Abstract
Purpose
Predicting feasibility of treatment in older patients with cancer is a major clinical task. The Initiative Geriatrische Hämatologie und Onkologie (IN-GHO®) registry prospectively collected data on the comprehensive geriatric assessment (CGA), physician’s and patient’s-self assessment of fitness for treatment, and the course of treatment in patients within a treatment decision aged ≥ 70 years.
Patients and methods
The registry included 3169 patients from 93 centres and evaluated clinical course and treatment outcomes 2–3 and 6 months after initial assessment. Fitness for treatment was classified as fit, compromised and frail according to results of a CGA, and in addition by an experienced physician’s and by patient’s itself. Feasibility of treatment (termed IN-GHO®-FIT) was defined as a composite endpoint, including willingness to undergo the same treatment again in retrospect, no modification or unplanned termination of treatment, and no early mortality (within 90 days).
Results
CGA classified 30.0% as fit, 35.8% as compromised, and 34.2% as frail. Physician’s and patient’s-self assessment classified 61.8%/52.3% as fit, 34.2%/42.4% as compromised, and 3.9%/5.3%, as frail, respectively. Survival status at day 180 was available in 2072 patients, of which 625 (30.2%) had died. After 2–3 months, feasibility of treatment could be assessed in 1984 patients. 62.8% fulfilled IN-GHO®-FIT criteria. Multivariable analysis identified physician’s assessment as the single most important item regarding feasibility of treatment.
Conclusion
Geriatricians were involved in 2% of patients only. Classification of fitness for treatment by CGA, and physician’s or patient’s-self assessment showed marked discrepancies. For the prediction of feasibility of treatment no single item was superior to physician’s assessment. However CGA was not performed by trained geriatricians.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is a disease of the older adults. In the United States, more than half of cancer patients are older than 65 years, and about 30% of all cancer deaths occur above the age of 80 (Siegel et al. 2014, 2021). However, due to active exclusion due to trial criteria, or passive non-inclusion by physicians, older patients are underrepresented in clinical trials (Hutchins et al. 1999). Data on feasibility, efficacy, and outcome of treatment in older patients with cancer outside clinical trials are scarce. Decision-making in older patients with cancer can be difficult, as both under- and overtreatment put patients at risk (Pallis et al. 2010a, b). While there are some data on the association of results of a comprehensive geriatric assessment (CGA) with treatment toxicity and mortality, there was limited evidence on how it impacts treatment decision. Recent data show an impact in about 30% of decisions (Hamaker, Te Molder et al. 2018).
Recently, some progress has been made, e.g., by better defining criteria for fitness for treatment, overcoming chronological age as the sole discriminator (Friedrich et al. 2003). A CGA has been advocated as an instrument that can help to identify individual limitations in a multidimensional approach (Maas et al. 2007; Pal et al. 2010; Pallis et al. 2011). Almost 2 decades ago, Hamerman had already gone one step further, and had linked classification according to CGA to treatment decisions (Hamerman 1999). “Fit” patients were deemed fit for standard treatment, whereas “compromised” patients were considered candidates for adapted treatment, and “frail” patients were thought to be largely unfit for cytotoxic treatment. However, until recently, the validity of this or any other classification to guide treatment decisions has, to our knowledge, been examined prospectively only once (Corre et al. 2016).
The “Initiative für Geriatrische Hämatologie und Onkologie” (Initiative for Geriatric Haematology and Oncology, IN-GHO®) is a working group of German-speaking oncologists and geriatricians exploring clinical aspects of treatment of older patients cancer patients. To this end, the group realized a large prospective registry for older patients with cancer. Besides clinical trials, registries are an important way of collecting data and knowledge on characteristics and outcome of patients with malignancies (Wildiers et al. 2013).
A registry allows collection of data from a clinically relevant subgroup of patients that would otherwise mostly not be taken into consideration. The aims of our registry were to demonstrate feasibility of CGA in the oncological setting, and to identify and analyse clinically important factors for decision-making, including feasibility of treatment. Even though the probability of a good or poor feasibility of treatment is of major importance in clinical decision-making, feasibility of treatment is so far an ill-defined endpoint in oncology (Wildiers et al. 2013, Laurent et al. 2014). To evaluate if treatment decision at baseline was adequate, a combined end-point was defined for feasibility of treatment. To be considered “fit” for the chosen treatment, all of the following criteria had to be fulfilled: (1) during the course of treatment, there was no modification of dose or intensity, and there was no unplanned termination of treatment; (2) the patient did not die within a follow-up period of 90 days (early mortality); and (3) both physician and patient declared at the first assessment at 8–12 weeks, that in retrospect, they would choose the same treatment again (without modifications).
Patients and methods
Study design and participants: The IN-GHO® registry collected data from patients with the following characteristics: age ≥ 70 years, diagnosis of a solid tumour or a haematological malignancy, and a pending treatment decision. This was either start of a new treatment, change of an existing treatment, or even the active decision against cytotoxic therapy. After registration, participating centres, either specialised oncology departments of hospitals (N = 22), two of them comprehensive cancer centres (2% = 2/93), or office-based specialised oncologists (N = 71), could access the web-based registry. Participating centres were advised to include consecutive patients. An external monitoring was not conducted. All centres were led by board certified oncologists or haematologists. The registry was approved by an institutional review board of the University of Hamburg and informed written consent to collect and analyse pseudonymised data was obtained from each eligible patient before participation. The registry was supported by Janssen-Cilag GmbH.
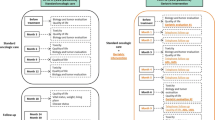
Data were collected prospectively at three different time points, unless observation was terminated prematurely due to patient’s withdrawal of consent, loss to follow-up, or death. Baseline characteristics were documented at inclusion and at two assessment points scheduled during follow-up, first within a window of 8–12 weeks, and again after 6 months (Fig. 1). At baseline, the following data were collected: demographic data (age, sex, weight, body height, body mass index, Karnofsky performance status = KPS), disease-specific information, and recent treatment decision (including modality and intensity of treatment, and palliative or curative intention). Furthermore, physicians, unaware of the results of the geriatric assessment, were asked to subjectively categorize patients’ fitness for treatment into one of three categories (“fit”, “compromised”, or “frail”), and patients’ self-assessment of resilience to stress in categories from 1 (“very resilient”) to 6 (“no resilience”) was documented. Physicians were board certified specialists for internal medicine and haematology/oncology, which included at least a training of 8 years.
Time schedule of the data collection in the IN-GHO® registry and definition of IN GHO®-FIT as a clinical endpoint. Abbreviations: ADL activity of daily living, BMI body mass index, CGA comprehensive geriatric assessment, IADL instrumental activity of daily living, IN-GHO® initiative for geriatric haematology and oncology, KPS Karnofsky performance score, MMSE mini-mental state examination, N number of patients, SCID Structured Clinical Interview for DSM-IV screening question
CGA
The CGA included in the first data set at baseline involved the following data and items: assessment of activities of daily living (ADL) (Mahoney and Barthel 1965), instrumental activities of daily living (IADL) (Lawton and Brody 1969), Charlson comorbidity score (Charlson et al. 1987), MMSE (Folstein et al. 1975), timed-up-and-go test (Podsiadlo and Richardson 1991), two screening questions for depression (Structured Clinical Interview for DSM-IV (SCID) depression screening) (Spitzer et al. 1992), co-medication, and history of previous falls. Items of CGA are described in more details elsewhere (Pallis et al. 2010a, b). CGA results were categorized as follows: charlson comorbidity index 0–2 vs. > 2, as most widely practised; comedication 0–3 vs. > 3, as median split; ADL score 100 vs. < 100, as classifying patients without and with limitations, IADL score 8 vs. < 8, as classifying patients without and with limitations; KPS 80–100 vs. < 80, as most widely practised; Timed-up-and-go test, as recommended by the authors; Mini Mental Status Examination 24–30 vs. < 24, as recommended by the authors; SCID as suggested by the manual (Cook et al. 2020, Scheubeck et al. 2021).
Classification according to CGA
Categorization of older patients with cancer by CGA has been proposed by Balducci and Extermann in 2000 (Balducci and Extermann 2000). Accordingly, we classified our patients as follows: group 1 were independent patients without relevant comorbidity. Group 2 comprised patients with one or two dependencies in IADLs and/or one or two comorbidities, while group 3 comprised frail patients, showing either 1 dependency in ADL, and/or ≥ 3 dependencies in IADL, and/or ≥ 3 comorbidities.
Feasibility of treatment
To evaluate if treatment decision at baseline was adequate, a combined end-point was defined for feasibility of treatment. To be considered “fit” for the chosen treatment (i.e., to re-assess retrospectively whether an adequate treatment was chosen for the individual patient), all of the following criteria had to be fulfilled: (1) during the course of treatment, there was no modification of dose or intensity, and there was no unplanned termination of treatment; (2) the patient did not die within a follow-up period of 90 days (early mortality); and (3) both physician and patient declared at the first assessment at 8–12 weeks, that in retrospect, they would choose the same treatment again (without modifications). The term “IN-GHO®-FIT” was coined for those patients fulfilling all 3 of these criteria.
Statistical considerations
Descriptive statistics for the overall cohort at baseline were calculated. Patients fulfilling IN-GHO®-FIT criteria were compared to patients failing those criteria by Chi squared test for relative data. Stepwise logistic regression models were used to analyse the association of physicians’ and patients’ assessments of fitness or resilience, respectively, and the variables of the CGA with the endpoint IN-GHO®-FIT. Inter-rater agreement between assessments was measured by Cohen’s Kappa. The statistical analyses were performed using SPSS 24. To elucidate which pre-therapeutic variables were predictive of IN-GHO®-FIT, uni- and multivariable analyses were performed including the following variables: sex, age, body mass index, diagnosis, stage of disease, prior tumour surgery, intention of treatment (curative versus palliative), combination versus single-agent therapy, adapted versus standard dose therapy, antibody or hormone treatment, patients’ and physicians’ assessment, and results of the different instruments of the CGA.
Results
Patients
The consort diagram reports availability of patient data. 3169 patients were included from 2005 to 2011. 47.3% were male, and 23.9% were ≥ 80 years. Mean age was 76.7 years, median age was 75.9 years. 77.9% were treated in an out-patients’ setting, 73.5% had a solid tumour. 63.5% of patients with a solid tumour had metastatic disease. In 54.0% of all patients, first diagnosis of cancer was ≤ 6 months before inclusion into the registry (0–1 month: 24.1%, > 1–6 months: 29.9%). 60.9% of patients had already received prior tumour-specific treatment, either surgery, radiotherapy, chemotherapy, endocrine therapy, or various combinations of those modalities (see supplementary Figure S1). In 49.9% of all patients, an interdisciplinary tumour board was involved in the treatment decision, whereas a geriatrician was involved in only 4.4% of cases. For further details of patients’ characteristics, Table 1 and Fig. 2.
Treatment
For 90.3% of patients, primary intention of treatment was captured, which was curative (mostly involving adjuvant therapy) in 30.1%. In 93.9% of patients, the treatment was tumour specific: this was chemotherapy in 86.7%, the rest comprised other treatment modalities (see supplementary Figure S2). 58.8% of patients with chemotherapy received combination and 41.2% single-agent therapy. 81.2% received standard dose and 18.8% dose-adapted treatment (see supplementary Figures S1, S3).
Assessment
Physicians’ assessment of fitness was as follows: 61.8% of patients were categorized as fit, 34.2% as compromised, and 3.9% as frail. Patients’ self-assessment of resilience to stress was as follows: good and sufficient resilience reported 20.4% and 31.9% of patients, respectively (combined: 52.3%), limited and clearly limited resilience reported 28.4% and 14.0% of patients, respectively (combined: 42.4%), and severely limited resilience or no resilience reported 4.3% and 1.0% of patients, respectively (combined: 5.3%), see supplementary Figure S4. The inter-rater agreement (Cohen’s Kappa) between physicians’ and patients’ assessment was 0.313, which is considered fair (see supplementary Table S1a).
Follow-up
Follow-up data after 8–12 weeks were available for 2520 patients (79.5% of all 3169 patients). In 72.0% of patients for whom follow-up data were available, treatment was performed as scheduled, in 15.5% it was modified, and in 12.5% it was either not started or there was unplanned termination of treatment. 83.5% of the patients who answered this question stated that they would choose the same treatment again without changes, 9.9% that they would choose it again with modifications, and 6.6% that they would not choose it again; physicians answers were similar, 82.7%, 12.0%, and 5.2%, respectively.
Rating feasibility of treatment
Furthermore, patients were grouped according to criteria based on CGA (Balducci and Extermann 2000). CGA classified 30.0% as fit, 35.8% as compromised, and 34.2% as frail, see supplementary Figure S4. The inter-rater agreement (Cohen’s Kappa) between CGA and physicians’ and patients’ assessment was 0.100 and 0.151, respectively, which is both considered poor (see supplementary Table S1b + c).
Using IN-GHO®-FIT criteria, treatment was feasible in 62.8% (1246 patients; data available for 1984 patients). By univariate analysis, the following results showed a significant positive association (p < 0.05) with “IN-GHO®-FIT”: female sex, non-metastatic disease, prior tumour surgery, antibody or hormone treatment, palliative approach, standard dose chemotherapy, both patients’ and physicians’ assessment of fitness or resilience better than compromised/limited, and classification according to Balducci as group 1. From the CGA, the following factors were associated with IN-GHO®-FIT: Charlson score 0, no dependency in ADL or IADL, KPS ≥ 80%, timed-up-and-go test < 10 s, MMSE score > 24, and negative depression screening by SCID. Tables 2, 3 report the results of the univariate analysis.
Using a stepwise logistic regression analysis, the following variables were tested for their association with IN-GHO®-FIT: physicians’ and patients’ assessment, Charlson comorbidity score, KPS, timed-up-and-go test, MMSE, and SCID depression screening. Physicians’ assessment of patients’ fitness was the best parameter in discriminating fit from unfit patients regarding IN-GHO®-FIT. The resulting logistic regression model (Table 3) was significant (p < 0.05). The model made a correct classification in 64.6% of all cases. Interestingly, adding information from the CGA did not help to improve the predictive value regarding feasibility of treatment for the overall cohort (Table 4).
In a second step, we tested different parameters to distinguishing fit from unfit patients in different subgroups of our registry, again using a stepwise regression analysis. Interestingly, in patients with haematological neoplasias (data available for 342 patients), discrimination using the MMSE (cut-off < 24 vs. 24–30) was the only parameter that was significantly (p < 0.05) associated with feasibility of treatment besides physicians’ assessment in multivariable analysis, whereas physicians’ assessment remained the only significant parameter in patients with solid tumours (Table 3).
Discussion
To our knowledge, we present data from one of the largest prospective registries of older patients with cancer to date. Data were collected from both specialised oncology practices and oncology departments from hospitals. There was a high proportion of patients both > 80 years and with comorbidities or impaired KPS, IADL, or ADL. Even though there is paucity of data regarding the referral practice of older patients with cancer to specialised care (Delva et al. 2012), we believe that this cohort represents a “real world” population of older patients with cancer.
Characteristics of patients enrolled in the registry are similar to a previous report from a German oncology practice (Wedding et al. 2007). Notably, we observed lower rates of geriatric problems compared to a large study from 10 Belgian hospitals, where the rate of patients showing geriatric problems was more than 50% (Kenis et al. 2014). This is most likely due to the higher rate of out-patients in our registry. The 180 days mortality rate in our study was 30.2%, which is in the range of that reported by Arnoldi, with 28.1% (Arnoldi et al. 2007), and Giantin, with 34.4% (Giantin et al. 2013). Soubeyran et al. reported a rate of 16.1%; however, they included patients with first-line treatment only (Soubeyran et al. 2012).
Puts et al. analysed data from studies that examined the impact of a CGA on treatment decisions, the relationship between CGA and toxicity, and correlation of CGA and prediction of mortality (Puts et al. 2012). Several authors identified CGA as a predictor of toxicity (Hurria et al. 2011; Extermann et al. 2012). To our knowledge, factors predicting feasibility of treatment have so far not been reported. Longitudinal reporting of treatment outcome and inclusion of both patients’ and physicians’ evaluation of the chosen treatment allowed us to create a new endpoint termed “IN-GHO®-FIT”. It consists of willingness to undergo the same treatment again, no need to dose-adapt the chosen treatment, no premature (unplanned) termination, and no early mortality (within 3 months) as a surrogate for treatment futility. We believe that this combined clinical endpoint is helpful in reassessing the initial treatment decision and can help to differentiate adequate treatment from inadequate or futile treatment, which is the most difficult part in caring for older adults with cancer.
Interesting, but somewhat unexpected, we did not find a single tool nor a combination of different tools from the CGA being superior to physicians’ general assessment of fitness for treatment in the prediction of feasibility of treatment (IN-GHO®-FIT). We think that this finding might be due to several factors. First, participating oncologists were all experienced clinicians, working in specialised oncology practices or departments, with a certain interest in the management of older patients with cancer. Second, some of the patients were already known to them, as inclusion criteria was not a newly diagnosed cancer but a pending treatment decision. Third, we investigated a very heterogeneous population. Whereas a physician can most likely accommodate to some extent for this heterogeneity by clinical experience, it seems that a single factor or even a combination of several factors is limited in the capacity to deal with this complexity. The finding of the predictive value of the MMSE in the subpopulation of patients with haematological neoplasia, which was not found in the overall population and the subpopulation of patients with solid tumours, could indicate that results might differ in different entities. Our finding is in accordance with two studies that reported cognitive impairment as a strong negative prognostic factor in older patients with haematological neoplasias (Dubruille et al. 2015, Goede et al. 2015). Forth, we did not only include patients at first diagnosis, and therefore many of the patients were already well known to their physicians. As some had already received cancer treatment before by the same physician, one can assume that the physician knew how these patients had fared under the stress of a previous cancer treatment. Fourth, we cannot exclude that the treating physician, who was not blinded to the results of the CGA, might have been influenced by the findings of the tests, thereby “diluting” a possible effect of the CGA.
It will eventually need randomised trials where treatment decision is guided by assessment tools versus physicians’ choice to get a real head-to-head comparison of different discriminators in geriatric oncology (Corre et al. 2016). A retrospective analysis suggests that in diffuse large B-cell lymphoma, CGA might be more accurately identifying patients who benefit from aggressive treatment than clinical assessment (Tucci et al. 2015), and data from a prospective trial in patients with diffuse large B-cell lymphoma indeed show promising results (Spina et al. 2012). Addition data for patients with multiple myeloma (MM) support this (Engelhardt et al. 2020). In a prospective trial in patients with MM Scheubeck et al. identified 4 of the 17 evaluated scores and functional tests as most relevant: the Revised Myeloma Comorbidity Index (R-MCI), Activity of Daily Living (ADL), the Mini-Mental State Examination (MMSE), and the quality-of-life 12-Item Short Form Health Survey Physical Composite Scale (SF-12 PCS) (Scheubeck, Ihorst et al. 2021). On the other hand, none of the studies included in a systematic review by Hamaker et al. used physicians’ assessment of fitness as an assessment tool (Hamaker et al. 2012a, b). In this review, none of the analysed screening method was able to predict impairment in a comprehensive geriatric assessment with sufficient quality.
Against this background, we believe that our data can be interpreted as follows: experienced oncologists are able to correctly choose “adequate treatment” (defined by the IN-GHO®-FIT criteria) in approximately two-thirds of older patients with cancer. Rather surprisingly, geriatric assessment tools were not able to improve physicians’ assessments in the overall population in this registry. Clearly, more research, possibly also including biological factors, is needed to better discriminate fit from unfit patients in geriatric oncology in the future (Pallis et al. 2013). A systematic review recently analysed the available data regarding the predictive value of a CGA for patients’ outcomes, and concluded that some variables are of predictive value, but the results were still somewhat inconsistent (Hamaker et al. 2012a, b).
Our registry has a number of limitations: (a) data completeness was lacking for this registry, (b) geriatric experts were hardly ever involved, (4.4% of patients), (c) nor did perform CGA, (d) that decision adapted therapy according to physician ratings vs. geriatric tests has not been established so far and/or (e) has not been shown to be necessarily better than wise physician ratings. Furthermore, it can be criticized that our definition of “adequate treatment” by the proposed IN-GHO®-FIT criteria might be adequate in a palliative setting, but less justified in a curative setting or when prolonging overall survival even at the cost of significant toxicity is the ultimate goal.
In conclusion, our study reports several new findings. We propose a novel endpoint, which we term “IN-GHO®-FIT”, for the assessment of adequate treatment in older patients with cancer. Judgement of patients’ fitness for treatment shows marked discrepancies between rating based on a geriatric assessment, and both physicians’ and patients’ assessment. No single parameter was superior to physician’s assessment in predicting feasibility of treatment. However, even this judgement was correct in only about two-thirds of patients. Interestingly, different subgroups (entities) seem to exist, in which elements of the CGA can contribute relevant information regarding feasibility of treatment. This might indicate the need to develop disease specific assessment tools in oncology/haematology rather than a “one size fits all approach”.
References
Arnoldi E, Dieli M, Mangia M, Minetti B, Labianca R (2007) Comprehensive geriatric assessment in elderly cancer patients: an experience in an outpatient population. Tumori 93(1):23–25
Balducci L, Extermann M (2000) Management of cancer in the older person: a practical approach. Oncologist 5(3):224–237
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383
Cook G, Larocca A, Facon T, Zweegman S, Engelhardt M (2020) Defining the vulnerable patient with myeloma-a frailty position paper of the European myeloma network. Leukemia 34(9):2285–2294
Corre R, Greillier L, Le Caer H, Audigier-Valette C, Baize N, Berard H, Falchero L, Monnet I, Dansin E, Vergnenegre A, Marcq M, Decroisette C, Auliac JB, Bota S, Lamy R, Massuti B, Dujon C, Perol M, Daures JP, Descourt R, Lena H, Plassot C, Chouaid C (2016) Use of a comprehensive geriatric assessment for the management of elderly patients with advanced non-small-cell lung cancer: the phase III randomized ESOGIA-GFPC-GECP 08–02 study. J Clin Oncol 34(13):1476–1483
Delva F, Soubeyran P, Rainfray M, Mathoulin-Pelissier S (2012) Referral of elderly cancer patients to specialists: action proposals for general practitioners. Cancer Treat Rev 38(7):935–941
Dubruille S, Libert Y, Roos M, Vandenbossche S, Collard A, Meuleman N, Maerevoet M, Etienne AM, Reynaert C, Razavi D, Bron D (2015) Identification of clinical parameters predictive of one-year survival using two geriatric tools in clinically fit older patients with hematological malignancies: major impact of cognition. J Geriatr Oncol 6(5):362–369
Engelhardt M, Ihorst G, Duque-Afonso J, Wedding U, Spat-Schwalbe E, Goede V, Kolb G, Stauder R, Wasch R (2020) Structured assessment of frailty in multiple myeloma as a paradigm of individualized treatment algorithms in cancer patients at advanced age. Haematologica 105(5):1183–1188
Extermann M, Boler I, Reich RR, Lyman GH, Brown RH, DeFelice J, Levine RM, Lubiner ET, Reyes P, Schreiber FJ 3rd, Balducci L (2012) Predicting the risk of chemotherapy toxicity in older patients: the chemotherapy risk assessment scale for high-age patients (CRASH) score. Cancer 118(13):3377–3386
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3):189–198
Friedrich C, Kolb G, Wedding U, Pientka L, der Interdisziplinare Arbeitsgruppe DD (2003) Comprehensive geriatric assessment in the elderly cancer patient. Onkologie 26(4):355–360
Giantin V, Valentini E, Iasevoli M, Falci C, Siviero P, De Luca E, Maggi S, Martella B, Orru G, Crepaldi G, Monfardini S, Terranova O, Manzato E (2013) Does the multidimensional prognostic index (MPI), based on a comprehensive geriatric assessment (CGA), predict mortality in cancer patients? Results of a prospective observational trial. J Geriatr Oncol 4(3):208–217
Goede V, Bahlo J, Chataline V, Eichhorst B, Durig J, Stilgenbauer S, Kolb G, Honecker F, Wedding U, Hallek M (2015) Evaluation of geriatric assessment in patients with chronic lymphocytic leukemia: results of the CLL9 trial of the German CLL study group. Leuk Lymphoma 57(4):789–796
Hamaker ME, Jonker JM, de Rooij SE, Vos AG, Smorenburg CH, van Munster BC (2012a) Frailty screening methods for predicting outcome of a comprehensive geriatric assessment in elderly patients with cancer: a systematic review. Lancet Oncol 13(10):e437-444
Hamaker ME, Vos AG, Smorenburg CH, de Rooij SE, van Munster BC (2012b) The value of geriatric assessments in predicting treatment tolerance and all-cause mortality in older patients with cancer. Oncologist 17(11):1439–1449
Hamaker ME, Te Molder M, Thielen N, van Munster BC, Schiphorst AH, van Huis LH (2018) The effect of a geriatric evaluation on treatment decisions and outcome for older cancer patients - A systematic review. J Geriatr Oncol 9(5):430–440
Hamerman D (1999) Toward an understanding of frailty. Ann Intern Med 130(11):945–950
Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, Lichtman SM, Gajra A, Bhatia S, Katheria V, Klapper S, Hansen K, Ramani R, Lachs M, Wong FL, Tew WP (2011) Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol 29(25):3457–3465
Hutchins LF, Unger JM, Crowley JJ, Coltman CA Jr, Albain KS (1999) Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med 341(27):2061–2067
Kenis C, Decoster L, Van Puyvelde K, De Greve J, Conings G, Milisen K, Flamaing J, Lobelle JP, Wildiers H (2014) Performance of two geriatric screening tools in older patients with cancer. J Clin Oncol 32(1):19–26
Laurent, M., E. Paillaud, C. Tournigand, P. Caillet, A. Le Thuaut, J. L. Lagrange, O. Beauchet, H. Vincent, M. Carvahlo-Verlinde, S. Culine, S. Bastuji-Garin, F. Canoui-Poitrine and E. S. Group (2014) Assessment of solid cancer treatment feasibility in older patients: a prospective cohort study. Oncologist 19(3):275–282
Lawton MP, Brody EM (1969) Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9(3):179–186
Maas HA, Janssen-Heijnen ML, Olde Rikkert MG, Machteld Wymenga AN (2007) Comprehensive geriatric assessment and its clinical impact in oncology. Eur J Cancer 43(15):2161–2169
Mahoney FI, Barthel DW (1965) Functional evaluation. Md Med J 14:61–65
Pal SK, Katheria V, Hurria A (2010) Evaluating the older patient with cancer: understanding frailty and the geriatric assessment. CA Cancer J Clin 60(2):120–132
Pallis AG, Fortpied C, Wedding U, Van Nes MC, Penninckx B, Ring A, Lacombe D, Monfardini S, Scalliet P, Wildiers H (2010a) EORTC elderly task force position paper: approach to the older cancer patient. Eur J Cancer 46(9):1502–1513
Pallis AG, Wedding U, Lacombe D, Soubeyran P, Wildiers H (2010b) Questionnaires and instruments for a multidimensional assessment of the older cancer patient: what clinicians need to know? Eur J Cancer 46(6):1019–1025
Pallis AG, Hatse S, Brouwers B, Pawelec G, Falandry C, Wedding U, Lago LD, Repetto L, Ring A, Wildiers H (2014) Evaluating the physiological reserves of older patients with cancer: the value of potential biomarkers of aging? J Geriatr Oncol 5(2):204–218
Pallis, A. G., A. Ring, C. Fortpied, B. Penninckx, M. C. Van Nes, U. Wedding, G. Vonminckwitz, C. D. Johnson, L. Wyld, A. Timmer-Bonte, F. Bonnetain, L. Repetto, M. Aapro, A. Luciani, H. Wildiers, R. European Organisation for and F. Treatment of Cancer Elderly Task (2011) EORTC workshop on clinical trial methodology in older individuals with a diagnosis of solid tumors. Ann Oncol 22(8):1922–1926
Podsiadlo D, Richardson S (1991) The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 39(2):142–148
Puts MT, Hardt J, Monette J, Girre V, Springall E, Alibhai SM (2012) Use of geriatric assessment for older adults in the oncology setting: a systematic review. J Natl Cancer Inst 104(15):1133–1163
Scheubeck S, Ihorst G, Schoeller K, Holler M, Moller MD, Reinhardt H, Wasch R, Engelhardt M (2021) Comparison of the prognostic significance of 5 comorbidity scores and 12 functional tests in a prospective multiple myeloma patient cohort. Cancer. https://doi.org/10.1002/cncr.33658
Siegel R, Ma J, Zou Z, Jemal A (2014) Cancer statistics, 2014. CA Cancer J Clin 64(1):9–29
Siegel RL, Miller KD, Fuchs HE, Jemal A (2021) Cancer statistics, 2021. CA Cancer J Clin 71(1):7–33
Soubeyran P, Fonck M, Blanc-Bisson C, Blanc JF, Ceccaldi J, Mertens C, Imbert Y, Cany L, Vogt L, Dauba J, Andriamampionona F, Houede N, Floquet A, Chomy F, Brouste V, Ravaud A, Bellera C, Rainfray M (2012) Predictors of early death risk in older patients treated with first-line chemotherapy for cancer. J Clin Oncol 30(15):1829–1834
Spina M, Balzarotti M, Uziel L, Ferreri AJ, Fratino L, Magagnoli M, Talamini R, Giacalone A, Ravaioli E, Chimienti E, Berretta M, Lleshi A, Santoro A, Tirelli U (2012) Modulated chemotherapy according to modified comprehensive geriatric assessment in 100 consecutive elderly patients with diffuse large B-cell lymphoma. Oncologist 17(6):838–846
Spitzer RL, Williams JB, Gibbon M, First MB (1992) The Structured Clinical Interview for DSM-III-R (SCID). I: history, rationale, and description. Arch Gen Psychiatry 49(8):624–629
Tucci A, Martelli M, Rigacci L, Riccomagno P, Cabras MG, Salvi F, Stelitano C, Fabbri A, Storti S, Fogazzi S, Mancuso S, Brugiatelli M, Fama A, Paesano P, Puccini B, Bottelli C, Dalceggio D, Bertagna F, Rossi G, Spina M, Italian Lymphoma F (2015) Comprehensive geriatric assessment is an essential tool to support treatment decisions in elderly patients with diffuse large B-cell lymphoma: a prospective multicenter evaluation in 173 patients by the Lymphoma Italian Foundation (FIL). Leuk Lymphoma 56(4):921–926
Wedding U, Kodding D, Pientka L, Steinmetz HT, Schmitz S (2007) Physicians’ judgement and comprehensive geriatric assessment (CGA) select different patients as fit for chemotherapy. Crit Rev Oncol Hematol 64(1):1–9
Wildiers H, Mauer M, Pallis A, Hurria A, Mohile SG, Luciani A, Curigliano G, Extermann M, Lichtman SM, Ballman K, Cohen HJ, Muss H, Wedding U (2013) End points and trial design in geriatric oncology research: a joint European organisation for research and treatment of cancer–Alliance for clinical trials in oncology-international society of geriatric oncology position article. J Clin Oncol 31(29):3711–3718
Acknowledgements
We thank the patients, relatives, investigators, and institutions involved in this study.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by Janssen-Cilag GmbH, who provided funding for the collection and analysis of data presented in this manuscript. The IN-GHO® patient registry is funded and owned by Janssen-Cilag GmbH, Germany.
Author information
Authors and Affiliations
Consortia
Contributions
Study concepts: FH, SH, RA, AL, CB, and UW; Study design: FH, SH, RA, WF, CF, GH, AL, BO, LP, ES-S, GKo, CB, and UW: Data acquisition: FH, WF, CF, GH, BO, ES-S, and UW; Quality control of data and algorithms: SH, RA, and AL; Data analysis and interpretation: FH, SH, CB, GKa, GKo, CB, and UW; Statistical analysis: GKa; Manuscript preparation: FH, GKa, and UW; Manuscript editing: FH and UW. Manuscript review: FH, SH, RA, WF, CF, GH, AL, BO, LP, ES-S, GKo, CB, and UW.
Corresponding author
Ethics declarations
Conflict of Interest
RA and SH are employees of Janssen-Cilag GmbH and shareholders of Johnson&Johnson shares; CB, FH and UW received honoraria from Janssen-Cilag for advisory boards and lectures; GKa is an employee of ARGUS GmbH, which was contracted for statistical analyses from Janssen-Cilag GmbH; GKo received honoraria from Janssen-Cilag for advisory boards; WF, CF, GH, BO, LP, and ESS have no conflicts of interest to declare.
Ethical approval
The registry was approved by an institutional review board of the University of Hamburg, and informed written consent to collect and analyse pseudonymised data was obtained from each eligible patient before participation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
An Initiative of Janssen-Cilag GmbH
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Honecker, F., Huschens, S., Angermund, R. et al. Patient assessment and feasibility of treatment in older patients with cancer: results from the IN-GHO® Registry. J Cancer Res Clin Oncol 147, 3183–3194 (2021). https://doi.org/10.1007/s00432-021-03714-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-021-03714-3