelihood with the R package “epitools” (Aragon 2020). Median follow-up time was calculated by the reverse Kaplan–Meier method. Poisson generalized linear models were used to compare the rates of newly diagnosed advanced NSCLC cases with high PD-L1 expression and their confidence intervals (CI) before and during COVID-19. These models were summarized with rate ratios comparing the pandemic period to pre-pandemic on a quarterly basis.
Kaplan–Meier curves from first pembrolizumab treatment were generated and groups compared by the log-rank test. Univariable and multivariable Cox proportional hazards models were used to examine the association of baseline covariates (including treatment with pembrolizumab before or during COVID-19) with OS. Results are summarized as hazard ratios and 95% CI.
Univariable and multivariable logistic regression models were used to determine the odds of experiencing a high-grade irAE within 6 weeks, 3 months, or 6 months of pembrolizumab initiation based on baseline characteristics and treatment period. As there is a wide range in time to onset of pembrolizumab-induced irAE, multiple timepoints were chosen. As a sensitivity analysis, calculations were repeated excluding individuals who died before each landmark as those poor prognosis patients might not have lived long enough to develop a high grade irAE.
All p values were based on two-sided hypotheses test and those < 0.05 were considered statistically significant. All analyses were conducted using R statistical software version 4.0.3 (R Core Team 2021).
Results
Patient characteristics
A total of 1627 advanced NSCLC cases with PD-L1 TPS ≥ 50% were diagnosed between June 2015 and December 2019 or March 11, 2020, and March 2021 (Fig. 1). Five hundred and twenty-eight patients met eligibility criteria for this study: 417 patients belonging to the pre-pandemic cohort and 111 patients to the pandemic cohort. Amongst the 528 patients, median age was 71 years (range 41–95), 276 patients (52.3%) were female, and 206 patients (39.0%) had ECOG performance status 2/3 at first pembrolizumab treatment. Patients in the pandemic cohort had a higher median Charlson Comorbidity Index score than those in the pre-pandemic cohort (3.0 vs. 1.0, p < 0.001) (Table 1). Individuals in the pre-pandemic cohort presented at first Medical Oncology consultation, more often with advanced NSCLC than individuals in the pandemic cohort (88.2 vs. 77.5%, p = 0.006). Otherwise, baseline demographic, pathologic and laboratory values for the two groups were similar.
Pembrolizumab treatment
Patients in the pandemic cohort (vs. pre-pandemic cohort) were more likely to receive pembrolizumab every 6 weeks instead of every 3 weeks (8.1 vs. 0.0%, p < 0.001). No significant differences were observed in the time from diagnosis of advanced NSCLC to first pembrolizumab infusion (i.e., time to treatment), median number of pembrolizumab doses administered, or receipt of post-progression treatments in the two groups.
Telemedicine
Telemedicine use was higher in the pandemic than pre-pandemic group. Individuals in the pandemic cohort were more likely to be assessed by telemedicine (vs. in-person) for the initial consultation (36.9 vs. 8.6%, p < 0.001). The rate of remote appointments during the 3-month interval after starting pembrolizumab was 3.25 times greater (rate ratio = 95% CI 2.61–4.07, p < 0.001) in the pandemic than pre-pandemic group.
Diagnosis of advanced NSCLC patients with PD-L1 TPS ≥ 50%
The average number of newly diagnosed advanced NSCLC cases with high PD-L1 expression per 3 month interval in 2018 and 2019 (baseline) was compared to similar three month intervals starting March 2020 (pandemic period.) The rates for new cases were lower from March to May 2020 (rate ratio = 0.52, 95% CI 0.40–0.73) and September to November 2020 (rate ratio = 0.72, 95% CI 0.54–0.97) than corresponding intervals in 2018–2019. However, the rates for new cases stabilized in June to August 2020 (rate ratio = 0.94, 95% CI 0.72–1.23) and December 2020 to February 2021 (rate ratio = 0.94, 95% CI 0.81–1.11) compared to baseline.
Changes in clinical management due to the COVID-19 pandemic
In the pre-pandemic cohort, 198 patients were alive after March 10, 2020. Nineteen of 198 patients (9.5%) had at least one alteration in management resulting from the pandemic, most commonly a delay in administration of ongoing chemotherapy (seven patients). Further, 14 of 111 patients (12.6%) in the pandemic cohort had oncologic care changed due to the pandemic: use of extended interval pembrolizumab dosing (eight patients), delay in ongoing pembrolizumab treatment (three patients), and delay in follow-up imaging (three patients).
Safety
Reverse Kaplan–Meier median follow-up times for patients in the pre-pandemic and pandemic groups were 38.6 month and 12.4 months, respectively. In the entire cohort of 528 patients during all follow-up, 230 (43.5%) patients experienced any irAE and 76 (14.4%) patients developed an irAE grade ≥ 3 (Tables 2, 3). 1.6% (5/309) of patients alive on or after March 11, 2020, were diagnosed with SARS-CoV-2 infection: three patients in the pre-pandemic cohort and two patients in the pandemic cohort. One patient died as a result of COVID infection.
As shown in Table 4, the risk of irAE grade ≥ 3 were numerically lower in the pandemic cohort but not statistically significant at the 6-week (risk ratio = 0.69, 95% CI 0.28–1.74), 3 month (risk ratio = 0.56, 95% CI 0.24–1.30), and 6 month (risk ratio = 0.48, 95% CI 0.22–1.05) landmarks. A sensitivity analysis excluding patients who died before each landmark yielded similar findings (Supplementary Table 1). Furthermore, on multivariable logistic regression the odds of developing a high grade irAE were not significantly different in the two cohorts at the 6-week (odds ratio, OR 0.62, 95% CI 0.17–1.75; p = 0.41), 3 month (OR = 0.56, 95% CI 0.18–1.39; p = 0.25), and 6 month (OR = 0.49, 95% CI 0.18–1.12; p = 0.12) landmarks (Table 5). A sensitivity analysis excluding poor prognosis patients who died before each landmark yielded similar results (Supplementary Table 2).
Survival outcomes
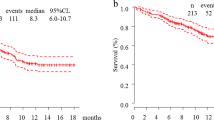
At last follow-up, 114/417 patients (27.3%) were alive in the pre-pandemic group and 62/111 patients (55.9%) were alive in the pandemic group. 5/528 patients (0.95%) in the total cohort were lost to follow-up. Median OS was numerically higher for patients in the pandemic than pre-pandemic group (17.8 months and 13.2 months; p = 0.34) but results were not significant (Fig. 2). Similarly, PFS was not significantly different between the pandemic and pre-pandemic groups (12.3 months vs. 7.0 months; p = 0.072). On multivariable analysis, ECOG performance status 2/3 (vs. 0/1, hazard ratio, HR = 2.3, 95% CI 1.9–2.9; p < 0.001) and serum NLR ≥ 6.4 (vs. < 6.4, HR = 1.8, 95% CI 1.5–2.3; p ≤ 0.001) were associated with shorter OS while being an active/former smoker (vs. non-smoker, HR = 0.67, 95% CI 0.45–0.99; p = 0.046) was associated with improved OS. No association between treatment period with pembrolizumab (before vs. during the COVID-19 pandemic) and OS was observed (Fig. 3).
Discussion
At the onset of the COVID-19 pandemic, significant alterations in cancer care delivery were required to minimize SARS-CoV-2 infection but still provide access to essential oncologic treatments. In this comprehensive analysis, we retrospectively evaluated the safety and survival outcomes for patients with advanced NSCLC receiving pembrolizumab before and during the pandemic. Remarkably, only 1.8% of patients treated with pembrolizumab on or after March 11, 2020, tested positive for COVID-19 disease. During the pandemic, telemedicine and extended interval immunotherapy dosing were both used more frequently. Receipt of pembrolizumab in the pandemic cohort was not associated with a higher risk of severe immune toxicity compared to the pre-pandemic cohort. Importantly, median OS and PFS were similar between the two groups.
Telemedicine utilization significantly increased during the pandemic. For instance, the proportion of new patient consultations conducted via telemedicine increased fourfold and telemedicine follow-up appointments within the first 3 months after starting pembrolizumab were 3.25 times higher than in the pre-pandemic cohort. While telemedicine permits physical distancing thereby reducing exposure to SARS-CoV-2, it is unclear whether remote consultations allow the practitioner to accurately assess for changes in performance status, symptoms of disease progression, and treatment toxicities (Elkaddoum et al. 2020). For instance, cutaneous irAE are common with immune checkpoint inhibitors and can include maculopapular eruptions, interface dermatitis (i.e., lichenoid dermatitis or psoriasis), granulomatous dermatitis (i.e., sarcoidal granulomatous dermatitis), and immunobullous reactions (i.e., bullous pemphigoid; Ellis et al. 2020). Determining the type and severity of dermatitis could be more challenging when conducting a clinical assessment remotely. Moreover, the severity of unrecognized and untreated irAE tends to worsen with continued pembrolizumab infusions.
To our knowledge, this is the first report comparing the safety of pembrolizumab for advanced NSCLC before and during the COVID-19 pandemic. We examined high grade adverse events as these are clinically important and demonstrate greater concordance in interobserver assessment than grade one or two irAE (Hsiehchen et al. 2019). Despite rapid and extensive changes in healthcare delivery, we did not observe any differences in the incidence of high grade irAE amongst the two cohorts. On multivariable logistic regression, treatment with pembrolizumab during the pandemic was not associated with the development of severe irAE at multiple landmarks. It is also noteworthy that the incidence of fatal irAE in the pandemic cohort was low (1 patient).
Between March 2020 and March 2021, the cumulative incidence of COVID-19 disease in British Columbia and Canada was 1.5% and 2.3%, respectively (Government of Canada 2022). By comparison, 1.8% (2/111) of patients treated with pembrolizumab on or after March 11, 2020, in the current study developed SARS-CoV-2 infection. Early on in the pandemic, BC Cancer proactively implemented strategies to reduce the risk of virus transmission amongst patients and healthcare workers (BC Cancer 2020). Key components of this policy (aside from a shift to virtual consultation and follow-up assessments) included: use of personal protective equipment, screening patients (both the day before and the day of their cancer clinic appointment) for symptoms of COVID-19 disease, and restricting building access to healthcare providers and patients. These findings could provide reassurance to patients with thoracic malignancies who are reluctant to start immunotherapy due to safety concerns as a result of the ongoing pandemic.
We observed significant declines in the number of new advanced NSCLC cases with PD-L1 TPS ≥ 50% between March and May 2020 (48% reduction) and September and November 2020 (28% reduction) compared to similar time intervals in 2018 and 2019. These timepoints correspond to the first and second waves of COVID-19 in British Columbia. As approximately 25% of advanced NSCLC have high PD-L1 expression, these findings suggest a general reduction in pathologic diagnoses of all lung cancers. A retrospective study in Quebec, Canada, observed a 34.7% decrease in all lung cancer diagnosed at a single institution between March 2020 and February 2021 (Kasymjanova et al. 2021). Similarly, in a bicenter retrospective study from Spain, new NSCLC and small cell lung cancer diagnoses were 38% lower between January and June 2020 than a baseline period of January to June 2019 (Reyes et al. 2021). Reasons for the reduction in lung cancer diagnoses could relate to patient fears of engaging health services or delays in diagnostic imaging and biopsies as healthcare resources were reprioritized for a projected surge in severe SARS-CoV-2 infections (Gourd 2020).
A number of studies have reported significant disruption to oncologic care as a result of the pandemic. For instance, a retrospective series from a thoracic oncology unit in Quebec, Canada, determined that 57% of patients receiving active treatment experienced alterations in clinical management due to COVID-19 (most commonly delay or cessation of chemotherapy) (Elkrief et al. 2020). Using data from the COVID-19 and Cancer Outcome Study, patients with lung cancer on active treatment were found more likely to have systemic therapy or imaging delays compared to individuals with other types of solid tumors (Bhalla et al. 2021). In the current study’s setting of a publicly funded cancer care institution with consensus-driven management guidelines and protocols, disruptions to cancer care were less common. For instance, the time from diagnosis of advanced NSCLC to first pembrolizumab therapy were similar in the pre-pandemic and pandemic cohorts. Through detailed chart review we found that pembrolizumab treatments were only delayed in 2.7% of patients in the pandemic cohort due to concerns about the pandemic. Last, duration of pembrolizumab treatments and receipt of post-progression systemic therapy were comparable in the two cohorts. It is plausible that survival outcomes (OS and PFS) for patients in the pre-pandemic and pandemic groups were similar as access to pembrolizumab was prioritized and treatment modifications minimized.
The current analysis provides important insights regarding prognostic factors amongst recipients of pembrolizumab for advanced NSCLC in a real world setting. Specifically, the studied cohort included patients who were older (median age 71 years) and had ECOG performance status 2/3 (39%). By comparison, the median age of patients enrolled onto KEYNOTE-24 was 65 years and individuals with poor ECOG performance status were excluded (Reck et al. 2016). Importantly, under-representation of these subgroups in clinical trials contributes to uncertainty regarding the risk–benefit ratio of pembrolizumab. Age ≥ 75 years (vs. < 75 years) was not associated with OS on multivariable analysis; moreover, advanced age was not associated with an increased risk of severe irAE. As such, these observations could be helpful when counseling older patients about the efficacy and toxicity of first-line pembrolizumab for advanced NSCLC. In line with other retrospective studies of pembrolizumab for advanced NSCLC, ECOG PS was associated with poorer prognosis on our multivariable analysis (Sehgal et al. 2021). To our knowledge PePS2 a single arm phase 2 trial, is the only prospective study examining the efficacy of pembrolizumab (any line) in patients with PD-L1 positive advanced NSCLC (Middleton et al. 2020). Importantly, the trial only enrolled 60 patients (15 patients with a PD-L1 TPS ≥ 50%). Given that approximately 40% of advanced NSCLC patients treated in contemporary practice have ECOG performance status 2/3, our data contribute insight into outcomes of patients encountered in daily practice while we await larger prospective trials to clarify the benefits and safety of pembrolizumab for advanced NSCLC patients with PD-L1 TPS ≥ 50%.
This study has several limitations, including patient and treatment selection biases inherent in retrospective studies. As the study population only included patients receiving pembrolizumab monotherapy, the results may not be generalizable to other treatments such as chemotherapy or tyrosine kinase inhibitors. Healthcare in British Columbia is publicly funded and further study is required to determine if similar findings are observed in regions without a universal healthcare system. Last, while we have detailed information regarding comorbidities (i.e., Charlson Comorbidity Index and history of autoimmune disease), we did not collect information regarding baseline medications such as corticosteroids which have been associated with reduced efficacy of immune checkpoint inhibitors.
Conclusion
The ongoing COVID-19 pandemic has challenged physicians to protect patients from SARS-CoV-2 infection yet still provide essential oncologic treatments. In the present study, patients with advanced NSCLC treated with pembrolizumab before and during the pandemic experienced a similar risk of high grade irAE. Furthermore, survival outcomes were comparable between the two groups. Our study provides support that life prolonging therapies such as pembrolizumab can be delivered safely to patients with advanced NSCLC while still following strict protocols to reduce the risk of SARS-CoV-2 transmission. Telemedicine for oncologic consultations and follow-up was not associated with inferior toxicity or survival outcomes, supporting its continued use for selected patients beyond the pandemic era.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BC:
-
British Columbia
- CCI:
-
Charlson comorbidity index
- CI:
-
Confidence interval
- COVID-19:
-
Coronavirus disease 2019
- ECOG PS:
-
Eastern Cooperative Oncology Group performance status
- HR:
-
Hazard ratio
- irAE:
-
Immune-related adverse events
- n :
-
Number of patients
- NSCLC:
-
Nonsmall cell lung cancer
- OR:
-
Odds ratio
- OS:
-
Overall survival
- PD-1:
-
Programmed death 1 inhibitor
- PD-L1:
-
Programmed death ligand 1
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- TPS:
-
Tumor proportion score
References
Aragon TJ (2020). Epitools: epidemiology tools. R package version 0.5-10.1. https://CRAN.R-project.org/package=epitools. Accessed 1 March 2022
BC Cancer (2020) Provincial cancer clinical management guidelines in pandemic situation (COVID-19). BC Cancer. http://www.bccancer.bc.ca/health-professionals-site/Documents/Provincial%20Cancer%20Therapy%20Clinical%20Management%20Guidelines%20in%20Pandemic%20situation%20%28COVID-19%29_April%2014%202020UPDATED.pdf. Accessed 5 January 2022.
Bhalla S, Bakouny Z, Schmidt AL et al (2021) Care disruptions among patients with lung cancer: a COVID-19 and cancer outcomes study. Lung Cancer 160:78–83. https://doi.org/10.1016/j.lungcan.2021.07.002
Curigliano G, Banerjee S, Cervantes A et al (2020) Managing cancer patients during the COVID-19 pandemic: an ESMO multidisciplinary expert consensus. Ann Oncol 31:1320–1335. https://doi.org/10.1016/j.annonc.2020.07.010
Elkaddoum R, Haddad FG, Eid R, Kourie HR (2020) Telemedicine for cancer patients during COVID-19 pandemic: between threats and opportunities. Future Oncol 16:1225–1227. https://doi.org/10.2217/fon-2020-0324
Elkrief A, Kazandjian S, Bouganim N (2020) Changes in lung cancer treatment as a result of the coronavirus disease 2019 pandemic. JAMA Oncol 6:1805–1806. https://doi.org/10.1001/jamaoncol.2020.4408
Ellis S, Vierra AT, Millsop JW et al (2020) Dermatologic toxicities to immune checkpoint inhibitor therapy: a review of histopathologic features. J Am Acad Dermatol 83:1130–1143. https://doi.org/10.1016/j.jaad.2020.04.105
Garassino MC, Whisenant JG, Huang L-C et al (2020) COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol 21:914–922. https://doi.org/10.1016/S1470-2045(20)30314-4
Gourd E (2020) Lung cancer control in the UK hit badly by COVID-19 pandemic. Lancet Oncol 21:1559. https://doi.org/10.1016/S1470-2045(20)30691-4
Government of Canada (2022) Interactive data visualizations of COVID-19. https://health-infobase.canada.ca/covid-19/?stat=num&measure=total&map=pt#a2. Accessed 5 April 2022
Hsiehchen D, Watters MK, Lu R et al (2019) Variation in the assessment of immune-related adverse event occurrence, grade, and timing in patients receiving immune checkpoint inhibitors. JAMA Netw Open 2:e1911519. https://doi.org/10.1001/jamanetworkopen.2019.11519
Kasymjanova G, Anwar A, Cohen V et al (2021) The impact of COVID-19 on the diagnosis and treatment of lung cancer at a Canadian Academic Center: a retrospective chart review. Curr Oncol 28:4247–4255. https://doi.org/10.3390/curroncol28060360
Ksienski D, Wai ES, Alex D, et al (2021) Prognostic significance of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for advanced non-small cell lung cancer patients with high PD-L1 tumor expression receiving pembrolizumab. Transl Lung Cancer Res 10:355–367. https://doi.org/10.21037/tlcr-20-541
Middleton G, Brock K, Savage J et al (2020) Pembrolizumab in patients with non-small-cell lung cancer of performance status 2 (PePS2): a single arm, phase 2 trial. Lancet Respir Med 8:895–904. https://doi.org/10.1016/S2213-2600(20)30033-3
Pan American Health Organization (2016) Framework for the implementation of a telemedicine service. Accessed 16 January 2022
Passaro A, Bestvina C, Velez Velez M et al (2021) Severity of COVID-19 in patients with lung cancer: evidence and challenges. J Immunother Cancer 9:e002266. https://doi.org/10.1136/jitc-2020-002266
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 15 March 2022
Reck M, Rodríguez-Abreu D, Robinson AG et al (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375:1823–1833. https://doi.org/10.1056/NEJMoa1606774
Reyes R, López-Castro R, Auclin E, et al (2021) MA03.08 Impact of COVID-19 pandemic in the diagnosis and prognosis of lung cancer. J Thorac Oncol 16:S141. https://doi.org/10.1016/j.jtho.2021.01.219
Sehgal K, Gill RR, Widick P et al (2021) Association of performance status with survival in patients with advanced non-small cell lung cancer treated with pembrolizumab monotherapy. JAMA Netw Open 4:e2037120. https://doi.org/10.1001/jamanetworkopen.2020.37120
U.S. Department of Health and Human Services (2017) Common terminology criteria for adverse events (CTCAE) version 5.0 Accessed 15 October 2021
Acknowledgements
This work was supported by a grant from the BC Cancer Foundation (Grant No. F21-02290).
Funding
This work was supported by an educational grant from the BC Cancer Foundation (Grant No. F21-02290).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation was provided by Dr. Alex and Ms. Clarkson. Data collection was done by Dr. Ksienski, Dr. Chan, Dr. Sonke, and Dr. Gupta. Data analysis was performed by Mr. Bone and Dr. Lesperance. The first draft of the manuscript was written by Dr. Ksienski, Mr. Bone, Dr. Truong, Dr. Lesperance, and Ms. Patterson. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Dr. D.K. has received educational grants from AstraZeneca and speaker honoraria from Merck and Bristol-Myers Squibb. Dr. A.C. has received speaker and consultant honoraria from Merck, Bristol-Myers Squibb, Novartis, and Pfizer. Dr. P.T. receives writing and royalty fees from UpToDate, Wolters Kluwer Health Publishing. The remaining authors have no relevant financial or non-financial interests to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the University of British Columbia Research Ethics Board (Protocol: H21-02241, Approval date: July 26, 2021), and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
The need for informed consent was waived by the University of British Columbia Research Ethics Board owing to the retrospective nature of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ksienski, D., Gupta, S., Truong, P.T. et al. Safety and efficacy of pembrolizumab for advanced nonsmall cell lung cancer: before and during the COVID-19 pandemic. J Cancer Res Clin Oncol 149, 2951–2961 (2023). https://doi.org/10.1007/s00432-022-04181-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-04181-0