Abstract
Background
There are currently few data on the outcome of acute myeloid leukemia (AML) in adolescents after allogeneic HSCT. The aim of this study is to describe the outcome and its specific risk factors for children, adolescents and young adults after a first allogeneic HSCT for AML.
Methods
In this retrospective study, we compared the outcome of AML patients receiving a first allogeneic HSCT between 2005 and 2017 according to their age at transplantation’s time: children (< 15 years, n = 564), adolescent and post-adolescent (APA) patients (15–25 years, n = 647) and young adults (26–40 years; n = 1434).
Results
With a median follow-up of 4.37 years (min–max 0.18–14.73 years), the probability of 2-year overall survival (OS) was 71.4% in children, 61.1% in APA patients and 62.9% in young adults (p = 0.0009 for intergroup difference). Both relapse and non-relapse mortality (NRM) Cumulative Incidence (CI) estimated at 2 years were different between the age groups (30.8% for children, 35.2% for APA patients and 29.4% for young adults—p = 0.0254, and 7.0% for children, 10.6% for APA patients and 14.2% for young adults, p < 0.0001; respectively). Whilst there was no difference between the three groups for grade I to IV acute GVHD CI at 3 months, the chronic GVHD CI at 2 years was higher in APA patients and young adults (31.4% and 36.4%, respectively) in comparison to the children (17.5%) (p < 0.0001). In multivariable analysis, factors associated with death were AML cytogenetics (HR1.73 [1.29–2.32] for intermediate risk 1, HR 1.50 [1.13–2.01] for intermediate risk 2, HR 2.22 [1.70–2.89] for high cytogenetics risk compared to low risk), use of TBI ≥ 8 Grays (HR 1.33 [1.09–1.61]), disease status at transplant (HR 1.40 [1.10–1.78] for second Complete Remission (CR), HR 2.26 [1.02–4.98] for third CR and HR 3.07 [2.44–3.85] for active disease, compared to first CR), graft source (HR 1.26 [1.05–1.50] for Peripheral Blood Stem Cells compared to Bone Marrow) and donor age (HR 1.01 (1–1.02] by increase of 1 year).
Conclusion
Age is an independent risk factor for NRM and extensive chronic GVHD. This study suggests that APA patients with AML could be beneficially treated with a chemotherapy-based MAC regimen and bone marrow as a stem cells source.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
There are currently few available data on the outcome of Adolescent and Post-Adolescent (APA) patients after allogeneic hematopoietic stem cell transplantation (HSCT) for acute myeloid leukemia (AML) (Jaime-Pérez et al. 2018; Tomizawa et al. 2017; Canner et al. 2013; Nasir et al. 2017; Pemmaraju et al. 2016).
Acute myeloid leukemia represents about 15–20% of childhood leukemia, approximately 33% of adolescent leukemia, and approximately 50% of adult leukemia. After a peak in the first 2 years of life, the annual incidence of AML increases slowly and gradually after the age of 9 years old (Appelbaum et al. 2006). Pediatric and adult AML patients overall share biological parameters although some differences have not been systematically reviewed to date. Acute myeloid leukemia treatment has considerably improved for all age’s groups over the last 20 years, particularly through the improvement of allogeneic HSCT techniques. However, outcome appears to worsen with increased patient age. In comparison with pediatric and adult groups, the data of allogeneic HSCT for AML in adolescents are rare since they usually represent a small percentage within the cohorts of adults or children. However, these data are important since APA patients are treated in both pediatric and adult hematology departments, using different conditioning regimens—either myeloablative conditioning (MAC) or reduced-intensity conditioning (RIC)—and different graft sources, which might influence the disease outcome. We conducted a large retrospective study based on the French speaking Society for Bone Marrow Transplantation and Cell Therapy (SFGM-TC) registry to analyze and compare the outcome of AML patients classified in three age groups: children (0–14 years), APA patients (15–25 years) and young adults up to 40 years (26–40 years), who received an allogeneic HSCT from January 2005 to December 2017. In addition, we determined the factors influencing Overall Survival (OS), Event-Free Survival (EFS), Non-Relapse Mortality (NRM), Graft Versus Host Disease (GVHD) and Relapse Free Survival (GRFS) in the three age groups.
Methods
This is a retrospective multicenter analysis using the data set from the Francophone Society of Bone Marrow Transplantation and Cellular Therapy (SFGM-TC) registry. The study protocol was approved by the scientific council of the SFGM-TC and complied with French regulatory requirements. The study was conducted according to the Declaration of Helsinki. All patients provided written informed consent authorizing the use of their personal information for research purposes.
We collected data from all patients up to the age of 40 years old included in the SFGM-TC registry from January 2005 to December 2017 who received a first allogeneic HSCT for treatment of AML.
Inclusion criteria were: patients younger than 41 years old who accepted to be registered in the SFGM-TC registry, treated with a first allogeneic HSCT for AML in first or further remission and also in refractory state. We included patients during the period from 2005 to 2017. The hematopoietic stem cell source was indifferently peripheral blood or bone marrow or cord blood. Forty-three centers accepted to participate in this study.
Risk staging of AML was reported according to the 2016 European Leukemia Net classification: low-risk was defined as CBF leukemia: t(8; 21)(q22; q22) RUNX1-RUNX1T1 or inv(16)(p13.1q22) CBFB-MYH11, or leukemia with biallelic mutations of CEBPA, or leukemia with normal karyotype and mutated NPM1 without FLT3-ITD. Intermediate risk 1 was defined as leukemia with normal karyotype with either mutated NPM1 and FLT3-ITD (mutant/wild-type mutation ratio > 0.3), or wild-type NPM1 and mutated FLT3-ITD, or wild-type NPM1 without FLT3-ITD. Intermediate risk 2 was defined as t(9; 11)(p21.3; q23.3) MLLT3-KMT2A or cytogenetic abnormalities not classified as favorable or adverse. High-risk group was defined as t(6; 9)(p23; q34.1) DEK-NUP214, t(v; 11q23) KMT2A rearranged, t(9; 22)(q34.1; q11.2) BCR-ABL1, inv(3)(q21.3; q26.2) or t(3; 3)(q21.3; q26.2) GATA2, MECOM (EVI1), complex karyotype (≥ 3), -5 or del (5q), − 7; − 17/abn(17p), mutated RUNX1, mutated ASXL1 (if not in low risk cytogenetics) and mutated TP53 (Stölzel et al. 2016). The conditioning regimen was considered Myeloablative Conditioning (MAC) if the total IV busulfan dose exceeded 12 mg/kg or the total fractionated body irradiation (TBI) dose exceeded 8 Grays. The combination of fludarabine 150 mg/m2 and IV busulfan 12.8 mg/kg (FB4) was defined as a reduced-toxicity MAC regimen. The other combinations have been defined as a Reduced-Intensity Regimen (RIC) (Bacigalupo et al. 2009).
Grading of acute GVHD was performed using the Glucksberg’s score (Glucksberg et al. 1974). Chronic GVHD was classified as limited or extensive according to previous published criteria (Filipovich et al. 2005).
Statistical analysis
For variable description, categorical variables were expressed as numbers and percentages and discrete/continuous variables by median and extremes (min–max) values.
Comparisons by age groups (i.e., children, APA patients and young adults) were performed using Chi-Squared or Fisher exact tests as appropriate for categorical variables and Kruskal–Wallis test for discrete/continuous variables.
Inter-groups comparisons for the following time-dependent variables were performed using Kaplan–Meier analyses: Overall Survival (OS), event-free survival (EFS) and GRFS.
Inter-groups comparisons for the following time-dependent variables were performed using Gray’s test for equality of Cumulative Incidence Functions (CIF): Non-Relapse Mortality (NRM) (Competitive Risk (CR) = relapse), acute GVHD (CR = death), grade II–IV acute GVHD (CR = death), grade III to IV acute GVHD (CR = death), chronic GVHD (CR = death) and extensive chronic GVHD (CR = death).
For the mentioned time-dependent events, probability of OS, EFS and GRFS at 2 years were computed and intergroup survival distributions were compared using log-rank tests. Cumulative incidences at the following time points (event) were estimated after considering death or relapse occurrence as competitive events: 3 months (acute GVHD), 1 year (chronic GVHD), 2 years (relapse, death not related to relapse and GRFS), and compared using Gray’s test.
For each time-dependent variable, independent risk factors were explored using multivariable Cox regression model after censoring the follow-up at time of competing event when appropriate. The following variables were considered as potential predictors for all models: age, pediatric or adult center, recipient’s gender, recipient’s CMV status, cytogenetics, extramedullary disease, previous autologous transplantation, delay between AML diagnosis and HSCT, myeloablative, reduced-intensity or sequential conditioning regimen, use of antithymoglobulins (ATG), total body irradiation (< 8 grays or ≥ 8 grays), use of methotrexate or mofetil mycophenolate, use of high dose cyclophosphamide after transplantation, disease status at transplantation, graft source, HLA matching, donor’s gender, age and CMV serology, acute GVHD occurrence and staging, chronic GVHD occurrence and staging, disease status at the time of last news, delay from HSCT to relapse, Donor Lymphocyte Infusions (DLI).
Proportional hazard assumption was checked for each time-to-event outcome—predictor combination, and if violated, a time-dependant interaction term was added in the model. Then, univariate analyses were conducted including the interaction term and after censoring the follow-up to the competitive event when required. Independent risk-factors of each time-to-event outcome were explored using multivariable Cox regression model with significance levels for entry (SLE) and for stay (SLS) of 0.20 and 0.05, respectively.
Statistical analyses were performed using SAS version 9.4. The level of statistical significance was set at 0.05.
Results
Transplantation characteristics and comparison of patients in the three age groups
We analyzed data from 2645 patients aged from 0 to 40 years old, who received a first allogeneic HSCT between January 2005 and December 2017 from 43 SFGM-TC centers. The patient’s characteristics are presented in Table 1.
The median follow-up of the study was 2.4 years (min–max 1 day–14.7 years), from the time of transplant to death or latest news date. Among alive patients, the median follow-up of the study was 4.37 years (min–max 0.18–14.73 years). Three age groups were assessed: 564 children aged from 0 to 14 years, 647 Adolescent and Post-Adolescent (APA) patients aged from 15 to 25 years, and 1434 young adult patients aged from 26 to 40 years.
The cytogenetics risk, the extramedullary involvement at diagnosis and the disease status at transplant were different in the three groups (p < 0.0001 for all analyses). The conditioning regimen was mainly myeloablative in the three groups (79% in APA patients, 76.1% in young adults and 95.3% in children), but APA patients and young adults received more often RIC regimen than the children (12.3 and 14.1% versus 3.6%). The use of TBI equal and over 8 Grays was different according to the age groups (p < 0.0001), APA patients and young adults received more TBI (25.7 and 25.6% respectively) than the children (7.5%). The use of Anti-Thymoglobulin (ATG) was different in the three groups (p = 0.0036): it was slightly less often used in APA patients and children compared to the young adults (42.8% and 42% versus 49%, respectively). Post-Transplant High Dose Cyclophosphamide (PT-Cy) was used in a minority of cases, 2.6% in children, 6.2% in APA patients and 6.1% in young adults (p = 0.0056 as intergroup comparison). PT-Cy was not only dedicated to haploidentical transplantations: 63.6% patients that received PT-Cy, underwent a haploidentical transplant and 62.7% haploidentical transplant were followed by PT-Cy. We observed some significant differences in stem cell source between the three groups (p < 0.0001). Bone marrow (BM) was the main source in children (63.2%, followed by cord blood in 27%, and peripheral blood stem cells (PBSC) in only 9.8%), PBSC were the major source of HSCT in young adults (62.3%, followed by bone marrow in 28.2%) while APA patients received bone marrow in 40.2%, PBSC in 45.9% and cord blood in 13.9%. The donor’s age was also different in the three age groups (p < 0.0001).
Engraftment and Graft-versus-Host Disease (GVHD)
The neutrophils recovery time up to 0.5 G/L differed between the three age groups (p < 0.0001) with a median (min–max) value of 20 days (4–61) in children, 19 days (1–66) in APA patients, and 18 days (1–108) in young adults. The platelets recovery time up to 20 G/L also differed in the three age groups (p < 0.0001) with a median (min–max) value of 21 days (3–181) in children, 18 days (1–124) in APA patients and 16 days (1–152) in young adults (Table 2).
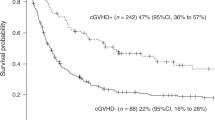
The grade I–IV acute GVHD cumulative incidence (CI) at 3 months was estimated to 55.7% for children, 49.3% for APA patients and 50.4% for young adults, the difference in the three age groups was close to being significant (p = 0.0534) (Table 2). Moreover, considering chronic GVHD, the CI at 2 years of follow-up showed a significant statistical difference in the three age groups (p < 0.0001), APA patients and young adults experiencing more chronic GVHD (31.4% and 36.4%, respectively) in comparison to the children (CI 17.5%) (Table 2, Fig. 1a, b).
The independent risk factors associated with grade II to IV acute GVHD were: the HLA matching (higher risk for mismatched unrelated donors followed by matched unrelated donors and haploidentical donors compared to the sibling donors), active disease at transplant time and the use of TBI ≥ 8 Grays. The protective factors were: the use of cord blood and PBSC, compared to the bone marrow, the use of ATG, the use of Post-Transplant High-Dose Cyclophosphamide (PT-Cy) and methotrexate in addition to cyclosporine in GVHD prophylaxis (Table 3).
Furthermore, independent risk factors associated with severe (grade III to IV) acute GVHD were: active disease at transplant time, HLA matching (higher risk in case of mismatched unrelated donors followed by matched unrelated donors and haploidentical donors compared to the sibling donors), and recipient CMV seropositivity. The use of ATG decreased the risk of severe acute GVHD (Table 3).
An independent risk factor of chronic GVHD was identified: the age group, adults being at highest risk, and then APA patients, compared to the children. The use of PT-Cy decreased the risk of chronic GVHD. As far as extensive chronic GVHD is concerned, the graft source (PBSC compared to bone marrow) and the increasing of donor’s age were also an independent risk factor in addition to the age group (young adults and APA patients, compared to the children). While the use of ATG or PT-Cy was an independent protective factor in extensive chronic GVHD (Table 3).
Relapse
With a median follow-up of 2.4 years (min–max: 1 day–14.7 years), AML relapse occurred after HSCT in 193 children (35.2%), 247 APA patients (39.1%) and 474 young adults (34.5%). Median (min–max) delay from HSCT to relapse was 165.5 (1–4377) days in children, 151 days (7–2457) in APA patients and 182 days (1–4305) in young adults.
The CI of relapse at 2 years differed in the three age groups (30.8% in children, 35.2% in APA patients and 29.4% in young adults—p = 0.0254) (Table 2, Fig. 2c).
The independent risk factors for relapse were: high cytogenetics risk, followed by intermediate risk 2 and 1 (compared to low cytogenetics risk), longer delay between diagnosis and HSCT, reduced-intensity conditioning regimens (compared to myeloablative conditioning regimens), active disease at transplant time, followed by third and second complete remission before HSCT (compared to the patients in first CR) (Table 4).
Donor lymphocyte infusions (DLI) were rarely used in this cohort, either in prophylaxis or as curative treatment. Thirty-four (6%) children, 61 (9.4%) APA patients and 180 (12.6%) young adults received at least one DLI.
Non-relapse mortality
The non-relapse mortality CI at 2 years was 7.0% in children, 10.6% in APA patients and 14.2% in young adults (p < 0.0001, Table 2, Fig. 2d) and the median (min–max) delay from HSCT to NRM was 0.34 (0.06–6.54) years, 0.33 (0.01–8.20) years and 0.45 (0–13.49) years, respectively.
The independent risk factors for NRM were: the age group (young adults followed by APA patients had a higher risk of NRM, compared to the children), the cytogenetics risk (high risk followed by intermediate risk 1and 2, compared to low risk), the disease status at transplant (third CR followed by active disease and second CR, compared to first CR), the HLA mismatch (haploidentical donors followed by mismatched unrelated donors and then matched unrelated donors, compared to the identical sibling donors) and the increasing of donor’s age (Table 4).
The causes of death (other than relapse) are described in Supplementary Table 1. Children mostly died of infections (n = 21, 10.7%), GVHD (n = 20, 10.2%) and pulmonary toxicity (n = 9, 4.6%). Adolescent and post-adolescent patients like young adults mostly died of infections (n = 53, 18.3% and n = 142, 21.9%; respectively), GVHD (n = 40, 13.8% and n = 125, 19.3%) and sinusoidal obstruction syndrome (n = 14, 4.8% and n = 21, 3.2%).
OS and EFS
In this cohort, 1513 patients were alive (57.2%) after a median follow-up of 4.37 years, 368 children (65.2%), 358 APA patients (55.3%) and 787 young adults (54.9%). The OS was significantly different between the three groups (p = 0.0003, Fig. 2a). At 2 years, the probability of OS was 71.4% in children, 61.1% in APA patients and 62.9% in young adults (p = 0.0009 as intergroup difference, Table 2). In the subgroup of patients who did not relapse (n = 1641 patients), the probability of 2-year OS also differed in the three age groups (p < 0.0001) with 89.2% in children, 82.5% in APA patients and 78.2% in young adults.
The independent risk factors for death were: high cytogenetics risk, followed by intermediate risk 1 and 2 (compared to low risk), the use of TBI ≥ 8 Grays, active disease at transplant time followed by the patients in 3rd CR and 2nd CR (compared to the patients in 1st CR), the use of PBSC (compared to bone marrow), and the increase of donor’s age (Table 4).
The EFS was also different in the three age groups (p = 0.013, Fig. 2b) at 2 years with a rate of 61.5% in children, 53.7% in APA patients and 55.8% in young adults, p = 0.0186 (Table 2).
The independent risk factors for death or relapse were: high cytogenetics risk followed by intermediate risk 1 and 2, TBI ≥ 8 Grays in the conditioning regimen, active disease at transplant time followed by 3rd CR and 2nd CR, and the increasing of donor’s age.
GRFS (Fig. 1c)
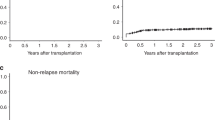
The GRFS, who was defined as survival without neither grade III-IV acute GVHD nor extensive chronic GVHD or relapse, was not significantly different in the three age groups (p = 0.0997, Fig. 1c). The probability of GRFS at 2 years was 47% in children, 40.1% in APA patients and 40.9% in young adults, p = 0.1107 (Table 2).
The independent protective factors for survival without neither disease nor GVHD were: CMV seronegative recipient (in particular the combination of positive donor and negative recipient), AML with low cytogenetics risk, male donor, transplant in 1st CR, bone marrow (compared to PBSC), younger and matched sibling donor (Table 4).
Additional analysis
To describe more precisely the impact of the conditioning regimen and the stem cell source on OS, NRM and chronic GvHD for APA patients, we compared in a subgroup study the APA patients who received a chemotherapy-based MAC regimen and bone marrow as stem cell source, i.e., 171 patients (6.5%), other APA patients, i.e., 449 patients (17.2%) and the children, i.e., 564 patients (21.5%) (Fig. 3). We found a better survival for APA patients who received a chemotherapy-based regimen and bone marrow (p < 0.0001) (Fig. 3a), and the NRM was lower for this subgroup of patients (p = 0.0153) (Fig. 3b). However, the incidence of chronic GvHD was still lower for children (p < 0.0001) (Fig. 3c). Moreover, the OS was the same for children and APA patients who received bone marrow, compared to APA patients who received other stem cells sources (Fig. 3d).
Better outcome of APA patients who received bone marrow grafts and a chemotherapy-based myeloablative conditioning regimen. a Overall Survival (Kaplan-Meïer curves) for APA patients who received a chemo-based MAC regimen and a bone marrow graft, compared to children and young adults. b Cumulative incidence of Non Relapse Mortality for APA patients who received a chemo-based MAC regimen and a bone marrow graft, compared to children and young adults. c Cumulative incidence of chronic GVHD for APA patients who received a chemo-based MAC regimen and a bone marrow graft, compared to children and young adults. d Overall survival (Kaplan-Meïer curves) for APA patients who received bone marrow grafts (whatever the conditioning regimen they received), compared to children and young adults
Discussion
This retrospective registry study from 2005 to 2017 showed that APA patients have a greater risk of NRM and chronic GVHD than children after allogeneic HSCT for AML. The relapse occurred slightly more frequently in the APA patient group, but age was not an independent factor for relapse. As far as NRM is concerned, we observed that the age group was an independent risk factor, but also the cytogenetics risk, the disease status, the HLA matching and the donor’s age; moreover, we observed a higher rate of chronic extensive GVHD in APA patients and young adults in comparison to children, with the age group as an independent risk factor.
Except for promyelocytic leukemia, APA patients with AML were described as presenting a poorer prognosis than children in a few studies due to a higher rate of relapse (Jaime-Pérez et al. 2018), higher risk of toxicity-related mortality (Tomizawa et al. 2017) owing to more frequent infections (Canner et al. 2013) and a higher early mortality rate (Nasir et al. 2017). Nevertheless APA patients had a better prognosis than adult AML patients (Pemmaraju et al. 2016). Age seems to be related to the outcome from childhood to adulthood (Appelbaum et al. 2006). Patients treated in pediatric trials had better outcomes than those treated on adult trials in an American study by the National Marrow Donor Program (NMDP), but age was a major confounding variable, making it harder to compare data sets by cooperative groups (Woods et al. 2013). Moreover, neither studies from the Nordic Society of Paediatric Haematology and Oncology (NOPHO), nor works from multi-center in Germany found no difference in outcomes for AML patients in overlapping age groups on pediatric versus adult protocols (Wennström et al. 2016; Büchner et al. 2009; Schlenk et al. 2003). Currently, there is a need for prospective studies to be able to issue a recommendation.
After allogeneic HSCT, a different survival rate for young AML patients was firstly reported from the International Bone Marrow Transplant Registry, from 1980 to 2005 (Majhail et al. 2012). Adolescent and post-adolescent patients were defined as aged 15 to 40 and had improved survival in comparison to older patients but also a worse prognosis compared to children of under 15 years of age. A further study from Minneapolis had reported no difference in children’s outcome compared to APA patients (aged 15–30) from 1995 to 2010, except for GVHD (Burke et al. 2014). A more recent study from the Japanese Group reported an inferior 5-year OS (54% versus 58%; p < 0.01) and an increased transplant-related mortality (TRM; 16% vs 13%, p = 0.02) in adolescent, post-adolescent and young adult patients (15–29 years) compared to children who received allogeneic HSCT for AML from 1990 to 2013 (Tomizawa et al. 2015). However, better HLA typing in recent years could eliminate this difference. Considering that last study, no difference in outcome of APA patients and children (OS, relapse-free survival and NRM) could be identified in the most recent period of their study between 2000 and 2013. This result is in contrast with our study on a more recent cohort of patients.
In our study, cytogenetics risk was strongly related to OS, EFS, NRM and GRFS in multivariable analysis. However, children had more often high-risk cytogenetics, but did not experience a higher incidence of relapse, and had a higher EFS, which was in concordance with the study of Alloin et al. that found a significant survival improvement for children with unfavorable karyotype due to the decrease of relapse risk over time (Alloin et al. 2017). Furthermore, through age groups, there are observable differences in mutated genes, somatic structural variants and DNA methylation patterns (Bolouri et al. 2018). From the study of Boulouri et al., it is important to notice, for instance, that the prevalence of gene fusions and focal deletions in MBNL1 and ZEB2 is much higher in young patients than in adults and the mutations in DNMT3A and TP53 are highly uncommon in children compared to adult patients. In the future, all of these genetic observations should allow targeted and age-suited treatment of AML (Bolouri et al. 2018).
Disease status remains a strong independent factor in relapse, toxicity and death after HSCT. In all recent studies, more advanced disease is still correlated with death for both adults (Konuma et al. 2018; Gaballa et al. 2017) and children (Bitan et al. 2017) in spite of improvements in salvage therapies (Rasche et al. 2018); it is the same for the minimal residual disease (MRD) which is a strong prognostic factor before HSCT (Gilleece et al. 2021; Candoni et al. 2017). Refractory AML has a very bad prognosis despite efforts to develop new strategies such as sequential regimen, except in patients with low medullar blast burden in primary refractory AML (Steckel et al. 2018). In our study, the disease status at the time of transplant was correlated with OS, EFS, NRM and GRFS.
Young adults and APA patients received myeloablative TBI more frequently in their conditioning regimen whilst almost all children received myeloablative chemotherapy without any irradiation. TBI was an independent risk factor of overall mortality. These results are consistent with previous studies reporting that the use of TBI in the conditioning regimen of AML patients, in comparison to busulfan-based MAC regimen, was deleterious for adults and children, despite this being the contrary in ALL studies (Champlin 2013; Bredeson et al. 2013). This deleterious effect of TBI compared to chemotherapy with busulfan was mostly explained by a higher rate of NRM (Berranger et al. 2014) and chronic GVHD incidence (Copelan et al. 2013; Nagler et al. 2013).
The increased NRM for APA (and adult) patients in our study was also possibly a result of the higher cumulative incidence of chronic GVHD compared to children under 15 years old. As far as extensive chronic GVHD is concerned, it was independently associated with PBSC, that were used as a stem cells source for more than 45% of APA patients and 60% of young adults, whereas children received mostly bone marrow and cord blood units. The observation of an increased incidence of chronic GVHD in APA patients has already been reported by Vignon et al. in a precedent study and is always an important matter due to the impact on quality of live (Vignon et al. 2017). As previously described, a high dose of Cyclophosphamide after HSCT reduced the risk of chronic GVHD, and also chronic extensive GVHD such as ATG did. Our results are consistent with previous studies on this point (Kröger et al. 2016; Martinez-Cibrian et al. 2020; Ruggeri et al. 2018; Luznik et al. 2012).
Moreover, we noted that allogeneic HSCT from 9/10 HLA matched unrelated donors resulted in a significantly worse OS than those from both 10/10 HLA matched unrelated donors and HLA identical sibling donors, which is mainly due to increasing NRM (Petersdorf et al. 2004; Flomenberg et al. 2004; Lee et al. 2009; Woolfrey et al. 2011; Horan et al. 2008). Cytomegalovirus serologic positivity for the recipient was also correlated to GRFS and grade III to IV acute GVHD, as previously described before the use of letermovir (Marty et al. 2017).
Donor age was higher in APA patients and young adults; besides, donor age was an independent factor in OS, EFS, NRM, GRFS and chronic extensive GVHD. According to previous publications, allogeneic HSCT from older donors could be associated with reduced OS (Kollman et al. 2001; Loren et al. 2006; Bastida et al. 2015; Ayuk et al. 2018; Shaw et al. 2018) for several reasons: on one hand, higher comorbidity and mobilization failure, on the other, to increased rates of acute and chronic GVHD, higher NRM and relapse rate (Kollman et al. 2001; Loren et al. 2006; Bastida et al. 2015). Recent study data from two works published in 2018 by Ayuk et al. and Shaw et al. showed the impact of the donor’s age and sex mismatch that could be comparable to a single HLA disparity (Ayuk et al. 2018; Shaw et al. 2018).
Conclusions
Adolescent and post-adolescent patients, like young adults, have a greater risk of NRM and chronic GVHD than children after allogeneic HSCT for AML. They also have a higher cumulative incidence of relapse, even if age is not an independent factor of relapse. Therefore, this study suggests that APA patients with AML could be beneficially treated with a conditioning regimen based on myeloablative chemotherapy associated with bone marrow graft. Moreover, donor age and HLA compatibility should also be carefully assessed prior to the procedure. A future prospective comparative study is needed to confirm these results and to assess this important issue of conditioning and stem cell source choice in APA patients who received an allogeneic HSCT for AML.
Availability of data and materials
The dataset used and analyzed during the current study is available from the corresponding authors on reasonable request.
Abbreviations
- AML:
-
Acute myeloid leukemia
- ATG:
-
Anti-thymoglobulin
- APA:
-
Adolescent and post-adolescent
- BM:
-
Bone marrow
- CBF:
-
Core binding factor
- CI:
-
Cumulative incidence
- CIF:
-
Cumulative incidence functions
- CMV:
-
Cytomegalovirus
- CR:
-
Complete remission
- CR:
-
Competitive Risk
- DLI:
-
Donor lymphocyte infusion
- EFS:
-
Event-free survival
- GRFS:
-
Graft-versus-host disease and relapse free survival
- GVHD:
-
Graft-versus-host disease
- HSCT:
-
Hematopoietic stem cell transplantation
- MAC:
-
Myeloablative conditioning
- MRD:
-
Minimal residual disease
- NMDP:
-
National marrow donor program
- NRM:
-
Non-relapse mortality
- OS:
-
Overall survival
- PBSC:
-
Peripheral blood stem cells
- PT-Cy:
-
Post-transplant high dose cyclophosphamide
- RIC:
-
Reduced intensity conditioning
- SFGM-TC:
-
Société francophone de greffe de moëlle et de thérapie cellulaire
- SLE:
-
Significance levels for entry
- SLS:
-
Significance levels for stay
- TBI:
-
Total body irradiation
- TRM:
-
Transplant-related mortality
References
Alloin A-L, Leverger G, Dalle J-H, Galambrun C, Bertrand Y, Baruchel A et al (2017) Cytogenetics and outcome of allogeneic transplantation in first remission of acute myeloid leukemia: the French pediatric experience. Bone Marrow Transplant 52:516–521
Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE et al (2006) Age and acute myeloid leukemia. Blood 107:3481–3485
Ayuk F, Beelen DW, Bornhäuser M, Stelljes M, Zabelina T, Finke J et al (2018) Relative impact of HLA matching and non-HLA donor characteristics on outcomes of allogeneic stem cell transplantation for acute myeloid leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant 24:2558–2567
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V et al (2009) Defining the Intensity of Conditioning Regimens: Working Definitions. Biol Blood Marrow Transplant 15:1628–1633
Bastida JM, Cabrero M, Lopez-Godino O, Lopez-Parra M, Sanchez-Guijo F, Lopez-Corral L et al (2015) Influence of donor age in allogeneic stem cell transplant outcome in acute myeloid leukemia and myelodisplastic syndrome. Leuk Res 39:828–834
Bitan M, Ahn KW, Millard HR, Pulsipher MA, Abdel-Azim H, Auletta JJ et al (2017) Personalized prognostic risk score for long-term survival for children with acute leukemia after allogeneic transplantation. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant 23:1523–1530
Bolouri H, Farrar JE, Triche T Jr, Ries RE, Lim EL, Alonzo TA et al (2018) The molecular landscape of pediatric acute myeloid leukemia reveals recurrent structural alterations and age-specific mutational interactions. Nat Med 24(1):103–112
Bredeson C, LeRademacher J, Kato K, Dipersio J, Agura E, Devine S et al (2013) Prospective cohort study comparing intravenous busulfan to total body irradiation in hematopoietic cell transplantation. Blood 122(24):3871–3878
Büchner T, Berdel WE, Haferlach C, Haferlach T, Schnittger S, Müller-Tidow C et al (2009) Age-related risk profile and chemotherapy dose response in acute myeloid leukemia: a study by the German Acute Myeloid Leukemia Cooperative Group. J Clin Oncol 27(1):61–69
Burke MJ, Gossai N, Cao Q, Macmillan ML, Warlick E, Verneris MR (2014) Similar outcomes between adolescent/young adults and children with AML following allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 49:174–178
Candoni A, De Marchi F, Zannier ME, Lazzarotto D, Filì C, Dubbini MV et al (2017) High prognostic value of pre-allogeneic stem cell transplantation minimal residual disease detection by WT1 gene expression in AML transplanted in cytologic complete remission. Leuk Res 63:22–27
Canner J, Alonzo TA, Franklin J, Freyer DR, Gamis A, Gerbing RB et al (2013) Differences in outcomes of newly diagnosed acute myeloid leukemia for adolescent/young adult and younger patients: a report from the Children’s Oncology Group. Cancer 119:4162–4169
Champlin RE (2013) Busulfan or TBI: answer to an age-old question. Blood 122:3856–3857
Copelan EA, Hamilton BK, Avalos B, Ahn KW, Bolwell BJ, Zhu X et al (2013) Better leukemia-free and overall survival in AML in first remission following cyclophosphamide in combination with busulfan compared with TBI. Blood 122:3863–3870
de Berranger E, Cousien A, Petit A, de Latour PR, Galambrun C, Bertrand Y et al (2014) Impact on long-term OS of conditioning regimen in allogeneic BMT for children with AML in first CR: TBI+CY versus BU+CY: a report from the Société Française de Greffe de Moelle et de Thérapie Cellulaire. Bone Marrow Transplant 49:382–388
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ et al (2005) National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 11:945–956
Flomenberg N, Baxter-Lowe LA, Confer D, Fernandez-Vina M, Filipovich A, Horowitz M et al (2004) Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood 104:1923–1930
Gaballa S, Saliba R, Oran B, Brammer JE, Chen J, Rondon G et al (2017) Relapse risk and survival in patients with FLT3 mutated acute myeloid leukemia undergoing stem cell transplantation. Am J Hematol 92:331–337
Gilleece MH, Labopin M, Yakoub-Agha I, Volin L, Socié G, Ljungman P et al (2021) Measurable residual disease, conditioning regimen intensity and age predict outcome of allogeneic hematopoietic cell transplantation for acute myeloid leukemia in first remission: a registry analysis of 2292 patients by the Acute Leukemia Working Party European Society of Blood Cancer J. Am J Hematol 11(5):88
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA et al (1974) Clinical manifestations of graft-versus-host disease in human recipients of marrow from HLA-matched sibling donors. Transplantation 18:295–304
Horan JT, Alonzo TA, Lyman GH, Gerbing RB, Lange BJ, Ravindranath Y et al (2008) Impact of disease risk on efficacy of matched related bone marrow transplantation for pediatric acute myeloid leukemia: the Children’s Oncology Group. J Clin Oncol off J Am Soc Clin Oncol 26:5797–5801
Jaime-Pérez JC, Padilla-Medina JR, Fernández LT, Herrera-Garza JL, Gutiérrez-Aguirre CH, Tarín-Arzaga L et al (2018) Outcomes of adolescents and young adults with acute myeloid leukemia treated in a Single Latin American Center. Clin Lymphoma Myeloma Leuk 18:286–292
Kollman C, Howe CW, Anasetti C, Antin JH, Davies SM, Filipovich AH et al (2001) Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood 98:2043–2051
Konuma T, Mizuno S, Kondo T, Yamaguchi H, Fukuda T, Uchida N et al (2018) Allogeneic hematopoietic cell transplantation in adult acute myeloid leukemia with 11q23 abnormality: a retrospective study of the Adult Acute Myeloid Leukemia Working Group of the Japan Society for Hematopoietic Cell Transplantation (JSHCT). Ann Hematol 97(11):2173–2183
Kröger N, Solano C, Wolschke C, Bandini G, Patriarca F, Pini M et al (2016) Antilymphocyte globulin for prevention of chronic graft-versus-host disease. N Engl J Med 374(1):43–53
Lee S, Kim Y-J, Chung N-G, Lim J, Lee D-G, Kim H-J et al (2009) The extent of minimal residual disease reduction after the first 4-week imatinib therapy determines outcome of allogeneic stem cell transplantation in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer 115:561–570
Loren AW, Bunin GR, Boudreau C, Champlin RE, Cnaan A, Horowitz MM et al (2006) Impact of donor and recipient sex and parity on outcomes of HLA-identical sibling allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant 12:758–769
Luznik L, O’Donnell PV, Fuchs EJ (2012) Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Semin Oncol 39(6):683–693
Majhail NS, Brazauskas R, Hassebroek A, Bredeson CN, Hahn T, Hale GA et al (2012) Outcomes of allogeneic hematopoietic cell transplantation for adolescent and young adults compared with children and older adults with acute myeloid leukemia. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant 18:861–873
Martinez-Cibrian N, Zeiser R, Perez-Simon JA (2020) Graft-versus-host disease prophylaxis: Pathophysiology-based review on current approaches and future directions. Blood Rev 26:100792
Marty FM, Ljungman P, Chemaly RF, Maertens J, Dadwal SS, Duarte RF et al (2017) Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation [Internet]. N Engl J Med. https://doi.org/10.1056/NEJMoa1706640 (Massachusetts Medical Society; 2017 [cited 2021 Jan 6])
Nagler A, Rocha V, Labopin M, Unal A, Ben Othman T, Campos A et al (2013) Allogeneic hematopoietic stem-cell transplantation for acute myeloid leukemia in remission: comparison of intravenous busulfan plus cyclophosphamide (Cy) versus total-body irradiation plus Cy as conditioning regimen–a report from the acute leukemia working party of the European group for blood and marrow transplantation. J Clin Oncol off J Am Soc Clin Oncol 31:3549–3556
Nasir SS, Giri S, Nunnery S, Martin MG (2017) Outcome of adolescents and young adults compared with pediatric patients with acute myeloid and promyelocytic leukemia. Clin Lymphoma Myeloma Leuk 17:126-132.e1
Pemmaraju N, Kantarjian H, Ravandi F, Nogueras-Gonzalez GM, Huang X, O’Brien S et al (2016) Patient Characteristics and outcomes in adolescents and young adults (AYA) with acute myeloid leukemia (AML). Clin Lymphoma Myeloma Leuk 16:213-222.e2
Petersdorf EW, Anasetti C, Martin PJ, Gooley T, Radich J, Malkki M et al (2004) Limits of HLA mismatching in unrelated hematopoietic cell transplantation. Blood 104:2976–2980
Rasche M, Zimmermann M, Borschel L, Bourquin J-P, Dworzak M, Klingebiel T et al (2018) Successes and challenges in the treatment of pediatric acute myeloid leukemia: a retrospective analysis of the AML-BFM trials from 1987 to 2012. Leukemia 32(10):2167–2177
Ruggeri A, Labopin M, Bacigalupo A, Afanasyev B, Cornelissen JJ, Elmaagacli A et al (2018) Post-transplant cyclophosphamide for graft-versus-host disease prophylaxis in HLA matched sibling or matched unrelated donor transplant for patients with acute leukemia, on behalf of ALWP-EBMT. Hematol Oncol 15:111–140
Schlenk RF, Benner A, Hartmann F, del Valle F, Weber C, Pralle H et al (2003) Risk-adapted postremission therapy in acute myeloid leukemia: results of the German multicenter AML HD93 treatment trial. Leukemia 17(8):1521–1528
Shaw BE, Logan BR, Spellman SR, Marsh SGE, Robinson J, Pidala J et al (2018) Development of an unrelated donor selection score predictive of survival after HCT: donor age matters most. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant 24:1049–1056
Steckel NK, Groth C, Mikesch J-H, Trenschel R, Ottinger H, Kordelas L et al (2018) High-dose melphalan-based sequential conditioning chemotherapy followed by allogeneic haematopoietic stem cell transplantation in adult patients with relapsed or refractory acute myeloid leukaemia. Br J Haematol 180:840–853
Stölzel F, Mohr B, Kramer M, Oelschlägel U, Bochtler T, Berdel WE et al (2016) Karyotype complexity and prognosis in acute myeloid leukemia. Blood Cancer J 6(1):e386
Tomizawa D, Watanabe T, Hanada R, Horibe K, Horikoshi Y, Iwamoto S et al (2015) Outcome of adolescent patients with acute myeloid leukemia treated with pediatric protocols. Int J Hematol 102:318–326
Tomizawa D, Tanaka S, Kondo T, Hashii Y, Arai Y, Kudo K et al (2017) Allogeneic Hematopoietic Stem Cell Transplantation for Adolescents and Young Adults with Acute Myeloid Leukemia. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant 23:1515–1522
Vignon M, Andreoli A, Dhédin N, Lengliné E, Masson E, Robin M et al (2017) Graft-versus-host disease in adolescents and young adults (15–24 Years Old) after allogeneic hematopoietic stem cell transplantation for acute leukemia in first complete remission. J Adolesc Young Adult Oncol 6(2):299–306
Wennström L, Wendtland Edslev P, Abrahamsson J, Maxwell Nørgaard J, Fløisand Y, Forestier E et al (2016) Acute Myeloid Leukemia in Adolescents and Young Adults Treated in Pediatric and Adult Departments in the Nordic Countries. Pediatr Blood Cancer 63(1):83–92
Woods WG, Franklin ARK, Alonzo TA, Gerbing RB, Donohue KA, Othus M et al (2013) Outcome of adolescents and young adults with acute myeloid leukemia treated on COG trials compared to CALGB and SWOG trials. Cancer 119:4170–4179
Woolfrey A, Klein JP, Haagenson M, Spellman S, Petersdorf E, Oudshoorn M et al (2011) HLA-C antigen mismatch is associated with worse outcome in unrelated donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant 17:885–892
Acknowledgements
The authors thank Alethea Pigott for proofreading the article, correcting and improving English.
Funding
This work had no funding.
Author information
Authors and Affiliations
Contributions
CP, MD, EA, TR and MTR contributed to the study design; CP, MD, ND, YC, EB, AS, EA, TR, MTR and SN contributed to the data interpretation, CP and MD wrote the manuscript; NR performed the data processing; EA and TR performed statistical analysis; CP, MD, JHD, GM, ND, UC, EB, EF, AS, FIL, MM, CR, IYA, FG, JOB, JK, JC, CEB, MA, SN, HLW, PC, FR, AB, YB, AH, ALM, PS, BN, CP and MTR were involved directly in the care of patients. All authors read and agreed on the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of or competing interest.
Ethics approval and consent to participate
Patients provided written informed consent authorizing the use of their personal information for research purposes. The study protocol was approved by the scientific council of the SFGM-TC and by the institutional review boards at each center participating. The study was in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pochon, C., Detrait, M., Dalle, JH. et al. Improved outcome in children compared to adolescents and young adults after allogeneic hematopoietic stem cell transplant for acute myeloid leukemia: a retrospective study from the Francophone Society of Bone Marrow Transplantation and Cell Therapy (SFGM-TC). J Cancer Res Clin Oncol 148, 2083–2097 (2022). https://doi.org/10.1007/s00432-021-03761-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-021-03761-w