Abstract
Purpose
This study aimed to evaluate the safety and efficacy of chimeric antigen receptor (CAR) disialoganglioside 2 (GD2)-specific (4SCAR-GD2) T cells for treatment of refractory and/or recurrent neuroblastoma (NB) in pediatric patients.
Experimental design
A phase I clinical study using 4SCAR-GD2 T cells for the treatment of NB in pediatric patients was conducted. This study was registered at www.clinicaltrials.gov (NCT02765243). A lentiviral CAR with the signaling domains of CD28/4-1BB/CD3ζ-iCasp9 was transduced into activated T cells. The response to 4SCAR-GD2 T-cell treatment, and 4SCAR-GD2 T-cell expansion and persistence in patients were evaluated. Toxicities were determined based on the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v4.03.
Results
Twelve patients were enrolled and finally ten patients were included in this clinical trial which started from January 1, 2016, to August 1, 2017. These patients had progressive disease (PD) before CAR T-cell infusion. After 4SCAR-GD2 T-cell treatment, 6 (6/10) had stable disease (SD) at 6 months, and 4 (4/10) remained SD at 1 year and alive after 3–4 years of follow-up. Six patients died due to disease progression by the end of July 1, 2020. The median overall survival (OS) time was 25 months (95% CI, 0.00–59.43), and the median progression-free survival (PFS) time was 8 months (95% CI, 0.25–15.75). Grade 3 or 4 hematological toxicities were the common adverse events frequently occurred after fludarabine and cyclophosphamide (Flu/cy) chemotherapy. Grade 1–2 toxicities such as cytokine release syndrome (CRS) and neuropathic pain were common, but were transient and mild.
Conclusions
The 4SCAR-GD2 T-cell therapy demonstrated antitumor effect and manageable toxicities, indicating its potential to benefit children with refractory and/or recurrent NB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroblastoma (NB) is the most prevalent extracranial pediatric solid tumor occurring typically in young children (Sherief et al. 2019). Patients with refractory and/or recurrent NB have a poor prognosis, even after intensive treatment with chemotherapy, surgery, stem cell transplantation, radiation, and/or antibody-based therapy (Mallepalli et al. 2019). Although metaiodobenzylguanidine (MIBG) and disialoganglioside 2 (GD2) monoclonal antibody treatment might be effective, they are difficult to access, resulting in worse prognosis of NB in China (Su et al. 2020).
Disialoganglioside 2 (GD2) is a surface antigen expressed on most neuroectoderm-derived cells including NB, melanoma, and others. GD2 is also expressed less predominantly in normal human tissue cells, such as neurons, skin melanocytes, and peripheral sensory nerve fibers (Ho et al. 2016). GD2 has been an effective target for NB immunotherapy and anti-GD2 monoclonal antibodies (MoAbs) based on the 3F8 and CH14.18 clones have been approved for clinical application (Yu et al. 2010; Kushner et al. 2011). The frequent and severe pain resulting from anti-GD2 antibody therapy is the main adverse event limiting dose escalation.
Chimeric antigen receptor (CAR) T cells, as a new therapeutic paradigm, have demonstrated remarkable antileukemic activity against B cell hematological malignancies (Part et al. 2018; Maude et al. 2018). However, their efficacy against solid tumors has not yet been clearly illustrated. In a previous study, the first- generation GD2 CAR T cells comprised of EBV- cytotoxic T lymphocytes (CTLs) and conventionally activated T cells showed no neurological toxicity, and some patients with active NB even achieved complete response (CR) (Pule et al. 2008; Louis et al. 2011). The third-generation GD2 CAR T cells had similar antitumor effects with significant expansion but the effect was transient. Furthermore, their effects were not greater than those earlier studies of the first-generation GD2 CAR T cells and the first-generation GD2-CAR EBVST (Heczey et al. 2017). The latest research, a second-generation GD2-CAR with CD28/CD3 signaling domain incorporating a humanized anti-GD2 single-chain variable fragment (scFv) based on the K666 antibody, revealed excellent safety and on-target off-tumor toxicity in patients, which illustrated transient antitumor effects (Straathof et al. 2020).
Here we developed a new generation of GD2-CAR T cells (4SCAR-GD2) to evaluate their safety and efficacy for refractory and recurrent NB treatment. The 4SCAR-GD2 used autologous T cells transduced with a lentiviral vector to express the anti-GD2 antibody domain scFv with a CD3-zeta domain, a 4-1BB (CD137) domain, a CD28 extracellular and intracellular domains and an inducible caspase 9 domain. In the 4SCAR-GD2 design, we devised a suicide gene, inducible caspase 9, into the vector construct, without impairing the antitumor efficacy of the CAR T cells. Accumulated studies showed that such design could prevent severe adverse effects (Budde et al. 2013, Gargett and Brown 2014a). Similar research-based an extended CD28 costimulatory signal plus a CD27 signaling domain with an inducible caspase 9 suicide gene, with target-specificity for CD19 surface antigen, has successfully applied in Chinese patients with relapsed or refractory B cell leukemia and achieved CR in up to 70–80% of patients (Xu et al. 2020; Zhang et al. 2018a).
Methods
Study design and participants
The study was approved by the Institutional Review Board of Zhujiang Hospital, affiliated with Southern Medical University (2016-EKZX-001). The study was registered at www.clinicaltrials.gov (NCT02765243). The protocol was conducted according to the principles of the Declaration of Helsinki. Written informed consents were provided by the guardians of all patients.
This was a single-cohort, phase I study of 4SCAR-GD2 T cells in children with refractory and/or recurrent NB. Patients received fludarabine and cyclophosphamide (Flu/cy) for lymphodepletion (Cy at 300 mg/m2/dose on days -4, -3, and-2 and Flu at 25 mg/m2/dose on days -4, -3, and -2 prior to 4SCAR-GD2 T-cell administration). The participants received once or more an intravenous infusion of modified 4SCAR-GD2 T cells and were closely followed-up for treatment-related responses. Additional treatment was not administrated after 4SCAR-GD2 T-cell treatment in patients without progression.
When the 4SCAR-GD2 T cells were not detectable in the peripheral blood in the follow-up, patients might opt to receive additional 4SCAR GD2 T-cell infusion. The additional CAR T-cell products were prepared independently using the cryo-preserved peripheral mononuclear cells or re-collection of PBMC from the patients.
Assessment of clinical response and toxic effects
The response to 4SCAR-GD2 T-cell infusion was evaluated by computed tomography (CT), positron emission tomography (PET) or magnetic resonance imaging (MRI) on the primary tumor sites, and a bone scan and bone marrow aspirate and biopsy were performed to evaluate metastatic sites after the initial infusion, according to the international neuroblastoma response criteria (INRC) (Tu et al. 2019). Further imaging was conducted again at 6 and 12 months after infusion. The response assessment results were recorded at 6 and 12 months after infusion. Overall survival (OS) and progression-free survival (PFS) were determined for evaluating the clinical response to 4SCAR-GD2 cells treatment. The patients had not received 123I‐MIBG scans as the latter was not easily accessible in China. Efficacy parameters included the following: stable disease (SD), partial response (PR) or progressive disease (PD); complete remission (CR); PFS (time from first treatment to disease progression) and OS. Toxicities were assessed through physical examinations, performance tests and laboratory tests of organ function weekly in the first month, each month in 6 months and at the end of the study. Adverse events were graded based on the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) v4.03 (https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf), and cytokine release syndrome(CRS) was graded according to a revised grading system (Park et al. 2018a).
GD2 expression by immunohistochemical (IHC) staining
Formalin-fixed paraffin-embedded tumor sections were immunohistochemically stained with mouse anti-human GD2 antibody (BD Biosciences, Cat# 554,272, 0.5 mg/mL). The isotype control was stained with purified mouse IgG2a (BioLegend, Cat# 400,202). All images were captured from tumor sections using a Zeiss Axio Vert.A1 microscope and Zeiss ZEN imaging software. The tissue staining intensity was compared with that from positive and negative controls and scored from 0 to 4 according to two components: staining intensity and the percentage of positive cells. Each sample was assessed and graded by two independent observers.
Construction of the 4SCAR-GD2 lentiviral vector
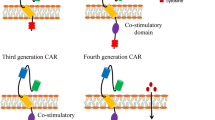
Lentiviral vectors were generated using the NHP/TYF lentiviral vector (LV) system as previously described (Lee et al. 2014; Chang et al. 2005). The DNA sequences of the anti-GD2 CAR clone hu3F8(a humanized mouse 3F8 scFv) were optimized for human codons, chemically synthesized, cloned into the lentiviral vector pTYF and packaged into lentiviral particles for gene transfer (Wang et al. 2006; Ahmed et al. 2014). The final lentiviral constructs were verified by restriction enzyme mapping and DNA sequencing. The GD2 CAR sequences were constructed with a lentiviral CAR design (4SCAR). The 4SCAR incorporated several intracellular T cells signaling motifs including the CD28 extracellular/transmembrane and cytoplasmic domains, the costimulatory 4-1BB intracellular TRAF-binding domain, the CD3ζ chain intracellular domain, and an inducible cell death-initiating caspase 9 genetic cassette (4SCAR-GD2) (Fig. 1). The iCasp9 system has been published in previous studies, and the small molecule dimerizer drug AP1903 could induce CAR T cells apoptosis (Cheung et al. 2012).
T-cell isolation and 4SCAR-GD2 T-cell production
Peripheral blood mononuclear cells (PBMCs) of patients were collected by apheresis and blood buffy coats were prepared. PBMCs were isolated using Ficoll-Paque plus (GE Healthcare). T cells were activated using anti-CD3(MACS CD3 MicroBeads 130-097-043) antibody-conjugated magnetic beads and anti-CD28 (Invitrogen anti-Hu CD28 clone 28.2 16-0289-85) antibody. T cells were maintained in AIM-V (Invitrogen, Thermo Fisher Scientific, Waltham, MA) supplemented with interleukin (IL)-2, IL-7 and IL-15, as previously described (Gargett and Brown 2014b). Phenotypic analysis of the activated cells was performed to confirm T-cell purity. After expansion for two-days, T cells were transduced with the lentiviral 4SCAR-GD2 vector and quality control/assurance assay for CAR T killing function was routinely performed as an integrated program. The 4SCAR-GD2 T cells were collected and infused five days after transduction. Transduction efficiency is reported as the percentage of CAR DNA copies positive per cellular genomes based on a standard single-cell clone tumor cell line with one copy of transgene per cell, and the internal house-keeping gene GAPDH; for example, 100% means 1 copy of CAR per T cell. Safety tests included mycoplasma detection and endotoxin analysis which were conducted and passed before CAR T-cell infusion.
Immunophenotyping of T-cell products
The following anti-human antibodies were used to evaluate the phenotype of the cells: anti-hCD3 PE (MACS, 130,113,129), anti-hCD4 Pacific Blue (BD Biosciences, 558,116), anti-hCD8 PerCp-Cy5.5 (BD Biosciences, 565,310), and anti-hCD8 PE-Cy7 (BD Biosciences, 557,747). Data acquisition was performed using a CytoFLEX flow cytometer (Beckman).
CAR detection by real-time quantitative PCR (qPCR)
The CAR copy number in the blood was determined by real-time qPCR, based on both SYBR and TaqMan probe methods, as previously described (Okada et al. 2015; Cui et al. 2003; Nair et al. 2019; Zhang et al. 2018b). Genomic DNA was harvested from blood cells using a Promega Genomic DNA Purification Kit (Promega Corp, Madison, WI, USA). The qPCR data were collected using MX3000P (Stratagene, Agilent Technologies, Santa Clara, CA, USA) after 4SCAR-GD2 T-cell treatment at 0, 1, 2, and 3 weeks and each month until the CAR copy number was at undetected level.
Cytokine analysis based on a cytokine bead array (CBA)
The serum levels of the cytokines and cytokine receptors TNF-α, IL-2R, IL-1β, IL-8, IL-6 and IL-10 were determined by using the BD CBA Human Soluble Protein Flex Set System at 0, 1, 2, 3 weeks and so on (Arican et al. 2005). The CBA system captures soluble analytes with beads of a known size and fluorescence and allows quantification using flow cytometry according to the manufacturer’s manual.
Statistical analysis
Descriptive statistics (means, ranges and standard deviations or standard errors) were used to summarize data. Correlation analysis was performed using Spearman’s rank correlation coefficient. OS and PFS were estimated by the Kaplan–Meier method. A two-sided p value < 0.05 was considered statistically significant. Analyses were performed with GraphPad Prism 6 (GraphPad Software) and IBM SPSS Statistics for Windows, version 21.0 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
From January 1, 2016, to August 1, 2017, a total of 12 patients were screened, and 10 patients with relapsed (2/10) or refractory (8/10) disease were enrolled in the study (Fig. 2). Seven of the 10 patients were male with a median age of 4.5 years (range 1.8–7 years). The previous treatments of the patients were chemotherapy, surgery, radiation and/or stem cell transplantation (Table S1 in the Supplementary Appendix). All patients were treated with 4SCAR-GD2 T cells (Table 1). Three patients had unresectable tumors at the primary site 1 in the spinal canal and 2 in the abdomen), three patients had bone marrow diseases, and the remaining three patients had multiple bone lesions, one of whom had lymph node involvement at the time of infusion. In the follow-up, when the 4SCAR-GD2 T cells could no longer be detected in the blood of patients No.1, 2 and 7, the guardians/families consented to additional CAR T-cell infusions. As a result, patient No.1 received 4SCAR-GD2 T cells for three times at an interval of 3 months, while patients No.2 and 7 received 4SCAR-GD2 T cells for two times at intervals of 6 months and 3 months, respectively.
Clinical response and survival assessments
Patient No.1 and 7 had NB bone marrow relapse, and CAR T cells expanded poorly after infusion of 4SCAR-GD2 T cells. Their CAR T-cell counts in the peripheral blood were lower than detection sensitivity. Therefore, they received multiple infusions but developed bone marrow relapse at the end.
Patients No. 2, 3, 4, and 9 had multiple skeletal metastatic lesions but negative in bone marrow. The efficacy of 4SCAR T cells treatment was assessed by observing survival because 123I‐MIBG scans evaluation was not available. Intracranial metastases occurred in both patients No.3 and 9. Patient No.3 had a posterior cranial fossa mass and received total surgical resection, but developed skeletal recurrence 1.5 years later. Patient No. 9 had intracranial metastases combined with intratumoral hemorrhage and died of brain herniation. Patients No. 2 and 4 were long-term survivors.
Patient No.6 had a huge abdominal occupancy with residual 50 × 42 × 23 mm on abdominal CT. The residual tumor was 54 × 33 × 22 mm on six months and one year later after 4SCAR-GD2 T-cell infusion. No significant abnormality in the radiological distribution of the residual tumor was seen on PET-CT. Patient No.8 had a spinal cord occupancy that could only be partially resected by surgery, and the residual tumor was slightly smaller than before after 4SCAR-GD2 T cells therapy. Both patients were long-term survivors.
Patient No.5 and 10 had NB soft tissue residuals and developed distal site recurrence after a period of remission after 4SCAR-GD2 T treatment.
The clinical responses to 4SCAR-GD2 T-cell therapy are summarized in Table 2. Of the 10 patients who had PD before CAR T-cell infusion, 6 achieved SD at 6 months, and the remaining 4 patients had SD at 1 year and alive at least 3–4 years of follow-up. The median overall survival (OS) time was 25 months (95% CI, 0.00 to 59.43), and the median progression-free (PFS) survival time was 8 months (95% CI 0.25–15.75). Six patients who relapsed again after 4SCAR-GD2 T-cell infusion received salvage chemotherapy, but these patients died due to disease progression. The survival curves for all patients are shown in Fig. 3.
Characterization, expansion, and persistence of 4SCAR-GD2 T cells
The dose of infused CAR T cells ranged from 0.13–34 × 106/kg body weight. The median lentiviral vector gene transfer efficiency was 36.53%, ranging from 3.59% to 596.08% (500% means 5 copies of lentiviral vector genes inserted in one cell). Cell products contained both CD4+ and CD8+ T cells (median ratio CD4:CD8 = 0.81; range, 0.15–4.27) (Table S2 in the Supplementary Appendix). The 4SCAR-GD2 T cells were released for infusion after a median preparation time of 7–8 days. The CAR DNA copies were measured to be 0.02%–22.4% in the 10 patients (Fig. 4). The number of 4SCAR-GD2 T cells in patient No.10 was markedly expanded when the disease re-relapsed after six months. Moreover, the 4SCAR-GD2 T cells in patient No.8 with 1.74% CAR DNA copies were detected in the blood for more than 1 year (in the 17th month).
Kinetics of 4SCAR-GD2 T cells in the peripheral blood (The percentage (%) of 4SCAR-GD2 T cells in peripheral blood mononuclear cells was assessed by qPCR before and after 4SCAR-GD2 infusion in ten enrolled patients). The 4SCAR-GD2 T cells expanded significantly when the disease relapsed in patient No.10 after six months
Safety assessment
Toxicities were mostly self-limited and resolved soon after treatment with the 4SCAR-GD2 T cells. There was no central neurotoxicity, and intervention or supportive care was not necessary (Table 3). No toxic event-related death was observed in this trial. The common treatment-related toxicities after Flu/cy chemotherapy conditioning were hematological events (neutropenia and thrombocytopenia) (Table S3 in the Supplementary Appendix). These events were fully reversible without any treatment in all patients within a median of 4 days (range, 0–22 days). Acute capillary leak syndrome (4/10) and CRS (9/10) of grades 1–2 were observed approximately 1 week after infusion. A febrile reaction (9/10) was the first and most common symptom observed. Fever without infection and febrile neutropenia occurred within a median of 10 days after infusion (range 0–18 days) and lasted for a mean of 5.9 days (range 0–9 days). The highest temperature was 40 °C and lasted more than 24 h in four patients after 4SCAR-GD2 T-cell infusion at the end of the first week. Hypotension (1/10) and neuropathic pain (3/10) were rare, transient, and mild in the 4SCAR-GD2 T-cell-treated patients. Cough (6/10) and acneiform rash (4/10) were common symptoms (median, 7 days; range, 5–20 days). Eosinophilia was observed in all patients in the phase of CAR T-cell expansion. There were no late-onset toxicities or long-term adverse events in the 4SCAR-GD2 T-cell-treated patients during follow-up.
The patient No.10 experienced extensive papules on the face, scalp, and extremities, that were accompanied with symptoms of pruritus and tenderness with deflorescence over one month. The symptoms receded without any treatment (Fig. 5). Pathological biopsy suggested immune-mediated reaction.
The cytokine profiles after 4SCAR-GD2 T-cell infusions were similar in all cases, with high secretion of IL-2R, IL-10, TNF-α, IL-8, IL-6 and IL-1β (Fig. 6). The serum levels of the cytokines were elevated when fever developed. The highest level of ferritin, IL-6 and IL-10 were 3417 ug/L, 1000 pg/ml and 8014 pg/ml, respectively (Table S3). There were no correlations between CAR T-cell expansion and serum cytokine levels, except for the level of IL-2R (r = 0.857; p = 0.014) (Fig. S1).
Discussion
In China, patients with refractory and/or recurrent NB have a poor prognosis in the late stages (Arican et al. 2005). This study first assessed GD2 CAR T-cell therapy on the basis of costimulatory signals with CD28-4-1BB plus an inducible domain caspase 9 in a lentiviral vector system. The results showed that 4SCAR-GD2 T cells were safe in children with relapsed/refractory NB. Previous studies were based on the first-, second- and third-generation CAR T cells with both CD28 and OX40 costimulatory domains (Pule et al. 2008; Louis et al. 2011; Heczey et al. 2017). Preclinical research indicated that a 3rd-generation CAR incorporating the CD28 and 4-1BB costimulatory domains improved antitumor efficacy compared with the combination of CD28.OX40 domains (Quintarelli et al. 2018). In addition, persistence or exhaustion of T cells may be the main factors contributing to the antitumor effectiveness of GD2 CAR T-cell therapies. CAR T-cell engineering with a combination of multiple signaling domains has been used to improve the safety and efficiency of CAR T cells and in vivo persistence (Jafarzadeh et al. 2020). Previous research showed that high-affinity GD2-specific E101K CAR T cells enhanced antitumor efficacy as compared with the m3F8 CAR T cells in vivo, yet it was concomitantly associated with lethal neurotoxicity more severe than the m3F8 CAR design (Richman et al. 2018, Richman and Milone 2018). Because a murine version of the scFv of the GD2 antibody could result in exhaustion of T cells in vivo thus limit antitumoral efficacy, we incorporated a humanized GD2 scFv antibody moiety in the 4SCAR-GD2 CAR design, which effectively suppressed tumor growth and prolonged patients’ survival without neurotoxicity as reported by others previously (Straathof et al. 2020).
It has been demonstrated that Flu/cy lymphodepleting chemotherapy could enhance CAR T-cell expansion and promote persistence (Louis et al. 2011; Heczey et al. 2017). In this study, 4SCAR-GD2 T-cell persistence was demonstrated to last longer than 6 months after infusion. Due to the limited number of patients, the correlation between the infusion dose and CAR T-cell expansion was not found, and there was no apparent correlation between CAR T-cell expansion and patient response. There was a correlation between early disease progression after CAR T-cell infusion and a poor prognosis. Furthermore, the patients with metastatic bone marrow disease and N-MYC amplification had the worst responses. The patient No.8 who had 1.74% CAR DNA copies detected in the blood for more than 1 year, experienced the longest survival time after the 3 years of follow-up suggesting that the increased 4SCAR-GD2 T-cell persistence could result in prolonged survival.
Previous clinical studies support that the 4SCAR design has a high safety profile and mild cytotoxicity (Xu et al. 2020; Tu et al. 2019). None of the patients in this study required the agent AP1903 to eliminate the CAR T cells because immediate severe toxicity at the time of administration and long-term toxicity were not observed.
CRS is the most common concern in CAR T-cell therapy, especially for the CD19-specific CAR T-cell therapy in patients with leukemia. Application of the anti-IL-6 receptor antibody tocilizumab is a routine management protocol (Titov et al. 2018; Mueller et al. 2018). In this study, CRS was moderate and no severe acute adverse effects were recorded. Although positive correlations between CRS, and tumor burden or CAR T-cell expansion in vivo have been reported in other CAR T-cell studies (Gardner et al. 2017), no such correlation was found in our study.
Toxicity of the hematopoietic system was reported after CAR T-cell therapy, especially after CD19 CAR T-cells therapy (Fried et al. 2019). Some researchers believe that this toxicity is attributed mostly to the lymphodepleting chemotherapy regimen and CRS, characterized by a biphasic effect (Nahas et al. 2020; Schaefer et al. 2019). In our study, cytopenia occurred after the lymphodepleting chemotherapy and also occurred lightly in the late period due to moderate CRS.
It is interesting that the 4SCAR-GD2 T cells markedly expanded when the disease re-relapsed (patient No. 10) after six months, suggesting that re-recurrence of the tumor could stimulate the proliferation of 4SCAR-GD2 T cells again. This phenomenon supports the existence of memory 4SCAR-GD2 T cells in patients, but the rate of tumor growth might be faster than CAR T-cell expansion. The high relapse rate after 4SCAR-GD2 T-cell therapy remains a major challenge. Many factors contribute to a high relapse rate, such as tumor immune escape mechanisms, the immunosuppressive tumor microenvironment, perturbation of antigen presentation, and metabolic alterations in tumor cells, to name a few (Vanichapol et al. 2018).
Because no relationship between infusion dose and T-cell expansion was observed, optimal dose of CAR T cells treatment is uncertain (Maude et al. 2018). The tumor relapse rate might be decreased by several approaches, such as optimizing CAR density, affinity and sensing, improving immunological synapse formation, and combining treatment with oncolytic viruses or PD-1 inhibitor in the future trials (Watanabe et al. 2018).
In conclusion, 4SCAR-GD2 T-cell therapy for relapsed and/or refractory NB patients delayed tumor progression with little to no toxicities, suggesting that 4SCAR-GD2 CAR T-cell treatment could be a safe and efficient approach for the management of NB.
Data availability
Available upon request.
References
Ahmed M, Hu J, Cheung NK (2014) Structure based refinement of a humanized monoclonal antibody that targets tumor antigen disialoganglioside GD2. Front Immunol 5:372
Arican O, Aral M, Sasmaz S et al (2005) Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm 2005:273–279
Budde LE, Berger C, Lin Y et al (2013) Combining a CD20 chimeric antigen receptor and an inducible caspase 9 suicide switch to improve the efficacy and safety of T cell adoptive immunotherapy for lymphoma. PLoS ONE 8:e82742
Chang LJ, Liu X, He J (2005) Lentiviral siRNAs targeting multiple highly conserved RNA sequences of human immunodeficiency virus type 1. Gene Ther 12:1133–1144
Cheung NK, Guo H, Hu J et al (2012) Humanizing murine IgG3 anti-GD2 antibody m3F8 substantially improves antibody-dependent cell-mediated cytotoxicity while retaining targeting in vivo. Oncoimmunology 1:477–486
Cui S, Schroeder CM, Zhang DY et al (2003) Rapid sample preparation method for PCR-based detection of Escherichia coli O157:H7 in ground beef. J Appl Microbiol 95:129–134
Fried S, Avigdor A, Bielorai B et al (2019) Early and late hematologic toxicity following CD19 CAR-T cells. Bone Marrow Transplant 54:1643–1650
Gardner RA, Finney O, Annesley C et al (2017) Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 129:3322–3331
Gargett T, Brown MP (2014a) The inducible caspase-9 suicide gene system as a “safety switch” to limit on-target, off-tumor toxicities of chimeric antigen receptor T cells. Front Pharmacol 5:235
Gargett T, Brown MP (2014b) The inducible caspase-9 suicide gene system as a “safety switch” to limit on-target, off-tumor toxicities of chimeric antigen receptor T cells. Front Pharmacol 5:235
Heczey A, Louis CU, Savoldo B et al (2017) CAR T cells administered in combination with lymphodepletion and PD-1 inhibition to patients with neuroblastoma. Mol Ther 25:2214–2224
Ho WL, Hsu WM, Huang MC et al (2016) Protein glycosylation in cancers and its potential therapeutic applications in neuroblastoma. J Hematol Oncol 9:100
Jafarzadeh L, Masoumi E, Fallah-Mehrjardi K et al (2020) Prolonged persistence of chimeric antigen receptor (CAR) T cell in adoptive cancer immunotherapy: challenges and ways forward. Front Immunol 11:702
Kushner BH, Kramer K, Modak S et al (2011) Successful multifold dose escalation of anti-GD2 monoclonal antibody 3F8 in patients with neuroblastoma: a phase I study. J Clin Oncol 29:1168–1174
Lee DW, Gardner R, Porter DL et al (2014) Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124:188–195
Li K, Dong K, Gao J et al (2012) Neuroblastoma management in Chinese children. J Invest Surg 25:86–92
Louis CU, Savoldo B, Dotti G et al (2011) Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 118:6050–6056
Mallepalli S, Gupta MK, Vadde R (2019) Neuroblastoma: an updated review on biology and treatment. Curr Drug Metab
Maude SL, Laetsch TW, Buechner J et al (2018) Tisagenlecleucel in children and young adults with B-Cell lymphoblastic leukemia. N Engl J Med 378:439–448
Mueller KT, Waldron E, Grupp SA et al (2018) Clinical pharmacology of tisagenlecleucel in B-cell acute lymphoblastic leukemia. Clin Cancer Res 24:6175–6184
Nahas GR, Komanduri KV, Pereira D et al (2020) Incidence and risk factors associated with a syndrome of persistent cytopenias after CAR-T cell therapy (PCTT). Leuk Lymphoma 61:940–943
Nair S, Patel V, Hickey T, et al. (2019) Real-Time PCR assay for differentiation of typhoidal and nontyphoidal salmonella. J Clin Microbiol 57
Okada S, Han S, Patel ES et al (2015) STAT3 signaling contributes to the high effector activities of interleukin-15-derived dendritic cells. Immunol Cell Biol 93:461–471
Park JR, Bagatell R, Cohn SL et al (2017) Revisions to the international neuroblastoma response criteria: a consensus statement from the national cancer institute clinical trials planning meeting. J Clin Oncol 35:2580–2587
Park JH, Riviere I, Gonen M et al (2018) Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med 378:449–459
Pule MA, Savoldo B, Myers GD et al (2008) Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med 14:1264–1270
Quintarelli C, Orlando D, Boffa I et al (2018) Choice of costimulatory domains and of cytokines determines CAR T-cell activity in neuroblastoma. Oncoimmunology 7:e1433518
Richman SA, Milone MC (2018) Neurotoxicity associated with a high-affinity GD2 CAR-response. Cancer Immunol Res 6:496–497
Richman SA, Nunez-Cruz S, Moghimi B et al (2018) High-affinity GD2-specific CAR T cells induce fatal encephalitis in a preclinical neuroblastoma model. Cancer Immunol Res 6:36–46
Schaefer A, Saygin C, Maakaron J et al (2019) Cytopenias after chimeric antigen receptor T-cells (CAR-T) infusion; patterns and outcomes. Biol Blood Marrow Transplant 25:S171
Sherief LM, Hassan TH, Zakaria M et al (2019) Tissue factor expression predicts outcome in children with neuroblastoma: a retrospective study. Oncol Lett 18:6347–6354
Straathof K, Flutter B, Wallace R, et al. (2020) Antitumor activity without on-target off-tumor toxicity of GD2-chimeric antigen receptor T cells in patients with neuroblastoma. Sci Transl Med 12
Su Y, Qin H, Chen C et al (2020) Treatment and outcomes of 1041 pediatric patients with neuroblastoma who received multidisciplinary care in China. Pediatr Investig 4:157–167
Titov A, Petukhov A, Staliarova A et al (2018) The biological basis and clinical symptoms of CAR-T therapy-associated toxicites. Cell Death Dis 9:897
Tu S, Huang R, Guo Z et al (2019) Shortening the ex vivo culture of CD19-specific CAR T-cells retains potent efficacy against acute lymphoblastic leukemia without CAR T-cell-related encephalopathy syndrome or severe cytokine release syndrome. Am J Hematol 94:E322–E325
Vanichapol T, Chutipongtanate S, Anurathapan U et al (2018) Immune escape mechanisms and future prospects for immunotherapy in neuroblastoma. Biomed Res Int 2018:1812535
Wang B, He J, Liu C et al (2006) An effective cancer vaccine modality: lentiviral modification of dendritic cells expressing multiple cancer-specific antigens. Vaccine 24:3477–3489
Watanabe K, Kuramitsu S, Posey AD Jr et al (2018) Expanding the therapeutic window for CAR T cell therapy in solid tumors: the knowns and unknowns of CAR T cell biology. Front Immunol 9:2486
Xu X, Zhao W, Yue Z et al (2020) 4SCAR-GD2-modified T-cell therapy in neuroblastoma with MYCN amplification: a case report with over 4-year follow-up data. Pediatr Investig 4:55–58
Yu AL, Gilman AL, Ozkaynak MF et al (2010) Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 363:1324–1334
Zhang JP, Zhang R, Tsao ST et al (2018a) Sequential allogeneic and autologous CAR-T-cell therapy to treat an immune-compromised leukemic patient. Blood Adv 2:1691–1695
Zhang C, Zheng X, Zhao C et al (2018b) Detection of pathogenic microorganisms from bloodstream infection specimens using TaqMan array card technology. Sci Rep 8:12828
Acknowledgements
The authors would like to thank all members of the study team, the patients and their families.
Funding
Dean’s Fund of Zhujiang Hospital, Southern Medical University, and funds from Science and Technology Planning Project of Shenzhen Municipality (JCYJ20170817172541842, JCYJ20170817172416991, and KQTD20140630143254906).
Author information
Authors and Affiliations
Contributions
Conception and design: LY, LH, DL, YL, LC, and LY. CAR T -cell production: YL, CJ and LJC. Administrative, technical: LY, LL, GZ, BW, ZW, and ST. Acquisition, analysis, or interpretation of data: LH, XL, LW, QH, LL, YZ, and GZ. Drafting of the manuscript: LY, DL, JL and LY. Writing, review, and/or revision of the manuscript: All authors.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no potential conflicts of interest.
Ethical statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Informed consent
All participants and/or their legal guardians provided written informed consent upon enrollment and approved of the dates to further publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yu, L., Huang, L., Lin, D. et al. GD2-specific chimeric antigen receptor-modified T cells for the treatment of refractory and/or recurrent neuroblastoma in pediatric patients. J Cancer Res Clin Oncol 148, 2643–2652 (2022). https://doi.org/10.1007/s00432-021-03839-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-021-03839-5