Abstract
Background
COVID-19 serologic response in patients with cancer may be lower than in the general population and may be influenced by the type of tumor or anticancer treatment. This study aims to analyze serological response prior and after vaccination of COVID-19 within the oncological population in Andorra. We set out to identify risk factors for a higher or lower serological response.
Patients and methods
Observational, unicentric, prospective cohort study of oncologic patients in Andorra. We calculated the seroprevalence of antibodies against SARS-CoV-2 (May 2020–June 2021) and analyzed the main demographic, oncologic features and factors associated with being seropositive.
Results
A total of 373 patients were analyzed, mainly with solid tumours (n = 334, 89.5%). At baseline, seroprevalence was 13%, increasing during follow-up to 19%; lower seroprevalence was observed in patients with hematologic malignancies (2.6% vs 14.2%; p = 0.041) and patients receiving biological therapies (0% vs 15%, p = 0.005). In the overall seroprevalence analysis, women (23% vs 11.9%; p = 0.006) and tumour-free patients (p = 0.034) showed higher seroprevalence. The multivariable analysis showed that odds of being seropositive were higher among women (OR: 2.44, 95% CI 1.28–4.64), and patients who underwent surgery (OR: 3.35, 95% CI 1.10–10.20). About 80% of the cohort received at least one dose of COVID-19 vaccination, showing a higher seroprevalence of patients who received ChAdOx1-S than those who received BNT162b2 (24.4% vs 6.4%: p = 0.001).
Conclusion
The seroprevalence of antibodies against SARS-COV-2 in oncologic patients in Andorra was higher among females and patients who received hormonal therapy and surgery while patients with hematologic malignancies and biologic therapies showed lower seropositivity without finding differences in the type of tumour or anticancer treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
On January 7th, 2020, Chinese authorities identified SARS-CoV-2, a virus in the Coronaviridae family, as the causative agent of a pneumonia outbreak in the Hubei Province (Wang et al. 2020). On March 11th, 2020, the World Health Organization (WHO) announced the disease coronavirus 2019 (COVID-19) as a pandemic (WHO 2020). To date, the impact of the pandemic has been devastating, affecting over 422 million people worldwide with more than 5.8 million related deaths as of 20 February 2022 (World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report 2022).
Throughout 2020, oncologic care has been greatly impacted by the COVID-19 pandemic, including treatment interruption due to both COVID-related morbidity as well as limited survival benefit (Ueda et al. 2020). Despite COVID-19 widespread involvement, little data on SARS-CoV-2 involvement in cancer patients was known initially. Early studies in oncologic patients suggested an increased risk of contracting this viral infection and developing complications related to COVID-19 (Liang et al. 2020; Dai et al. 2020; Miyashita et al. 2020; Yu et al. 2020; Subbiah 2020 ). Moreover, mortality from COVID-19 is higher within the oncologic population, with patients treated with chemotherapy having up to a 20% mortality rate (Saini et al. 2019; Wise-Draper et al. 2020; Wiersinga et al. 2020; Daniel et al. 2020).
Notwithstanding the recent published studies in oncologic patients, information about the humoral response in this population is scarce and further studies are therefore needed to confirm whether the immune response to SARS-COv2 is influenced by recent cancer treatments as well as by the pathophysiology of the disease itself. Some studies pointed out a lower serological response in cancer patients receiving anticancer treatment (Solodky et al. 2020) but whether this serological response is lower in immunosuppressed patients and its length is little known. Hence, the evolution of serological response over time within the oncologic population needs to be analysed.
This study aims to analyse serological response over a period of time of 12 months prior and after vaccination of COVID-19 within the oncological population with active follow-up in Andorra according to tumour type and treatment received. We set out to identify risk factors for a higher or lower serological response.
Methods
Study population, setting, and data collection
We conducted a prospective observational study in Hospital Nostra Senyora Meritxell, the main hospital in Andorra. Overall, the patient pool represents all oncologic patients followed by Hospital Nostra Senyora Meritxell (Andorra) during the accrual period. This study included all patients ≥ 18 years of age with a diagnosis of solid tumour or haematological malignancy in the last 5 years (from January 2016–May 2020) who participated in the nationwide SARS-CoV-2 population screening in May 2020 (Royo-Cebrecos et al. 2021) with means of serological testing and who provided informed oral consent. Patients who failed to provide informed oral consent had been cancer-free for the last 5 years or deceased before the start of the study from a non-COVID-19 related death were excluded. Anonymized use of data was collected as per standard of care.
Patients' clinical and personal data were obtained from medical records (HCIS, software SAAS) and inputted into a database created for the purpose of this study (https://forms.universaldoctor.com/form/study/covoncoand) with software named Epidemix build to reinforce pandemic studies. Patient characteristics data included age, gender, comorbidities (notably COVID-19 risk factors) and drug history. Laboratory tests and microbiological results (cultures and non-culture diagnostics such as fungal biomarkers and viral PCR results) were also collected. Moreover, oncological characteristics included cancer diagnostic, stage, remission, and treatment (divided into active and naïve treatment, type and line of treatment). Lastly, COVID-19 characteristics included IgM and IgG serological results, PCR results (if available), symptomatology, confirmed COVID-19 contact, hospitalisation and treatment.
Definitions
Overall seroprevalence was defined as the number of individuals who had a positive result of IgG and/or IgM at any of the two surveys. Consequently, to calculate the overall proportion of seronegative individuals we used a numerator which includes those participants with a negative result in both surveys, an inconclusive result and one inconclusive result, or with just one negative result if the individual only participated in one survey. Seroconversion was defined as a transition of the test results (IgM or IgG) from negative to positive. Seroreversion was defined as a transition of the test results for IgG or IgM against SARS-CoV-2 from positive to negative results during the study. Inconclusive results were those that could not be interpreted correctly. Active oncological treatment was defined as patients receiving systemic anticancer agents (including chemotherapy, radiotherapy, biologic therapy, hormonal therapy, immunotherapy, surgery and combinations) 3 months prior to May 2020. Treatment-naïve was defined as patients with an oncologic diagnosis who were not receiving any treatment for their oncologic diagnosis 3 months prior to May 2020.
Study timeline
Accrual period was from May 2020 to July 2021. To analyse serological response over time, the established accrual period was divided into four subgroups: Baseline seroprevalence (S0): May 2020 (during Andorra’s nationwide serologic screening programme) (Royo-Cebrecos et al. 2021), seroprevalence at 6 months (S6), up to 6 months after baseline serologies (November 2020–December 2021) seroprevalence at 12 months (S12), serologies performed up to 12 months after baseline (May–June 2021) and overall seroprevalence. The vaccination campaign in Andorra started in January 2021.
Study procedures
In May 2020, through mass media and social media announcements, the Andorran population was invited to participate in a nationwide serology screening. Online registration for this screening was conducted through http://coronavirus.govern.ad/. Oncologic population who participated in the nationwide screening campaign were invited to participate in the COVONCO study during their regular visits to the Oncologic Department. Plasma serological tests were performed 6 and 12 months after the initial serological test, coinciding with the follow-up analyses of the oncology process.
Serologic test
Livzon® rapid tests were employed during May 2020 (as part of the May 2020 national screening campaign), a diagnostic kit for IgM/IgG antibody detection against SARS-Cov-2 based on a lateral flow assay (nCOV 2019 IgG/IgM- Zhuhai Livzon Diagnostics, Inc.—IgM and IgG kits, Colloidal gold). The test was selected based on a list of recommended tests from FIND (Foundation for Innovative Diagnostics) (https://www.finddx.org/sarscov2-eval-antigen/). It detects IgM and IgG on the same test providing a maximum combined sensitivity and specificity of 90.6% and 99.2%, respectively (according to the manufacturer). The combined sensitivity (IgM-IgG) ranged from 0.72–0.78 depending on the days since symptoms onset (7 or 14 days) 0.71–0.81 when positive samples were PCR-confirmed. Specificity ranged from 0.98 to 0.99 (Royo-Cebrecos et al. 2021).
Antigen-specific humoral immune response was analysed using two commercial immunoassays: first, in June 2020, Elecsys Anti-SARS-CoV-2 assay (Roche Diagnostics, Mannheim, Germany), an electrochemiluminescence immunoassay was used in vitro qualitative detection of total (IgG + IgA + IgM) nucleocapsid (N) -specific antibodies in patient serum. In December 2020, a quantitative electrochemiluminescence assay was added to detect total antibodies (IgG + IgA + IgM) against SARS-CoV-2 spike protein (S) receptor-binding domain (RBD) (Elecsys Anti-SARS-CoV-2 S assay, Roche Diagnostics, Mannheim, Germany). Results were automatically reported as the analyte concentration in U/mL, considering positive results ≥ 0.80 U/mL, with a measuring range from 0,4 U/mL to 250 U/mL. Correlation between U/mL and BAU/mL (WHO International Standard Binding Antibody Units) was U is 0,972 BAU. Both techniques were performed on the Cobas e601 module (Roche Diagnostics, Mannheim, Germany).
Statistical analysis
Descriptive statistics for quantitative variables are expressed as the median and interquartile range (IQR) or mean and standard deviation (SD). Categorical variables are expressed as absolute and relative frequencies. In the bivariate analysis, quantitative variables were compared with a student’s t test or a Wilcoxon test depending on data characteristics. For categorical variables, a chi-squared test was used. Variables with a significance level of < 0.10 in the bivariate analysis were included in the multivariate analysis. The final model was obtained using a stepwise backward elimination process. Odds ratios (OR) with their 95% CI were calculated. The analysis was performed with software SAS v9.4 (SAS Institute Inc., Cary, NC, USA). The significance level was set at p < 0.05.
Ethical considerations
The protocol of the study was approved by the relevant Andorran regulatory agencies and the local Research Ethics Committee. Participation in the study was entirely voluntary. The Institutional Review Board of the Servei Andorrà Atenció Sanitaria (SAAS) approved the study.
Results
Demographics, oncologic features, and outcomes
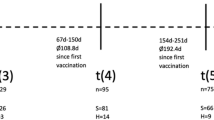
Of 477 oncologic patients who meet inclusion criteria, we finally analysed a total of 373 patients, see Fig. 1. Clinical and demographic features of this patient population are described in Table 1. Most of the patients were women (n = 213, 57.1%) with a mean (± SD) age of 60.19 (± 13.3) years (range 22–89). Solid tumours (n = 334, 89.5%) predominated over hematologic malignancies, with breast cancer representing the most common primary site (n = 114, 30.6%) followed by colorectal cancer (n = 73, 19.6%), genitourinary cancer (n = 34, 9.1%) and lung cancer (n = 23, 6.2%). Among hematologic malignancies, lymphoma (n = 25; 6.7%) and multiple myeloma (n = 9; 2.4%) were the most common. Comorbidities were present in 224 patients (60.1%), hypertension (n = 102, 45.5%) being the most prevalent, followed by chronic cardiovascular disease (n = 51, 22.8%) and diabetes (n = 47, 21.0%). Around half of the cohort (n = 208, 55.8%) was tumour-free at database lock. Most patients were outpatients, (n = 101, 64.7%) presented an ECOG performance status of 0 and 70 (18.8%) presented metastatic disease. A total of 171 (45.8%) were undergoing active treatment during the study period; chemotherapy (n = 79, 21.2%) was the most common treatment, followed by hormonal therapy (n = 69, 18.5%), biologic treatments (n = 48, 12.9%) and radiotherapy (n = 22, 5.9%). Only 29 patients (7.8%) presented symptoms at onset of test, being fever (n = 18, 62.1%), cough (n = 17,58.6) and malaise (n = 18,62.1%) the most prevalent symptoms. Only 3 (0.8%) patients presented pneumonia.
Regarding outcomes, 50 (4.8%) patients required hospital admission with 5 (0.5%) patients being admitted to ICU. Moreover, 8 patients (0.8%) reported complications, with 3 (4.3%) of them being hospital-acquired infections. Twenty-four patients (2.3%) passed away, of which 5 were in palliative care.
Seroprevalence at S0
Seroprevalence in oncologic features at S0 is represented in Table 2. Forty-eight (13%) patients were seropositive at S0. Women presented with higher seropositivity (17% vs 7.6%, p = 0.008), with no differences observed in age, comorbidities, or previous treatment received. Patients with haematologic malignancies presented lower seropositivity compared to solid tumours (2.6% vs 14.2%; p = 0.041). Nevertheless, no statistically significant differences were found between types of cancer at S0. Higher percentages of seropositivity were observed in patients with ECOG score 0–1, although these differences are not statistically significant (p = 0.082). Regarding tumour status, the percentage of positive serologies were higher among tumour-free patients (p = 0.006). Higher seroprevalence was also observed in patients with COVID-19 related symptoms (38% and 11% respectively, p < 0.001), especially in patients with cough (p = 0.047). Interestingly, lower seropositivity was observed among patients undergoing biological therapies (0% vs 15%, p = 0.005). Conversely, patients with hormonal therapy presented higher seropositivity than patients with other treatments (20% and 11%, p = 0.045). Similar results were observed in patients who had undergone surgery (29% and 12% respectively, p = 0.039). No statistically significant differences were found between S0 and other anticancer treatments. Patients who received one or two lines of treatment tended to present a higher percentage of positive serologies (p = 0.084).
Seroprevalence over time
Seroprevalence throughout the study is described in Fig. 1. About half of the cohort only has one serology measurement, 27.6% of the cohort have two measures and 20.9% of the cohort have three measures. A total of 305 patients (81.8%) were negative in all serologies, 23 patients (6.2%) remain seropositive throughout the study, 20 patients (5.4%) were seroconverted and 25 (6.7%) seroreverted. Similar to S0 serologies, seropositivity in S6 was higher among women (14.5% and 1.5%, p = 0.011) and among patients with a COVID-19 symptoms (p < 0.001). Conversely, no statistically significant differences were observed in the rest of analysed variables. In S12 we observed higher seropositivity associated with COVID-19 symptoms (p < 0.001) but did not observe any differences in sex or other variable. In the overall seroprevalence analysis, 68 (18%) patients were seropositive, see Table 2. Women also showed higher seroprevalence (23% vs 11.9%; p = 0.006) as well as having an ECOG score 0–1 (p = 0.031), being tumour-free (p = 0.034) and having COVID-19 related symptoms (62.1% vs 14.5%; p < 0.001). Patients who received biological therapy showed trends to be lower in overall seropositivity (p = 0.057) with no statistical differences observed in the rest of the variables.
Regarding serology evolution during follow-up, statistical differences in sex were observed (p = 0.036) and persisted over time (p = 0.194), Fig. 2a. This difference was also observed in patients with COVID-19 related symptoms (p < 0.001) and persisted over time (p = 0.282), Fig. 2b. Conversely, these differences were not observed in the rest of the analysed variables.
Multivariable analysis of factors affecting seroprevalence
Table 3 describes bivariate and multivariable analysis of overall seroprevalence. The odds of being seropositive were higher among women (OR: 2.44, 95% CI 1.28–4.64), and have received a surgery (OR: 3.35, 95% CI 1.10–10.20). In contrast, lymphocytes values or tumour status did not show a significant risk of being seropositive.
COVID-19 vaccination
Two hundred ninety-five (79.1%) patients received a first dose of vaccine, 91 (30.8%) patients receiving Pfizer-BioNTech (BNT162b2) and 204 (69.2%) Oxford/AstraZeneca (ChAdOx1-S) while 289 (77.5%) patients received a second dose, 118 (40.8%) patients BNT162b2 and 171 (59.2%) ChAdOx1-S. Interestingly, at S12 analysis, patients who received a first dose of vaccination with ChAdOx1-S presented higher seroprevalence than those who received BNT162b2 (24.4% vs 6.4%: p = 0.001) as well as a second dose with ChAdOx1-S (29.0% vs 6.8%; p = 0.002). These differences were not observed at S6 analysis coinciding with the start of the vaccination campaign. Regarding serology over time, no differences were observed in serology between the first dose of the COVID-19 vaccine nor second dose of the vaccine. Otherwise, statistically significant differences were observed in patients who received a second dose of vaccine, with a higher seroprevalence in those who received ChAdOx1-S in S12 analysis (p = 0.029), Fig. 2d. This difference was not observed in the first dose of the vaccine.
Discussion
To our knowledge, this study is one of the largest COVID-19 seroprevalence studies with an oncologic cohort in vaccine-naive and post-vaccine patients. Moreover, this study is unique in analysing a nationwide oncological population, with 373 patients and a follow-up of over 12 months. Seroprevalence at S0 was 13%, increasing during follow-up to 19% coinciding with the start of Andorra’s vaccination campaign. Interestingly, we observed higher seroprevalence among women. Haematologic patients presented with lower seroprevalence than patients with solid tumours, without observing differences between types of tumours. We observed higher seroprevalence among patients who underwent surgery and those who received hormonal therapy in contrast to those who received biologic therapy. In the overall analysis, only the presence of surgery and female sex were independent risk factors for increased seropositivity. Finally, 77.5% of the cohort received two doses of the COVID-19 vaccine, without observing differences in seroprevalence and number of vaccines. Patients with a second dose of ChAdOx1-S vaccine had higher seropositivity in the S12 analysis.
We observed a seroprevalence of 13% at S0, higher than the general Andorran population (Royo-Cebrecos et al. 2021) and other seroprevalence studies conducted at the same time (Royo-Cebrecos et al. 2021; Snoeck et al. 2020; Salje et al. 2020; Stringhini et al. 2020; Xu et al. 2020; Pollán et al. 2020; Cabezón-Gutiérrez et al. 2020; Cantini et al. 2021). Seroprevalence increased up to 17.9% in S12 analysis after the vaccination campaign and overall seroprevalence was 18.2%. To our knowledge, a few seroprevalence studies have focused on the oncologic population prior to vaccination. Cabezón-Guiterrez et al. (Cabezón-Gutiérrez et al. 2020) concluded that the seroprevalence of the oncological population was 31.4% and higher than the general population (Cabezón-Gutiérrez et al. 2020), which is similar to Zambelli et al. (Zambelli et al. 2020), with a seroprevalence of 31%. Other studies within the oncology population focus on the prevalence of COVID-19 infection confirmed with RT-PCR (Solodky et al. 2020; Cabezón-Gutiérrez et al. 2020).
Differing from other studies that observed a higher COVID-19 involvement in the male sex, we found that females presented higher seroprevalence (Royo-Cebrecos et al. 2021; Jaillon et al. 2019). A striking finding was that these differences were observed throughout the study and persist over time. Moreover, in the multivariate analysis, females were a risk factor for seropositivity. This fact could be explained by the general hypothesis that females develop stronger innate and adaptive immune responses than males (Thakkar et al. 2021). Thus, females might present with positive serology and less clinical impact than males. Conversely, while other studies identified older age as a risk factor for a positive serology, we did not observe differences between age groups or over time.
Recent studies similarly found a lower rate of seropositivity within the haematological malignancies cohort after vaccination (Tran et al. 2021; Liu et al. 2021). This may be explained by the greater immunosuppression presented by hematologic malignancy itself and treatments received, conferring a worse prognosis in those patients (Cabezón-Gutiérrez et al. 2020; Aschele et al. 2021). The fact that we did not observe a significant difference between serology and tumour type might be explained by the overrepresentation of breast cancer within our cohort, as this cancer tends to present in younger fitter patients. Similarly, our findings of comorbidities not being associated with a positive serology might also be related with most of our cohort being younger than 65 years old. However, our finding of tumour-free patients being more likely to have a positive serology is likely linked to over half of our study cohort being tumour-free.
Interestingly, we found lower seropositivity in patients who received biologic therapies in the S0 analysis, probably caused by increased immunosuppression in these patients. Some studies already point to an increased risk of infection and severity in patients receiving these treatments (Aschele et al. 2021). However, firm conclusions cannot be drawn due to the small sample size and these findings not being confirmed in the overall analysis. Contrastingly, we found higher seropositivity in patients with hormonal therapy as well as patients who underwent surgery, although only patients who had received surgery demonstrated a higher risk in the multivariate analysis. Consistent with other studies, in which patients receiving hormonal therapies demonstrated high seroconversion after vaccination, it has been also postulated that androgen deprivation therapy may have a beneficial role in viral replication due to an androgen-regulated serine protease (Tran et al. 2021; Montopoli et al. 2020). However, we did not find differences in seroprevalence with other anticancer treatments seen in other studies suggesting that the type of treatment does not influence the severity or the increase in mortality from COVID-19 (Pinato et al. 2020). Conversely, another study concluded that chemotherapy led to higher mortality in patients with haematological malignancies (Cabezón-Gutiérrez et al. 2020). Similarly, chemotherapy has been linked to a higher risk of developing COVID-19 (Aschele et al. 2021). Therefore, randomised multicentre studies are needed to clarify these concerns.
Cancer status also plays an important role in patient immunosuppression, as observed in the S0 analysis as tumour-free patients showed a higher seropositivity compared to those with tumour located or metastatic cancers. This difference was also observed in the overall analysis but was not related as an independent risk factor for seropositivity.
Andorra’s nationwide vaccination programme started throughout our study period. This is further evident as 79.1% of our cohort received the first COVID-19 dose while 77.5% received the second dose and seroprevalence increased thorughout the study. These percentages are slightly higher than the general Andorran population at that time since oncologic patients were an initial target for the vaccination campaign. One interesting finding in our study was a higher serological response among patients who had received a second dose of ChAdOx1-S vaccine, especially in S12 analysis. A recently published metanalysis showed seroconversion rates after COVID-19 vaccination in cancer patients involving 17 studies; only 4 studies included the ChAdOx1-S vaccine with BNT162b2 and only concerning patients with hematologic malignancies (Guven et al. 2021). While some studies showed no differences in seroconversion according to which vaccine was received (Gavriatopoulou et al. 2021; Bird et al. 2021; Lim et al. 2021; Chowdhury et al. 2021), only one study showed higher antibody titters after the first and second dose of BNT162b2 among patients with lymphoma (Chowdhury et al. 2021). Nevertheless, these studies are limited to patients with certain haematological malignancies and cannot be extrapolated to the general oncology population. Whether the serological response after vaccination in solid tumours patients and influence of treatments received is not yet clarified so further studies are needed.
This study has several strengths. Given the nationwide size of our cohort, it is one of the largest studies observing serologic response during one year in cancer patients. Moreover, when compared to similar studies, the broad representation of multiple solid tumours within our oncologic cohort stands out. Additionally, access to a single database with all the country’s medical records allowed for thorough detail into specific characteristics as well as homogeneity within records.
However, this study has several limitations. First, its lack of a matched control group prevented us from directly comparing between the oncologic population and non-oncological population. Second, the heterogeneity of the cohort, which limits the conclusions obtained, especially when comparing different oncologic treatments. Thirdly, missings during follow-up study. Additionally, patients being vaccinated during our follow-up period might have altered the results of serologies.
In conclusion, we analysed a large cohort of oncologic patients observing a higher seroprevalence among females and patients who received hormonal therapy and surgery while patients with hematologic malignancies and biologic therapies showed lower seropositivity without finding differences in the type of tumour or anticancer treatment. Prospective study and larger samples are needed to better understand the effect of humoral response among oncologic patients. Particular attention is required in the response of oncologic patients to SARS-COV2 infection to decide on subsequent vaccinations.
References
Aschele C, Negru ME, Pastorino A, Cavanna L, Zagonel V, Barone-Adesi F et al (2021) Incidence of SARS-CoV-2 infection among patients undergoing active antitumor treatment in Italy. JAMA Oncol 7(2):304
Bird S, Panopoulou A, Shea RL, Tsui M, Saso R, Sud A et al (2021) Response to first vaccination against SARS-CoV-2 in patients with multiple myeloma. Lancet Haematol 8(6):e389–e392
Cabezón-Gutiérrez L, Custodio-Cabello S, Palka-Kotlowska M, Oliveros-Acebes E, García-Navarro MJ, Khosravi-Shahi P (2020) Seroprevalence of SARS-CoV-2–specific antibodies in cancer outpatients in Madrid (Spain): A single center, prospective, cohort study and a review of available data. Cancer Treat Rev 90:102102
Cantini L, Bastianelli L, Lupi A, Pinterpe G, Pecci F, Belletti G et al (2021) Seroprevalence of sars-cov-2–specific antibodies in cancer patients undergoing active systemic treatment: A single-center experience from the marche region, italy. J Clin Med 10(7):1503
Chowdhury O, Bruguier H, Mallett G, Sousos N, Crozier K, Allman C et al (2021) Impaired antibody response to COVID-19 vaccination in patients with chronic myeloid neoplasms. Br J Haematol 194(6):1010–1015
Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z et al (2020) Patients with cancer appear more vulnerable to SARS-COV-2: a multi-center study during the COVID-19 outbreak. Cancer Discovery. https://doi.org/10.1158/2159-8290.CD-20-0422
Daniel P, Oran A, Eric J, Topol M (2020) Prevalence of Asymptomatic SARS-CoV-2 Infection. Ann Int Med
Gavriatopoulou M, Terpos E, Kastritis E, Briasoulis A, Gumeni S, Ntanasis-Stathopoulos I et al (2021) Low neutralizing antibody responses in WM, CLL and NHL patients after the first dose of the BNT162b2 and AZD1222 vaccine. Clin Experim Med. https://doi.org/10.1007/s10238-021-00746-4
Guven DC, Sahin TK, Kilickap S, Uckun FM (2021) Antibody responses to COVID-19 vaccination in cancer: a systematic review. Front Oncol 4:11
Jaillon S, Berthenet K, Garlanda C (2019) Sexual dimorphism in innate immunity. Clin Rev Allergy Immunol 56(3):308–321
Liang W, Guan W, Chen R, Wang W, Li J, Xu K et al (2020) Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 21(3):335–337
Lim SH, Campbell N, Johnson M, Joseph-Pietras D, Collins GP, O’Callaghan A et al (2021) Antibody responses after SARS-CoV-2 vaccination in patients with lymphoma. Lancet Haematol 8(8):e542–e544
Liu H, Yang D, Chen X, Sun Z, Zou Y, Chen C et al (2021) The effect of anticancer treatment on cancer patients with COVID-19: A systematic review and meta-analysis. Cancer Med 10(3):1043–1056
Miyashita H, Mikami T, Chopra N, Yamada T, Chernyavsky S, Rizk D et al (2020) Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann Oncol 31(8):1088–1089
Montopoli M, Zumerle S, Vettor R, Rugge M, Zorzi M, Catapano CV et al (2020) Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532). Ann Oncol 31(8):1040–1045
Pinato DJ, Zambelli A, Aguilar-Company J, Bower M, Sng CCT, Salazar R et al (2020) Clinical portrait of the SARS-CoV-2 epidemic in european patients with cancer. Cancer Discov 10(10):1465–1474
Pollán M, Pérez-Gómez B, Pastor-Barriuso R, Oteo J, Hernán MA, Pérez-Olmeda M et al (2020) Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. The Lancet 396(10250):535–544
Royo-Cebrecos C, Vilanova D, López J, Arroyo V, Pons M, Francisco G et al (2021) Mass SARS-CoV-2 serological screening, a population-based study in the Principality of Andorra. Lancet Region Health Europe. https://doi.org/10.1016/j.lanepe.2021.100119
Saini KS, Tagliamento M, Lambertini M, McNally R, Romano M, Leone M et al (2020) Mortality in patients with cancer and coronavirus disease: A systematic review and pooled analysis of 52 studies. Eur J Cancer. https://doi.org/10.1016/j.ejca.2020.08.011
Salje H, Tran Kiem C, Lefrancq N, Courtejoie N, Bosetti P, Paireau J et al (2020) Estimating the burden of SARS-CoV-2 in France. Science 369(6500):208–211
Snoeck CJ, Vaillant M, Abdelrahman T, Satagopam VP, Turner JD, Beaumont K, et al. Prevalence of SARS-CoV-2 infection in the Luxembourgish population-the CON-VINCE study. Available from: https://doi.org/10.1101/2020.05.11.20092916
Solodky ML, Galvez C, Russias B, Detourbet P, N’Guyen-Bonin V, Herr A-L et al (2020) Lower detection rates of SARS-COV2 antibodies in cancer patients versus health care workers after symptomatic COVID-19. Ann Oncol 31(8):1087–1088
Stringhini S, Wisniak A, Piumatti G, Azman AS, Lauer SA, Baysson H et al (2020) Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. The Lancet 396(10247):313–319
Subbiah V (2020) A global effort to understand the riddles of COVID-19 and cancer. Nature Cancer 1(10):943–945
Thakkar A, Gonzalez-Lugo JD, Goradia N, Gali R, Shapiro LC, Pradhan K et al (2021) Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell 39(8):1081-1090.e2
Tran S, Truong TH, Narendran A (2021) Evaluation of COVID-19 vaccine response in patients with cancer: An interim analysis. Eur J Cancer 159:259–274
Ueda M, Martins R, Hendrie PC, McDonnell T, Crews JR, Wong TL et al (2020) Managing cancer care during the COVID-19 pandemic: agility and collaboration toward a common goal. J Natl Comprehens Cancer Network. https://doi.org/10.6004/jnccn.2020.7560
Wang C, Horby PW, Hayden FG, Gao GF (2020) A novel coronavirus outbreak of global health concern. Lancet. 395(10223):470–473. https://doi.org/10.1016/S0140-6736(20)30185-9
WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020 [Internet]. [cited 2020 Jun 2]. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC (2020) Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19). JAMA 324(8):782
Wise-Draper TM, Desai A, Elkrief A, Rini BI, Flora DB, Bowles DW et al (2020) LBA71 Systemic cancer treatment-related outcomes in patients with SARS-CoV-2 infection: A CCC19 registry analysis. Ann Oncol. https://doi.org/10.1016/j.annonc.2020.08.2312
World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report. Available at: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---22-february-2022.
Xu X, Sun J, Nie S, Li H, Kong Y, Liang M et al (2020) Seroprevalence of immunoglobulin M and G antibodies against SARS-CoV-2 in China. Nat Med 26(8):1193–1195
Yu J, Ouyang W, Chua MLK, Xie C (2020) SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol 6(7):1108
Zambelli A, Fotia V, Bosetti T, Negrini G, di Croce A, Moro C, Poletti PL, Bettini AC, Arnoldi E, Messina C, Merelli B, Callegaro AP, Chiudinelli L, Mosconi S, Tondini C (2020) 1676MO Prevalence and clinical impact of asymptomatic or mildly symptomatic SARSCoV-2 infection among actively treated cancer patients during COVID-19 pandemic in Italy. Ann Oncol 31:S994. Available from: http://www.annalsofoncology.org/article/S0923753420417375/fulltext
Funding
Andorran Health Services.
Author information
Authors and Affiliations
Contributions
All authors were involved in the study concept. CRC, SA, EB, FC and DV were involved in study design. CRC, IR, EM, LBC, JSP, JP, FC and SA were involved in data acquisition. CRC, DV, EB, JMB and OV were involved in data analysis or interpretation. CRC, IR, DV, EB, JMP, JPS, FC and drafted and revised the manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Royo-Cebrecos, C., Robert-Montaner, Ï., Vilanova, D. et al. Seroprevalence study prior and post vaccination in cancer patients in principality of Andorra (COVONCO study). J Cancer Res Clin Oncol 149, 2883–2892 (2023). https://doi.org/10.1007/s00432-022-04141-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-04141-8