Abstract
Purpose
The aim of this retrospective study was to evaluate the prognostic impact of global health status assessment tools in elderly patients with endometrial cancer (EC) on survival.
Methods
Preoperative frailty status was assessed by the G8 geriatric screening tool (G8 Score), Lee Schonberg prognostic index, Charlson Comorbidity index and American Society of Anesthesiologists Physical Status System in women older than 60 years with EC. Univariable and multivariable Cox-regression analyses, as well as Kaplan–Meier survival analyses were performed to determine the prognostic impact. Statistical analyses were adjusted for cancer entity-specific risk factors such as conventional histopathological tumor characteristics and relevant anamnestic life style parameters.
Results
153 patients with all stages of EC who were operated at the University Medical Center Mainz between 2008 and 2019 were included. In multivariable analyses, only the G8 Score retained independent significance as a prognostic factor for disease-specific survival (DSS) (HR:4.58; 95% CI [1.35–15.51]) and overall survival (OS) (HR:2.89; 95% CI [1.31–6.39]. 92 patients (61.3%) were classified as G8-non-frail with a significantly increased DSS and OS rate compared to the 58 G8-frail patients (DSS:93.8% vs. 60.8%; p < 0.001 and OS:88.2% vs. 49.7%; p < 0.001; respectively).
Conclusions
This is the first study demonstrates the substantial clinical and prognostic impact of the G8 Score on survival in elderly women with EC. Assessing the frailty status to estimate the individual vulnerability of elderly cancer patients could be useful in preoperative decision-making to individualize treatment plans such as the surgical radicality and to improve pre- and postoperative morbidity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endometrial Carcinomas (EC) are the most frequent gynecological malignancies in developed countries. With a prevalence of 1–2% in all women and an incidence peak between the ages of 60 and 70 years (142,000 new cases in the world per year) (Sung et al. 2021). EC has become”the fifth leading cause of death from cancer in women” (Bourgin et al. 2017). The incidence and the associated mortality are currently rising, potentially due to various factors, including an increasing prevalence of obesity and diabetes as well as the decreasing use of progestin-based postmenopausal hormone treatment (Lu and Broaddus 2020) (Cote et al. 2015). To further specify EC and to make decisions concerning the treatment and prognosis of a patient, conventional risk factors such as histopathological tumor characteristics including tumor stage (TNM and FIGO classification), histological type and grading are used (Felix et al. 2010). Moreover, the postoperative residual tumor burden has been found to be a decisive predictor for surgical quality and consequently of the risk of recurrence (Cree et al. 2020), (Emons et al. 2018).
Increased age is often associated with more aggressive and advanced diseases (Driver and Viswanathan 2017), (Dumas et al. 2016), (Saegusa et al. 2002). The incidence of all cancer entities is statistically overrepresented in the group of the elderly with approximately 54% and with 70% mortality (Siegel et al. 2019). The worse outcome of the population older than 65 years appears to be associated with a lesser likelihood to apply all types of standardized oncological investigations and radical operative treatment strategies (Freedman et al. 2017), (Merchant et al. 1996), (Rauh-Hain et al. 2015). However, the role of biological-calendaric age alone as a perioperative prognostic factor remains controversial (Bourgin et al. 2017), (Creutzberg et al. 2000), (Fleming et al. 2011), (Polanczyk et al. 2001), (Quaglia et al. 2009). Therefore, to increase the chance of cure in older women, an adapted standardized therapy algorithm modified to take the special needs of the older adults is required. Multimorbidity, a dysregulated stress response system and decreased physical performance status with representative symptoms such as fatigue, low activity, weight loss, weakness or low gait and physiologic reserves must be taken into account to modify the current treatment guidelines (Lachance et al. 2006), (Alektiar et al. 2003; Walston 2004). Especially in cancer patients, the offered multimodal therapies, as well as the cancer itself are significant additional stressors that challenge the patient’s physiological reserve (Ethun et al. 2017). The increased vulnerability in frail older adults contributes that this population is less able to tolerate acute stressors, e.g., major surgical interventions with the necessity of multivisceral resections (Birkelbach et al. 2019; Clegg et al. 2013). In general, frailty is associated with increased mortality (Hoogendijk et al. 2021).
In fact, the worse prognosis and postoperative outcome of elderly cancer patients appear to depend on multifactorial geriatric conditions (Ahmed et al. 2018). The basis of heterogeneity due to the aging process contains different domains of frailty and deficiency including comorbid conditions and decreased organ-specific physiological reserves (Balducci and Beghe 2000). The objective of an individual preoperative evaluation of the global health status is to identify frail patients expected to have more treatment-related side effects and toxicities (Clark et al. 2016). Currently, two different approaches exist in geriatric oncology to detect frail patients: the intensive multidisciplinary Comprehensive geriatric assessments (CGA) and the concept of a simple screening tool to identify patients who could benefit from a full subsequent detailed CGA (Hamaker et al. 2012). A CGA is a time-consuming systematic process that objectively assesses somatic, functional and psychosocial domains in elderly patients, which contribute to frailty (Honecker et al. 2011). Thus, more comprise screening tools have been developed, such as the G8 geriatric screening tool (G8 Score) which is one of the most used common global health status screening assessment tools (Kenis et al. 2014), (van Walree et al. 2019). The G8 Score proved to be a practical and time-saving preoperative screening test, especially for elderly cancer patients prior to a major abdominal surgical intervention to identify the frail patients who could benefit from a preoperative multidisciplinary assessment (Honecker et al. 2011), (Soubeyran et al. 2011). Besides the G8 Score, other global health status assessment tools exists: the Lee Schonberg prognostic index (Lee-Index), the American Society of Anesthesiologists Physical Status System (ASA PS) and the original Charlson Comorbidity Index (CC-Index) (Mohile et al. 2018), (Hilditch et al. 2003), (Molto and Dougados 2014).
The objective of this single-institution retrospective cohort study was to determine the prognostic influence of various global health status assessment tools on survival in a consecutive group of women older than 60 years of age with EC, who received radical surgical therapy.
Materials and methods
Study population
Women with EC older than 60 years of age irrespective of the histopathological tumor characteristics who underwent primary staging operation in the University Medical Center Mainz between 2008 and 2019 were assessed in this study. Standardized staging operations regardless of the surgical approach (traditional laparotomy or minimally invasive techniques) included a hysterectomy, and bilateral salpingo-oophorectomy, with or without pelvic and para-aortic lymph-node dissection, depending on the currently national guidelines. The surgical treatment was followed by adjuvant radiation or chemotherapy, when appropriate. Long-term follow-up data were collected through telephone calls, written inquiries to the patients or their physicians, and by checking the available patient clinical records up to February 2021. The G8 Score, Lee-Index, ASA PS and CC-Index were retrospectively assessed to determine the individual preoperative global health status.
Global health status assessment tools
The G8 geriatric screening tool (G8 Score) established by Bellera et al. in 2011 is one of the most commonly used rapid geriatric screening test (5 min duration). The test is validated in accordance to predict treatment-related toxicity in elderly cancer patients with systematic chemotherapy. The screening aims to identify frail patients, who could benefit from a full CGA (Soubeyran et al. 2011). As an extension of the Mini Nutritional Assessment questionnaire {item 1–7} (Vellas et al. 1999), the G8 Score also consists of the biological-calendaric age {item 8} (Appendix A1) (Bellera et al. 2012),(Bongue et al. 2017),(Hamaker et al. 2014). The G8 Score ranges from 17 points (not at all impaired—G8-non-frail) to zero points (heavily impaired—G8-frail) with the original cut-off value of ≤ 14 points as an indication for frailty (Hamaker et al. 2012).
The Lee Schonberg prognostic index (Lee-Index) is a 4-year life span calculator calibrated in community-dwelling adults older than 50 years. The prognostic index combines in 15 selected questions self-reported comorbidities and functional measures with sex and age (Lee et al. 2006), (Yourman et al. 2012).
The American Society of Anesthesiologists Physical Status System (ASA PS) is an international commonly used instrument, developed in 1941 by Meyer Saklad et al. with the title “Grading of Patients for Surgical Procedures” to categorize the preoperative medical health status of adult patients (Fitz-Henry 2011). The classification system ranges from ASA 1—healthy patient to ASA 6—a brain-dead patient whose organs are removed for organ donation (Doyle and Garmon 2018).
The Charlson Comorbidity index (CC-Index) was developed and validated to measure 1-year mortality risk and burden of disease especially as a predictor of surgical mortality. Sixteen conditions have been included in the CC-Index with varying degrees of severity (from one to six) depending on their association with mortality risk and disease severity. The sum adds up to the total CC-Index with a maximum of five points (Molto and Dougados 2014).
Data collection
General patient information was gathered from our hospital database including histopathological tumor characteristics (tumor stage (TNM and FIGO), histological grading and subtype), as well as postoperative residual tumor burden recorded into the official documentation of our interdisciplinary tumor conference. Relevant lifestyle parameters such as age, smoking status, medical history and comorbidities were collected from a physician preoperatively. Furthermore, physical performance status, cognitive functioning and nutritional status were evaluated preoperatively by the oncology nurse specialist together with the patient and the accompanying person through a standardized health status self-assessment questionnaire. Furthermore, perioperative clinical and surgical complications were derived according to the International Statistical Classification of Diseases and Related Health Problems (ICD-10) (Organization, 2004). The G8 geriatric screening tool (G8 Score) consists of eight questions with predefined answer options. Seven items correspond with the MNA (Vellas et al. 1999), (Rubenstein et al. 2001), (Guigoz 2006). The several items assessed in the G8 Score are routinely recorded through a standardized health status self-assessment questionnaire in accordance with the MNA as a standard procedure during the presurgical consultation. Adding the missing item “biological-calendaric age” allows us to calculate the G8 Score retrospectively for each individual patient on this basis with the validated cut-off of ≤ 14 points as an indicator for frailty. The routinely utilized and reproducible Lee-Index was modified afterward by following the calculation without the cancer diagnosis. The CC-Index is calculated with the sum of 16 different conditions according to ICD-10 codes (Organization 2004), all recorded from the physician during preoperative consultation and were deposited in the hospital's internal patient database. All patients undergoing elective surgery were examined at the anesthesia preoperative clinic of the Department of Anesthesiology, where the ASA PS was collected.
In case of ambiguous or missing answers, the total score and estimation of the frailty status was not be performed. The determined frailty definitions and subclassifications of the used global health assessment tools with the numbers of analyzed patients were summarized in Table 4.
Statistical analyses
The manuscript was written in accordance with the STROBE-cohort checklist of the EQUATOR network reporting guidelines (Von Elm et al. 2007).
Statistical analyses were performed using the SPSS statistical software program, version 23.0 V5 R (SPSS Inc, Chicago, IL, USA). Patients’ characteristics were given in absolute and relative numbers (categorical data), and continuous data were reported as mean and standard deviation or median and their quartiles. First, we compared the histopathological tumor characteristics, as valuated and international recognized prognostic parameters, with unique anamnestic life style parameters and postoperative events of the patients, preoperatively classified as G8-frail and G8-non-frail, using the Chi-square test for categorical or ordinal variables. Anamnestic life style parameters were collected on the basis of the patients' self-assessment in comparison to their peers.
The frailty status of all patients was measured in addition to the G8 Score with the three further global health assessment tools and the results were variously categorized to increase the feasibility in clinical work routine: The Lee-Index and ASA PS divide the study population into four groups, of the one part based on their 4-year mortality probability (< 10%, 10– < 20%; 20– < 30%, > 30%) and the patient’s preanesthesia medical comorbidities, of the other part. The CC-Index describes three groups based on their comorbidity status in relation to the expected 1-year mortality. In addition, we dichotomized the age in a group younger and older than the mean age of study population, to assess the usability in a clinical context.
The Cox-proportional hazard regression model was used to determine the prognostic influence of the selected preoperative global health status assessment tools (G8 Score, Lee-Index, CC-Index and ASA PS). Furthermore, other established risk factors like histopathological tumor characteristics (tumor stage [FIGO, TNM], histological subtype and grade as well as postoperative residual tumor burden) on survival were evaluated to control for these potential confounders likely to influence the prognosis. In addition, unique anamnestic life style parameters (smoker status, physical performance and cognitive functioning) and the influence of biological-calendaric age were included in the model. First, univariate Cox-regression analysis for every single variable was performed. Second, variables with a p value < 0.05 entered the multivariable Cox-regression analyses with a variable selection via backward elimination. All associations were given as hazard ratios (HR) with their 95% confidence interval (95% CI) and p values. Kaplan–Meier estimations were used to describe progression free survival (PFS), disease-specific survival (DSS) as well as overall survival (OS). All patients routinely received a computer tomography (CT) either preoperatively to determine the clinical tumor stage or, in the case of non-complete resection a CT scan as baseline imaging postoperatively. Consequentially, PFS was defined as the length of time after the primary operation that a patient lives without a relapse. In case of residual tumor burden, PFS was defined as time after surgery till clinical or radiological progression of disease. DSS was defined as the length of time after the primary operation until the death due to EC. OS was measured from the date of operation to the date of death or last follow-up. The log-rank test was used to compare the survival curves. All associations were given as HRs with their 95% CI and p values. A p value of < 0.05 was regarded as statistically significant.
Results
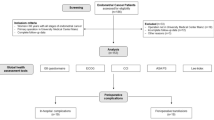
A total of 185 patients were screened. 32 patients were excluded, because they did not undergo primary staging operation in our institution (n = 20) or because of incomplete follow-up information (n = 12). 153 patients were included in the final retrospective cohort study (Fig. 1). The mean age of the cohort was 71.0 ± 7.4 years. The median follow-up time was 31.0 (8.0–68.5) months. 25 relapses and 20 deaths occurred during the study period (Table 1). All patients were classified with the G8 geriatric screening tool in G8-frail (n = 58) and G8-non-frail (n = 92), using the previously evaluated cut-off value of ≤ 14 points for being at risk. All conventional histopathological tumor characteristics did not differ between the two cohorts, whereas unique anamnestic life style parameters (e.g., smoker status, physical performance status and cognitive functioning) were statistically more frequent in the G8-frail cohort.
In the univariable Cox-regression analyses, all examined histopathological tumor characteristics were associated with PFS, DSS and OS except the TNM-tumor stage and the histological subtype for PFS (Table 2). Moreover, preoperative classification as G8-frail was associated with a significantly decreased survival (Table 2 and 3). In contrast, unique anamnestic life style parameters as well as mean age did not predict postoperative survival (Table 2).
In the multivariable analyses, only FIGO stage and the preoperative frailty validation with G8 Score retained their independent significance for DSS and OS in patients older than 60 years with EC (G8 – DSS: HR: 4.58; 95% CI [1.35–15.51], G8 – OS: HR: 2.89; 95% CI [1.31–6.39]) (Table 3). All other conventional histopathological tumor characteristics (TNM-tumor stage, histological subtype and grading as well as postoperative residual tumor burden) and further global health status assessment tools (Lee-Index, CC-Index and ASA PS) were not independently associated with the postoperative prognosis (all p values > 0.05).
In Kaplan–Meier estimations, all global health assessment tools showed statistically significant differences in terms of 5-year OS (Table 4). Mean age showed no significant difference for 5-year OS (younger than average 79.2% vs. older than average 71.4%; p = 0.321) (Fig. 2).
Kaplan–Meier plots of global health status assessment tools and age. G8 geriatric screening tool (a), (b), Lee Schonberg prognostic index (c), (d), American Society of Anesthesiologists Physical Status System (e), (f), Charlson Comorbidity Index (g), (h), and mean age (i), (j) for disease-specific survival (a, c, e, g, i) and overall survival (b, d, f, h, j)
In univariate Cox-regression analyses, four out of eight items of the G8 Score (functional status {item 4}, cognitive status {item 5}, polypharmacy {item 6} and increased age over 80 years {item 8}) predicted OS (HR: 0.47, 95% CI [0.26–0.86]; HR: 0.49, 95% CI [0.27–0.90]; HR: 0.33, 95% CI [0.16–0.71] and HR: 0.43, 95%CI [0.25–0.75]; respectively) (Supplementary Table).
Discussion
To the best of our knowledge, we report an independent prognostic impact of the G8 geriatric screening tool on DSS and OS for women older than 60 years with all tumor stages of EC for the first time. The preoperatively used G8 Score demonstrated this impact independent of established histopathological tumor characteristics in multivariable analyses. In contrast, other global health assessment tools, e.g., Lee-Index, CC-Index and ASA PS, were not able to stratify the cohort into subgroups with a statistically significant and independent survival difference. Furthermore, various single clinically meaningful anamnestic life style parameters (e.g., smoking status, physical performance status or cognitive functioning) were also not reliable prognostic factors. One reason might be that frailty is nowadays regarded as an age-related complex and multifactorial syndrome of marked vulnerability, which is inadequately reflected by single anamnestic factors (Fried 2001),(Bandeen-Roche et al. 2006). Clegg et al. defined frailty as “the most problematic expression of population aging. It is a state of vulnerability to poor resolution of homoeostasis after a stressor event and is a consequence of cumulative decline in many physiological systems during a lifetime” (Clegg et al. 2013).
Moreover, older cancer patients have been shown to be less able to tolerate and adapt to stressors like acute illnesses or surgical interventions (Linn et al. 1982),(Youl et al. 2021). One could be explained by the excellent prognostic impact of preoperative evaluation with G8 Score because of the combination of items with the main focus on nutrition status, physical performance as well as cognitive function and social status. The G8 Score can specially assess the contribution to daily functioning and patients’ resilience to the major stressor, surgery (van Gestel et al. 2013). Moreover, the relationship between increasing age and frequently associated comorbidities results in loss of physical capacity and > 60% decline in postoperative self-care in older patients (Hamaker et al. 2015).
Our findings are in line with other reports. Driver et al. reported a strong prognostic value for the presence of selected frailty markers including weight loss and BMI < 20 kg/m2, as well as ECOG > 2 and laboratory pathologies for 3-year DSS (HR: 2.21; 95% CI: 1.02–4.80) and for a shortened OS (HR: 2.34; 95% CI: 1.08–5.03) in a prospective cohort study with 88 consecutive women with EC in 2017 (Driver and Viswanathan 2017). Decoster et al. postulated in their systematic literature review that geriatric screening tools are recommended to identify those patients in need of further evaluation for a multidisciplinary approach in a busy clinical practice (Decoster et al. 2015). Moreover, in another retrospective cohort study, our study group could demonstrated that the G8 score independently predicted PFS in older ovarian cancer patients regardless of maximal surgical effort (Anic et al. 2021).
To individualize therapy for the very heterogeneous older population, a global health validation with validated geriatric assessment tools to identify frailty status before extended oncological surgery should be applied as opposed to age alone (Nadaraja et al. 2018),(Fried 2001). With reference to the existing evidence of age-related conditions and deficits, and not biological-calendaric age itself, preoperative global health assessment tools should be used to consider risks and benefits of adjuvant cancer treatment. In contrast, a number of studies have shown that increasing age could be a risk factor for poorer tolerance especially regarding adjuvant chemotherapy just as radiation, but data for the predictive power before operations are missing (Nadaraja et al. 2018).
Contrastingly, Nadaraja et al. published a prospective randomized controlled study focused on the systematically oncologic treatment decision based on the G8 Score followed by CGA in women older than 70 years of age with various cancer entities. The results were not be able to show an influence on completion rate of oncologic treatment or survival compared to a therapy decision based on standard assessment (Nadaraja et al. 2020). Reasonably, their findings refer to a small and heterogenous cohort of only 21 G8-frail patients with different cancer entities. Moreover, the presented results concern a cohort with predominantly elderly patients in good health condition with a majority of only grade 1–2 of chemotherapy-related toxicities in their population. Nevertheless, the patients with presurgical geriatric screening had a borderline significantly lower incidence of grade 3–4 toxicity (88% vs. 20%; p = 0.055).
Limitations could arise apart from the retrospective and single-institution character of the study from the fact that a small amount of individual data were lost due to the calculation of the frailty assessment tools based on various standardized multiple-choice questionnaires. In case of ambiguous or missing answers, the total score and estimation of the frailty status were not be performed. However, with the completeness of data from 94.8 to 100% (see Tables 1 and 4), the risk of bias due to missing data is minimal. This may be also relevant especially in terms of incomplete follow-up information which was successfully reduced to a minimum of 12 patients by reaching out to patients and physicians through different ways of communication and an extensive review of clinical records. One advantage of our study is the comparability with regards to baseline characteristics of the G8-frail and G8-non-frail cohort. Moreover, the conventional histopathological tumor characteristics, the surgical procedures, the postoperative residual tumor burden as well as surgical parameters were comparable in the G8-frail and G8-non-frail subgroup. These facts underline the independent prognostic impact on DSS and OS of the G8 Score in our cohort. In contrast, the biological-calendaric age alone did not predict DSS nor OS after 5 years, although it was established as an independent risk factor in numerous prior publications. This might be explained by the fact that patients younger than 60 years have been excluded from our study, to enlarge the cohort with a higher number of frail patients.
In conclusion, the G8 Score can be used to predict DSS and OS prior to surgery in elderly women with EC. Preoperative assessment and treatment decisions should not solely rely on a simplistic view of biological-calendaric age or anamnestic life style parameters, but should incorporate more sophisticated tools such as the G8 Score. Making individualized treatment decisions for elderly patients based on preoperative global health status assessment tools through standardized screenings would be desirable. Further research should also focus on preoperative interventions to increase the global health status of G8-frail patients and their potential to improve postoperative outcomes.
Data availability
All data generated or analyzed during this study are included in this article and its supplementary material files. Further enquiries can be directed to the corresponding author.
Abbreviations
- ASA PS:
-
American society of anesthesiologists physical status system
- CC-Index:
-
Charlson comorbidity index
- CGA:
-
Comprehensive geriatric assessments
- CT:
-
Computer tomography
- 95% CI:
-
Confidence interval
- DSS:
-
Disease-specific survival
- EC:
-
Endometrial cancer
- FIGO:
-
International federation of gynecology and obstetrics
- G8 Score:
-
G8 geriatric screening tool
- HR:
-
Hazard ratio
- Lee-Index:
-
Lee Schonberg prognostic index
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- TNM:
-
Tumor staging system
References
Ahmed A, Deng W, Tew W, Bender D, Mannel RS, Littell RD, DeNittis AS, Edelson M, Morgan M, Carlson J (2018) Pre-operative assessment and post-operative outcomes of elderly women with gynecologic cancers, primary analysis of NRG CC-002: an NRG oncology group/gynecologic oncology group study. Gynecol Oncol 150(2):300–305
Alektiar KM, Venkatraman E, Abu-Rustum N, Barakat RR (2003) Is endometrial carcinoma intrinsically more aggressive in elderly patients? Cancer 98(11):2368–2377
Anic K, Birkert S, Schmidt MW, Linz VC, Heimes A-S, Krajnak S, Schwab R, Schmidt M, Westphalen C, Hartmann EK (2021) G-8 geriatric screening tool independently predicts progression-free survival in older ovarian cancer patients irrespective of maximal surgical effort: results of a retrospective cohort study. Gerontology. https://doi.org/10.1159/000520328
Balducci L, Beghe C (2000) The application of the principles of geriatrics to the management of the older person with cancer. Crit Rev Oncol Hematol 35(3):147–154
Bandeen-Roche K, Xue Q-L, Ferrucci L, Walston J, Guralnik JM, Chaves P, Zeger SL, Fried LP (2006) Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci 61(3):262–266
Bellera C, Rainfray M, Mathoulin-Pelissier S, Mertens C, Delva F, Fonck M, Soubeyran P (2012) Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol 23(8):2166–2172
Birkelbach O, Mörgeli R, Spies C, Olbert M, Weiss B, Brauner M, Neuner B, Francis RC, Treskatsch S, Balzer F (2019) Routine frailty assessment predicts postoperative complications in elderly patients across surgical disciplines–a retrospective observational study. BMC Anesthesiol 19(1):1–10
Bongue B, Buisson A, Dupre C, Beland F, Gonthier R, Crawford-Achour É (2017) Predictive performance of four frailty screening tools in community-dwelling elderly. BMC Geriatr 17(1):1–9
Bourgin C, Lambaudie E, Houvenaeghel G, Foucher F, Lévêque J, Lavoué V (2017) Impact of age on surgical staging and approaches (laparotomy, laparoscopy and robotic surgery) in endometrial cancer management. Eur J Surg Oncol (EJSO) 43(4):703–709
Clark LH, Jackson AL, Gehrig PA, Bae-Jump V, Van Le L, Ko EM (2016) Adjuvant treatment and clinical trials in elderly patients with endometrial cancer: a time for change? Int J Gynecol Cancer 26(2):282
Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K (2013) Frailty in elderly people. Lancet 381(9868):752–762
Cote ML, Ruterbusch JJ, Olson SH, Lu K, Ali-Fehmi R (2015) The growing burden of endometrial cancer: a major racial disparity affecting black women. Cancer Epidemiol Prev Biomark 24(9):1407–1415
Cree IA, White VA, Indave BI, Lokuhetty D (2020) Revising the WHO classification: female genital tract tumours. Histopathology 76(1):151–156
Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Wárlám-Rodenhuis CC, De Winter KA, Lutgens LC, van den Bergh AC, van de Steen-Banasik E (2000) Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. Lancet 355(9213):1404–1411
Decoster L, Van Puyvelde K, Mohile S, Wedding U, Basso U, Colloca G, Rostoft S, Overcash J, Wildiers H, Steer C (2015) Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations. Ann Oncol 26(2):288–300
Doyle DJ, Garmon EH (2018) American Society of Anesthesiologists classification (ASA class). StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK441940.
Driver JA, Viswanathan AN (2017) Frailty measure is more predictive of outcomes after curative therapy for endometrial cancer than traditional risk factors in women 60 and older. Gynecol Oncol 145(3):526–530
Dumas L, Ring A, Butler J, Kalsi T, Harari D, Banerjee S (2016) Improving outcomes for older women with gynaecological malignancies. Cancer Treat Rev 50:99–108
Emons G, Steiner E, Vordermark D, Uleer C, Bock N, Paradies K, Ortmann O, Aretz S, Mallmann P, Kurzeder C (2018) Interdisciplinary diagnosis, therapy and follow-up of patients with endometrial cancer. Guideline (S3-Level, AWMF registry number 032/034-OL, April 2018)–Part 2 with recommendations on the therapy and follow-up of endometrial cancer, palliative care, psycho-oncological/psychosocial care/rehabilitation/patient information and healthcare facilities. Geburtshilfe und Frauenheilkunde 78(11):1089–1109
Ethun CG, Bilen MA, Jani AB, Maithel SK, Ogan K, Master VA (2017) Frailty and cancer: implications for oncology surgery, medical oncology, and radiation oncology. CA: Cancer J Clin 67(5):362–377
Felix AS, Weissfeld JL, Stone RA, Bowser R, Chivukula M, Edwards RP, Linkov F (2010) Factors associated with Type I and Type II endometrial cancer. Cancer Causes Control 21(11):1851–1856
Fitz-Henry J (2011) The ASA classification and peri-operative risk. Ann R Coll Surg Engl 93(3):185–187
Fleming ND, Lentz SE, Cass I, Li AJ, Karlan BY, Walsh CS (2011) Is older age a poor prognostic factor in stage I and II endometrioid endometrial adenocarcinoma? Gynecol Oncol 120(2):189–192
Freedman RA, Foster JC, Seisler DK, Lafky JM, Muss HB, Cohen HJ, Mandelblatt J, Winer EP, Hudis CA, Partridge AH (2017) Accrual of older patients with breast cancer to alliance systemic therapy trials over time: protocol A151527. J Clin Oncol 35(4):421
Fried LP (2001) Frailty in older adults: evidence for a phenotype. J Gerontol Medical Sciences 56:M146–M156
Guigoz Y (2006) The Mini Nutritional Assessment (MNA) review of the literature--What does it tell us?. Journal of Nutrition Health and Aging 10(6):466
Hamaker ME, Jonker JM, de Rooij SE, Vos AG, Smorenburg CH, van Munster BC (2012) Frailty screening methods for predicting outcome of a comprehensive geriatric assessment in elderly patients with cancer: a systematic review. Lancet Oncol 13(10):e437–e444
Hamaker ME, Mitrovic M, Stauder R (2014) The G8 screening tool detects relevant geriatric impairments and predicts survival in elderly patients with a haematological malignancy. Ann Hematol 93(6):1031–1040
Hamaker ME, Prins MC, Schiphorst AH, van Tuyl SA, Pronk A, van den Bos F (2015) Long-term changes in physical capacity after colorectal cancer treatment. J Geriatr Oncol 6(2):153–164
Hilditch W, Asbury A, Jack E, McGrane S (2003) Validation of a pre-anaesthetic screening questionnaire. Anaesthesia 58(9):874–877
Honecker F, Wedding U, Huschens S, Bokemeyer C, Board I-GA (2011) Incorporation of geriatric assessment into oncology practive: views and attitudes of physicians participating in the IN-GHO registry. J Clin Oncol 29(15_suppl):e19607–e19607
Hoogendijk EO, Stolz E, Oude Voshaar RC, Deeg DJ, Huisman M, Jeuring HW (2021) Trends in frailty and its association with mortality: results from the longitudinal aging study Amsterdam (1995–2016). Am J Epidemiol 1:kwab018
Kenis C, Decoster L, Van Puyvelde K, De Greve J, Conings G, Milisen K, Flamaing J, Lobelle J-P, Wildiers H (2014) Performance of two geriatric screening tools in older patients with cancer. J Clin Oncol 32(1):19–26
Lachance J, Everett E, Greer B, Mandel L, Swisher E, Tamimi H, Goff B (2006) The effect of age on clinical/pathologic features, surgical morbidity, and outcome in patients with endometrial cancer. Gynecol Oncol 101(3):470–475
Lee SJ, Lindquist K, Segal MR, Covinsky KE (2006) Development and validation of a prognostic index for 4-year mortality in older adults. JAMA 295(7):801–808
Lewin SN (2011) Revised FIGO staging system for endometrial cancer. Clin Obstet Gynecol 54(2):215–218
Linn BS, Linn MW, Wallen N (1982) Evaluation of results of surgical procedures in the elderly. Ann Surg 195(1):90
Lu KH, Broaddus RR (2020) Endometrial cancer. N Engl J Med 383(21):2053–2064
Merchant TE, McCormick B, Yahalom J, Borgen P (1996) The influence of older age on breast cancer treatment decisions and outcome. Int J Radiat Oncol Biol Phys 34(3):565–570
Mohile SG, Dale W, Somerfield MR, Schonberg MA, Boyd CM, Burhenn PS, Canin B, Cohen HJ, Holmes HM, Hopkins JO (2018) Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol 36(22):2326
Molto A, Dougados M (2014) Comorbidity indices. Clin Exp Rheumatol 32(5 Suppl 85):131–134
Nadaraja S, Jørgensen TL, Matzen L-E, Herrstedt J (2018) Impact of age, comorbidity, and FIGO stage on treatment choice and mortality in older danish patients with gynecological cancer: a retrospective register-based cohort study. Drugs-Real World +es 5(4):225–235
Nadaraja S, Matzen L-E, Jørgensen TL, Dysager L, Knudsen AØ, Jeppesen SS, Möller S, Herrstedt J (2020) The impact of comprehensive geriatric assessment for optimal treatment of older patients with cancer: a randomized parallel-group clinical trial. J Geriatr Oncol 11(3):488–495
Polanczyk CA, Marcantonio E, Goldman L, Rohde LE, Orav J, Mangione CM, Lee TH (2001) Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgery. Ann Intern Med 134(8):637–643
Quaglia A, Tavilla A, Shack L, Brenner H, Janssen-Heijnen M, Allemani C, Colonna M, Grande E, Grosclaude P, Vercelli M (2009) The cancer survival gap between elderly and middle-aged patients in Europe is widening. Eur J Cancer 45(6):1006–1016
Rauh-Hain JA, Pepin KJ, Meyer LA, Clemmer JT, Lu KH, Rice LW, Uppal S, Schorge JO, Del Carmen MG (2015) Management for elderly women with advanced-stage, high-grade endometrial cancer. Obstet Gynecol 126(6):1198–1206
Rubenstein LZ, Harker JO, Salva A, Guigoz Y, Vellas B (2001) Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF). J Gerontol A Biol Sci Med Sci 56(6):M366–M372. https://doi.org/10.1093/gerona/56.6.M366
Saegusa M, Machida D, Okayasu I (2002) Age-dependent differences in tumor cell polarity in endometrial carcinomas. J Cancer Res Clin Oncol 128(4):205–213
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA: Cancer J Clin 69(1):7–34
Soubeyran P, Bellera C, Goyard J, Heitz D, Cure H, Rousselot H, Albrand G, Servent V, Saint Jean O, Roy C (2011) Validation of the G8 screening tool in geriatric oncology: the ONCODAGE project. J Clin Oncol 29(15_suppl):9001–9001
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin 71(3):209–249
van Gestel YR, Lemmens VE, de Hingh IH, Steevens J, Rutten HJ, Nieuwenhuijzen GA, van Dam RM, Siersema PD (2013) Influence of comorbidity and age on 1-, 2-, and 3-month postoperative mortality rates in gastrointestinal cancer patients. Ann Surg Oncol 20(2):371–380
van Walree IC, Scheepers E, van Huis-Tanja L, Emmelot-Vonk MH, Bellera C, Soubeyran P, Hamaker ME (2019) A systematic review on the association of the G8 with geriatric assessment, prognosis and course of treatment in older patients with cancer. J Geriatr Oncol 10(6):847–858
Vellas B, Guigoz Y, Garry PJ, Nourhashemi F, Bennahum D, Lauque S, Albarede J-L (1999) The mini nutritional assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 15(2):116–122
Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP (2007) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Bull World Health Organ 85:867–872
Walston J (2004) Frailty–the search for underlying causes. Sci Aging Knowl Environ 2004(4):pe4–pe4
WH Organization (2004) The international statistical classification of diseases and health related problems ICD-10: tenth revision. Volume 1: tabular list (Vol 1). World Health Organization
Youl PH, Theile DE, Moore J, Harrington J, Philpot S (2021) Outcomes following major resection for colorectal cancer in patients aged 65+ years: a population-based study in Queensland, Australia. ANZ J Surg 91(5):932–937
Yourman LC, Lee SJ, Schonberg MA, Widera EW, Smith AK (2012) Prognostic indices for older adults: a systematic review. JAMA 307(2):182–192
Acknowledgements
Parts of the here presented results derive from the doctoral thesis at Johannes Gutenberg University in Mainz of Ms. Christin Altehoefer.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study received no external funding.
Author information
Authors and Affiliations
Contributions
Design of the work: KA and MJB. Data acquisition: KA, MJB, CA, SK, VCL, MWS, RS, MS, CW, EKH and AH. Data analysis: KA and MJB. Writing of paper: KA and MJB. All authors critically and substantively revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Annette Hasenburg reports honoraria and expenses from AstraZeneca, FBA Frauenärzte BundesAkademie GmbH, KlarigoVerlag, MedConcept, Med public GmbH, Med update GmbH, Medicultus, Pfizer, Promedicis GmbH, Pierre Fabre Pharma GmbH, Softconsult, Roche Pharma AG, Streamedup! GmbH, Tesaro Bio Germany GmbH. I am consultant to PharmaMar, Promedicis GmbH, Pierre Fabre Pharma GmbH, Roche Pharma AG and Tesaro Bio Germany GmbH. I have received funded research from Celgene. Roxana Schwab reports honoraria and expenses from Roche Pharma AG and AstraZeneca GmbH. Marcus Schmidt reports personal fees from AstraZeneca, BioNTech, Eisai, Lilly, MSD, Novartis, Pantarhei Bioscience, Pfizer, Roche, and SeaGen outside the submitted work. Institutional research funding from AstraZeneca, BioNTech, Eisai, Genentech, German Breast Group, Novartis, Palleos, Pantarhei Bioscience, Pierre-Fabre, and Roche. Travel reimbursement from Pfizer and Roche. In addition, M.S. is named inventor on patent EP 2390370 B1 and patent EP 2951317 B1 issued. Marco Battista reports honoraria and expenses from Pharma Mar, Astra Zeneca, Tesaro, GSK, Roche, Clovis Oncology. The other authors state that no conflicts of interests are to declare.
Ethical approval
The retrospective cohort study will be conducted in accordance with the “Ethical principles for medical research involving human subjects” of the current version of the Declaration of Helsinki. Data collected for this study were obtained as part of routine medical care. Ethical approval for use of these samples for research purposes was not required for this study in accordance with local/ national guidelines.
Consent to participate
Written informed consent from participants was required in accordance with local/national guidelines.
Consent for publication
Written consent for publication of data was obtained from all participants prior to study enrollment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Anic, K., Altehoefer, C., Krajnak, S. et al. The preoperative G8 geriatric screening tool independently predicts survival in older patients with endometrial cancer: results of a retrospective single-institution cohort study. J Cancer Res Clin Oncol 149, 851–863 (2023). https://doi.org/10.1007/s00432-022-03934-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-03934-1