Abstract
Purpose
Several studies have evaluated the role of delayed initiation of adjuvant chemotherapy (AC) in breast cancer (BC), but the results have remained controversial and an optimal time has not been defined. Our aim was to determine the effect of time to starting AC from the date of surgery on survival of BC patients, based on estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) status, using data from the National Cancer Database (NCDB).
Methods
A total of 332,927 Stage I–III BC patients who received AC from 2010 to 2016 were analyzed. We included all ER, PR and HER2 statuses and excluded patients with stage 4 and stage 0 (DCIS) disease. The cohort was divided into five groups based on the time of initiating AC from the date of the most definitive surgery i.e., ≤ 30 days, 31–60 days, 61–90 days, 91–120 days and > 120 days. They were further divided into five subgroups based on the receptor status.
Results
Hazard ratio (HR) estimates and Kaplan–Meier (KM) analysis shows that starting AC by 31–60 days shows the best survival outcome in all the subtypes, except in hormone positive/HER2 negative BC in which 31–60 days and 61–90 days have similar outcomes.
Conclusions
After surgery for BC, it takes around 4–6 weeks to begin AC and delay in initiating the same leads to poor outcomes. Our results are particularly significant in triple-negative breast cancer (TNBC), similar to prior studies showing a benefit to starting AC as early as possible after surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) is one of the commonest causes of cancer-related mortality in women (Sun et al. 2017). In 2017, in the United States alone, 30% of all new cancer diagnosis in women was breast cancer (Siegel et al. 2017). BC is a very complex disease and has distinct clinical entities based on immunohistochemistry markers such as estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2). This along with clinico-pathological variables such as size, nodal involvement, histologic subtype and surgical margins have the treatment and prognostic implications. The implications of delayed initiation of adjuvant chemotherapy (AC) in BC is subtype dependent, as each subtype behaves differently in regard to clinical and biological response (Dai et al. 2016; Lebert et al. 2018).
The time to instituting adjuvant therapy, surgical and medical management strategies, particularly in early-stage breast cancer has been frequently discussed, as delays in initiation portend worse outcomes. Several studies have sought to evaluate the role of delayed initiation of chemotherapy on survival, but the results have remained controversial and an optimal time has not been defined (Zhan et al. 2017). In general practice, clinicians prefer to begin AC by around 4–6 weeks after surgery (Chavez-MacGregor et al. 2016). For women, less than 70 years with Stage I–III, ER/PR negative BC, starting systemic chemotherapy within 120 days is considered a quality metric by the Centers for Medicare and Medicaid services (Chavez-MacGregor et al. 2016; Department of Health and Human Services 2014; de Gagliato et al. 2014; Raphael et al. 2016).
Given the paucity and lack of clarity in data with regard to the ideal time in starting AC, especially among the clinically distinct biologic subtypes of the disease (Cold et al. 2005; Kupstas et al. 2019; Early Breast Cancer Trialists’ Collaborative Group 2005; Bleicher et al. 2016; Howlader et al. 2014), we decided to perform a comprehensive analysis using the National Cancer Database (NCDB) database to determine the effect of time to start chemotherapy from the date of surgery on the outcome and survival of different BC subtypes and identify an ideal time period to start AC. We also hoped to see if factors like patient demographics, comorbidities and tumor characteristics play a role in affecting this time.
Methods
Data source
The NCDB is a very large database managed by the American College of Surgeons, comprising information from Commission on Cancer (CoC)-accredited facilities. The database has been used in several cancer-focused studies (Losk et al. 2016; Kelley and Tsikitis 2019). Data were obtained from the NCDB Participant Use Data File (PUF). The PUF contains information on patient demographics and comorbidities, tumor characteristics, treatments employed and mortality data (Boffa et al. 2017; Bilimoria et al. 2008).
Patient cohort and stratification
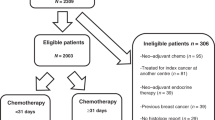
The dataset that we used included BC patients diagnosed between 2010 and 2016 which is closely representative of current breast cancer management strategies and advances. Previous years were not included as HER2 data from prior years were not available. We included patients with Stage I–III Breast cancer, aged > 18 years. All ER, PR and HER2 statuses were included. We excluded patients with Stage IV and Stage 0 (DCIS). We only included patients who received systemic AC after their definitive surgery and excluded patients who received neoadjuvant chemotherapy. The study population was divided into five groups based on the time of initiating AC from the date of the most definitive surgery i.e., ≤ 30 days, 31–60 days, 61–90 days, 91–120 days and > 120 days (Table 1). They were further divided into five subgroups [triple negative breast cancer (TNBC), hormone positive/HER2 negative BC, hormone positive/HER2 positive BC and hormone negative/ HER2 positive BC] based on the ER, PR and HER2 receptor status as represented in Table 1 and Fig. 1. A general scheme of the methodology is represented in Fig. 1.
Statistical analysis
All analyses were performed using SAS 9.4. Patients demographics and clinical characteristics were summarized by frequencies and proportions by the five groups derived from the time to AC therapy after surgery, and the marginal association between each factor and the time to AC therapy was assessed by Pearson chi-square test. Logistic regression was further performed to model the probability of starting AC therapy after 60 days of surgery and determine the factors that are associated with the delay while adjusting the confounding effects of other relevant factors. Kaplan–Meier (KM) survival curves were used to illustrate the survival difference between different time groups, by ER, PR and HER2 subgroups. Log–log transformation was used to calculate the 95% confidence intervals (CI) for the survival rate at 60 and 90 months. Cox regression model was further applied to confirm the impact of the delay of AC therapy on survival. All factors that are associated with the delay of AC therapy as identified in the logistic model are included in the Cox model. Hazard ratio (HR) was reported as the measure of association and the 95% CI was calculated from the profile likelihood ratio. Propensity score (inverse probability of treatment) weighting was used to adjust the confounding effects caused by the factors listed above in Table 1a and estimate the effect of delayed treatment (> 60 days vs ≤ 60 days) on survival via Cox regression.
Results
A total of 332,927 BC patients from 2010 to 2016 were analyzed for our study. Table 1a summarizes the distribution, demographic and pathological characteristics of the study population.
The odds ratio estimates for the various factors associated with a delay in getting AC are represented in Table 1b. Delay was defined as getting AC beyond 60 days from the date of surgery. 60 days was selected as 31–60 days group had the best results in most of the biologic subgroups as described in the subsequent sections. After adjusting for all the other factors listed in Table 1a, factors that were associated with a greater likelihood of AC being delayed included older age group, African American race and other race compared to the Caucasian race, Academic/research Facility, Uninsured patients and Government insurance compared to private insurance, Stage I compared to II and Charlson Deyo Score of 3, 2, 1 and 0 in that order. Hormone positive/HER negative BC had the highest likelihood of being associated with a delay, followed by hormone positive/HER2 positive and hormone negative/HER2 positive BC, with TNBC having the least likelihood for the delay.
The survival estimates from KM analysis for the overall BC population is shown in Table 2a and Fig. 2a. The 31–60 days group had the best survival estimate at 90 months (81.2%) followed by 61–90 days (79.5%), ≤ 30 days (79.2%), 91–120 days (77.7%) and > 120 days (74.4%) in that order.
Kaplan–Meier survival curves at different points of time in months of the various groups and subgroups in the study. a All Stage I–III breast cancer patients. b Triple negative breast cancer patients. c Hormone positive/HER2 negative breast cancer patients. d Hormone positive/HER2 positive breast cancer patients. e Hormone negative/HER2 positive breast cancer patients
Table 3a shows the comparison of adjusted HR for survival between the groups for the overall population, after adjusting for factors listed in Table 1a. The 31–60 days group had the best survival with the least adverse outcomes, compared to the other groups. This was followed by the 61–90 days group. ≤ 30 days group had a better outcome than > 120 days group and no difference when compared to the 91–120 days group. 91–120 days had a better HR estimate than the > 120 days group. The hazard for the 31–60 days group was 84.2% of the < 30 days group, 90% of the 61–90 days group, 80.2% of the 91–120 days group and 71% of the > 120 days group.
The TNBC subgroup had a total of 68,598 patients who met the inclusion criteria. The survival estimates among TNBC patients from KM analysis is shown in Table 2b and Fig. 2b. The 31–60 days group had the best survival estimate at 90 months (77%), followed by ≤ 30 days (72.9%), 61–90 days (71.9%), > 120 days (70.4%) and 91–120 days (68.2%) in that order.
Table 3b represents the HR comparison between the various groups in TNBC, after adjusting for factors shown in Table 1a. The 31–60 days group had the best overall survival compared to the other groups. The 61–90 days had better results compared to the ≤ 30 days and no difference when compared to 91–120 days and > 120 days. There was no significant difference in comparisons between ≤ 30 days, 91–120 days and > 120 days groups. The hazard for the 31–60 days group was 81.6% of the < 30 days group, 89.2% of the 61–90 days group, 79.1% of the 91–120 days group and 77.7% of the > 120 days group.
KM survival estimate for hormone positive/HER2 negative BC at 90 months were of the following order; 31–60 days (82%), 61–90 days (81.8%), 91–120 days (80%), ≤ 30 (79.2%) days and > 120 days (74%) (Table 2c and Fig. 2c).
The adjusted HR and CI of the various groups for hormone positive/HER2 negative BC are summarized in Table 3c. 31–60 and 61–90-days groups had the best HR outcomes compared to others and comparison between the two groups showed no significance. 91–120 days was better than > 120 days group but showed no difference when compared to ≤ 30 days. > 120 days had worse outcomes compared to ≤ 30 days. After adjusting for the factors in Table 1a, the hazard for the 31–60 days group was 84.6% of the < 30 days group, 86.4% of the 91–120 days group and 72.5% of the > 120 days group. The 61–90 days group had a hazard that was 87.5% of the < 30 days group, 89.3% of the 91–120 days group and 75% of the > 120 days group.
KM analysis and survival estimates of the hormone positive/HER2 positive subgroup is represented in Table 2d and Fig. 2d. The survival estimates at 90 months is as follows: ≤ 30 days (87.3%), 31–60 days (83.5%), 61–90 days (79.1%), > 120 days (78.4%) and 91–120 days (77.9%). KM analysis and survival estimates of BC patients with hormone negative/HER2 positive BC is represented in Table 2e and Fig. 2e. The survival estimates at 90 months is as follows: ≤ 30 days (82.8%), 31–60 days (82.4%), 61–90 days (79.6%), 91–120 days (77%) and > 120 days (75.1%).
After adjusting for the factors in Table 1a, the HR and CI of the various groups in hormone positive/HER2 positive and hormone negative/ HER2 positive BC patients are summarized in Table 3d. The 31–60-days group seemed to have a better outcome in both subgroups compared to all other groups. The comparison between the other groups showed no significance except that ≤ 30 days and 61–90 days had better outcomes when individually compared to > 120 days in the hormone positive/HER2 positive subgroup. In hormone positive/HER2 positive BC, the hazard for the 31–60 days group was 85.4% of the < 30 days group, 81.1% of the 61–90 days group, 68% of the 91–120 days group and 56.2% of the > 120 days group. In hormone negative/HER2 positive BC, the hazard of the 31–60 days group was 84%of the < 30 days group, 77.1% of the 61–90 days group and 72.1% of the > 120 days group.
For hormone positive/HER2 positive patients, the potential survival benefit of having AC between 31 and 60 days is seen before 80 months from KM survival curves (Fig. 2d). At 60 months, the survival estimates for the group < 30 days is 0.919 (95% CI, 0.912–0.925) and 31–60 days is 0.926 (95% CI, 0.921–0.930). However, survival estimate at 90 months for the group < 30 days is 0.873 (95% CI, 0.857–0.887) and is better than the 31–60 days group at 0.835 (95% CI, 0.815–0.853). After adjusting for other factors listed in Table 1, the hazard for the 31–60 days group appears to be 85.4% and 84% respectively of that for the < 30 days group as shown in the Table 3d. Thus, we conclude that overall, 31–60 days had a better survival than ≤ 30 days in both hormone positive/HER2 positive and hormone negative/HER2 positive BC patients. The unadjusted HR for all the groups and subgroups can be found in the supplement.
Discussion
The Early Breast Cancer Trialists Collaborative Group showed that adjuvant therapy, especially anthracycline-based chemotherapy and hormone therapy in ER-positive BC significantly reduces the 5-year recurrence and 15-year mortality rates (Early Breast Cancer Trialists’ Collaborative Group 2005; Bleicher et al. 2016). Delayed adjuvant therapy is believed to lead to poor outcomes based on preclinical data. This included the rapid growth of micro metastasis following removal of the primary tumor, increased angiogenesis and development of resistance to adjuvant therapy (Miller et al. 2014; Tremont et al. 2017; Pernas and Tolaney 2019; Wahba and El-Hadaad 2015; Fisher et al. 1989; Gunduz et al. 1979). From a large cohort of patients, our observation shows that the ideal time to start AC is 31–60 days following definitive surgery in all major BC subtypes, expect in hormone positive/HER2 negative BC (31–90 days).
Factors shown to be associated with a delay in AC include African American and Hispanic race, residents of rural areas, low socioeconomic status, unmarried individuals, medicare, medicaid or military insurance, uninsured individuals and those undergoing mastectomies instead of breast-conserving surgery (Chavez-MacGregor et al. 2016; Losk et al. 2016). Studies have shown that TNBC, Stage II or III disease are associated with a lesser chance of delay. The poorer prognosis associated with aggressive subtypes may have promoted physicians to be aggressive in their management strategies. In a study of 523 patients who received AC following surgery, factors associated with a delay of AC beyond 42 days after surgery were highlighted. They include ER or PR positivity, HER2 positivity, Stage I disease, mastectomy and delay of pathological sign-out beyond 10 days. They also conclude that in hormone-positive BC patients, a higher proportion of delay was found in those who had oncotype Dx testing done compared to those who did not have the same (Losk et al. 2016). This was in most part in line with our results represented in Table 1b. Our analysis reveals that older patients, non-caucasian race, uninsured or government insurance and those with multiple comorbidities as indicated by the Charlson Deyo Score had a more likelihood of a delay. It is interesting to note that in our study, similar to the above analysis, lower stage disease and receptor positivity (ER/PR > HER2 > TNBC) were associated with more chances for delayed AC. This data may help providers identify target groups for whom the timely start of AC can be emphasized more.
Kupstas et al. used the data from 2010 to 2014 NCDB and analyzed the effect of the type of surgery on delayed AC initiation and its impact on survival on Stage I–III BC patients. Besides concluding that the type of surgery impacts time to AC, with breast reconstruction resulting in considerable delay, multivariable Cox proportional hazard model to determine the impact of delayed AC on overall survival was also studied. However, Kupstas et al.’s study mainly dealt with time to AC from date of diagnosis, whereas the focus of our study was time to AC from the date of surgery. In their study, delay in AC, defined as >120 days from diagnosis, was associated with poor prognosis across all biological subtypes. HR analysis comparing 0–60 days to 61–90, 91–120 and >120 days revealed only >120 days to have a statistically significant worse risk. Aside from, the difference in defining the delay in AC, our study had 0–30 and 31–60 days instead of 0–60 days alone and had a newer dataset (2010–2016). The type of surgery was not a focus in our analysis (Kupstas et al. 2019). Several studies have suggested a cut-off of 61 days or 91 days (Chavez-MacGregor et al. 2016; de Gagliato et al. 2014). Some meta-analyses have recommended a timeframe as short as 4 weeks beyond which survival may be worse (Zhan et al. 2017; Raphael et al. 2016). Although many reports have repeatedly identified the detrimental effects of delayed initiation of AC, data that may challenge the same include that of the Danish Breast Cancer Cooperative Group (DBCG) involving 7501 BC patients. Using nationwide clinical data, it was shown that there was no clinical benefit in initiating chemotherapy within 2- or 3-months following surgery (Cold et al. 2005). However, most of such studies were under-powered or were not applicable in clinically relevant thresholds (Kupstas et al. 2019).
de Gagliato et al. studied the clinical effect of delaying AC from the date of definitive surgery among 6827 Stage I–III BC patients from 1997 to 2011 and showed that a delay of ≥61 days had a 19% greater increase of death when compared ≤ 30 days. Comparison between ≤ 30 days and 31–60 days showed no significance. Stage I disease showed no association between the outcome parameters and delay. In Stage II disease, starting chemotherapy ≤ 30 days had a lower risk of distant relapse compared to 31–60 days and ≥61 days with no difference in the risk of death. In Stage III disease, ≥ 61 days had a higher risk for death and relapse than ≤ 30 days, but there was no difference between ≤ 30 and 31–60 days. When patients were divided into subtypes, hormone positive BC showed no difference in outcomes based on delay in starting chemotherapy. In HER2 positive cancers, 31–60 days had a higher risk of relapse than ≤ 30 days, but no difference in risk of death. In TNBC, both 31–60 days and ≥ 61 days had a higher risk of death than ≤ 30 days (de Gagliato et al. 2014). In our study, the 31–60 days group had the best outcome across all receptor subtypes except in the hormone positive group where the time frame was more lenient. In TNBC, the 31–60 days group had a 18.4% and 10.8% lesser risk of death than the ≤ 30 days and 61–90 days groups respectively. This shows a striking difference when compared to above referenced analysis and should encourage further research. Prospective trials would be ideal but may not be realistic. In hormone positive/HER2 negative breast cancer, it was 15.4% lesser than the ≤ 30 days group with no difference compared to 61–90 days. This number was 14.6% and 18.9% for hormone negative/HER2 positive group and 16% and 22.9% for hormone positive/HER2 positive group. Overall, in all Stage I–III BC, starting AC by 31–60 days had a 15.8% and 10% lesser risk of death than starting chemotherapy by ≤ 30 days and 61–90 days respectively.
Chavez-MacGregor et al. used 24,843 patients from the California cancer registry from 2005 to 2010 and found that a delay greater than 91 days from the time of surgery had adverse effects and poor overall and cancer-specific survival when compared to those receiving chemotherapy within 31 days. There was no difference in outcome when chemotherapy was started between 30 and 90 days. On subtype analysis, TNBC showed worse overall survival when chemotherapy was delayed for 91 days, but no effect in hormone positive or HER2 positive patients (Chavez-MacGregor et al. 2016). Studies such as that of Lohrisch et al. with data from British Columbia (2594 patients), Nurgalieva et al. (14,380 patients) and Hershman et al. (5003 patients) who used the SEER-Medicare data defined a duration of around 90 days beyond which outcomes are less ideal (Lohrisch et al. 2006; Nurgalieva et al. 2013; Hershman et al. 2006).
Our study has the advantage of having a much larger sample size than the above-mentioned studies (Chavez-MacGregor et al. 2016; Gagliato Dde et al. 2014). Limitations of our analysis include the fact that the results are from observational data that is not population based. Despite using propensity score weighting, there may be confounding that may not always be adjusted for by the multivariate model. The other drawback is the lack of specific chemotherapy related information like the type of regime and disease specific survival.
From our analysis, we can say that 31–60 days may be considered an ideal time frame to start AC in Stage I–III BC in all receptor subtypes, except in hormone positive/HER2 negative BC patients where 31–90 days may be considered the right window. It is well established that receptor status is an independent predictor of outcomes in BC (Grann et al. 2005; Dunnwald et al. 2007; Aysola et al. 2013; Abdollahi and Etemadi 2016). In our study, no major difference was noted with regard to the effect of delayed AC on the two HER2 subgroups, despite them known to have variable response to treatment in clinical practice (Dai et al. 2016; Lebert et al. 2018; Howlader et al. 2014; Wang and Xu 2019). The fact that 31–60 days and 61–90 days groups showed no difference in hormone positive/HER2 negative BC, points to an important hypothesis that delay caused due to performing oncotype Dx may not significantly impact outcomes in this group (Losk et al. 2016; Schmidt 2014; He et al. 2017). Also, in the event of a pandemic like the Covid-19 situation, delaying AC and considering radiation may be a reasonable alternative in hormone positive/HER2 negative BC patients. Starting AC very close to surgery may lead to patients not tolerating it well, whereas delaying it beyond a certain period may lead to poor outcomes secondary to reasons like rapid growth of micrometastasis following surgery (Schmidt 2014; He et al. 2017). Given the limitations of our study, larger population-based studies are needed to accurately determine an ideal time frame to start AC in BC patients.
Conclusions
Our study analyzes the effect of delay in starting chemotherapy from the date of surgery in BC based on the receptor status, using a large national database, the NCDB. Overall survival and adverse outcome results are better when AC is started by 31–60 days after the date of surgery in hormone positive/HER2 positive, hormone negative/HER2 positive BC and TNBC and 31–90 days in hormone positive/HER2 negative BC patients.
Availability of data and material/code availability
The datasets analyzed during the current study are not publicly available as they were provided by the American College of Surgeons. They are available through an application process to investigators in CoC- accredited cancer programs.
References
Abdollahi A, Etemadi M (2016) Pathological characteristics of triple-negative breast cancer at Main Referral Teaching Hospital, April 2014 to April 2015, Tehran. Iran Int J HematolOncol Stem Cell Res 10(4):200–205
Aysola K, Desai A, Welch C, Xu J et al (2013) Triple negative breast cancer—an overview. Hereditary Genet 2013(Suppl 2):001
Bilimoria KY, Stewart AK, Winchester DP, Ko CY (2008) The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann SurgOncol 15(3):683–690
Bleicher RJ, Ruth K, Sigurdson ER, Beck JR et al (2016) Time to surgery and breast cancer survival in the United States. JAMA Oncol 2(3):330–339
Boffa DJ, Rosen JE, Mallin K, Loomis A et al (2017) Using the national cancer database for outcomes research: a review. JAMA Oncol 3(12):1722–1728
Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, Giordano SH (2016) Delayed initiation of adjuvant chemotherapy among patients with breast cancer. JAMA Oncol 2(3):322–329
Cold S, Düring M, Ewertz M, Knoop A et al (2005) Does timing of adjuvant chemotherapy influence the prognosis after early breast cancer? Results of the Danish Breast Cancer Cooperative Group (DBCG). Br J Cancer 93(6):627–632
Dai X, Xiang L, Li T, Bai Z (2016) Cancer hallmarks, biomarkers and breast cancer molecular subtypes. J Cancer 7(10):1281–1294
de Gagliato MD, Gonzalez-Angulo AM, Lei X, Theriault RL et al (2014) Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J ClinOncol 32(8):735–744
Department of Health and Human Services (2014) Center for medicare and medicaid services. 42 CFR Parts 405, 412, 413, 415, 422, 424, 485 and 488. https://www.govinfo.gov/content/pkg/FR-2014-08-22/pdf/2014-18545.pdf. Accessed 3 September 2020
Dunnwald LK, Rossing MA, Li CI (2007) Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res 9(1):R6
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365(9472):1687–1717
Fisher B, Gunduz N, Coyle J, Rudock C et al (1989) Presence of a growth-stimulating factor in serum following primary tumor removal in mice. Cancer Res 49(8):1996–2001
Grann VR, Troxel AB, Zojwalla NJ, Jacobson JS et al (2005) Hormone receptor status and survival in a population-based cohort of patients with breast carcinoma. Cancer 103(11):2241–2251
Gunduz N, Fisher B, Saffer EA (1979) Effect of surgical removal on the growth and kinetics of residual tumor. Cancer Res 39(10):3861–3865
He X, Ye F, Zhao B, Tang H et al (2017) Risk factors for delay of adjuvant chemotherapy in non-metastatic breast cancer patients: a systematic review and meta-analysis involving 186982 patients. PLoS One 12(3):e0173862
Hershman DL, Wang X, McBride R et al (2006) Delay of adjuvant chemotherapy initiation following breast cancer surgery among elderly women. Breast Cancer Res Treat 99(3):313–321
Howlader N, Altekruse SF, Li CI et al (2014) US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 106(5):dju055
Kelley KA, Tsikitis VL (2019) Review of colorectal studies using the national cancer database. Clin Colon Rectal Surg 32(1):69–74
Kupstas AR, Hoskin TL, Day CN, Habermann EB et al (2019) Effect of surgery type on time to adjuvant chemotherapy and impact of delay on breast cancer survival: a national cancer database analysis. Ann SurgOncol 26(10):3240–3249
Lebert JM, Lester R, Powell E, Seal M et al (2018) Advances in the systemic treatment of triple-negative breast cancer. CurrOncol 25(Suppl 1):S142–S150
Lohrisch C, Paltiel C, Gelmon K, Speers C et al (2006) Impact on survival of time from definitive surgery to initiation of adjuvant chemotherapy for early-stage breast cancer. J ClinOncol 24(30):4888–4894
Losk K, Vaz-Luis I, Camuso K, Batista R et al (2016) Factors associated with delays in chemotherapy initiation among patients with breast cancer at a comprehensive cancer center. J Natl ComprCancNetw 14(12):1519–1526
Miller E, Lee HJ, Lulla A, Hernandez L et al (2014) Current treatment of early breast cancer: adjuvant and neoadjuvant therapy. F1000Res 3:198
Nurgalieva ZZ, Franzini L, Morgan RO, Vernon SW et al (2013) Impact of timing of adjuvant chemotherapy initiation and completion after surgery on racial disparities in survival among women with breast cancer. Med Oncol 30(1):419
Pernas S, Tolaney SM (2019) HER2-positive breast cancer: new therapeutic frontiers and overcoming resistance. TherAdv Med Oncol 11:1758835919833519
Raphael MJ, Biagi JJ, Kong W, Mates M et al (2016) The relationship between time to initiation of adjuvant chemotherapy and survival in breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat 160(1):17–28
Schmidt M (2014) Chemotherapy in early breast cancer: when, how and which one? Breast Care (Basel) 9(3):154–160
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67(1):7–30
Sun YS, Zhao Z, Yang ZN, Xu F et al (2017) Risk factors and preventions of breast cancer. Int J BiolSci 13(11):1387–1397
Tremont A, Lu J, Cole JT (2017) Endocrine therapy for early breast cancer: updated review. Ochsner J 17(4):405–411
Wahba HA, El-Hadaad HA (2015) Current approaches in treatment of triple-negative breast cancer. CancerBiol Med 12(2):106–116
Wang J, Xu B (2019) Targeted therapeutic options and future perspectives for HER2-positive breast cancer. Signal Transduct Target Ther 4:34
Zhan QH, Fu JQ, Fu FM, Zhang J et al (2017) Survival and time to initiation of adjuvant chemotherapy among breast cancer patients: a systematic review and meta-analysis. Oncotarget 9(2):2739–2751
Acknowledgement
Our sincere gratitude to the Department of Hematology Oncology at SUNY Upstate Medical University for all the support. Our thanks to the American College of Surgeons and the American Cancer Society for providing us with the National Cancer Database PUF file without which our project would have not been possible.
Funding
The study was funded by the Division of Hematology-Oncology, Upstate Cancer Center, of the Upstate Medical University.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Prashanth Ashok Kumar, Shweta Paulraj, Dongliang Wang, Danning Huang and Abirami Sivapiragasam. The first draft of the manuscript was written by Prashanth Ashok Kumar and Shweta Paulraj and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no relevant financial or non-financial interests to disclose. Prashanth Ashok Kumar declares that he has no conflict of interest. Shweta Paulraj declares that she has no conflict of interest. Dongliang Wang declares that he has no conflict of interest. Danning Huang declares that she has no conflict of interest. Abirami Sivapiragasam declares that she has no conflict of interest. The National Cancer Database (NCDB) is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The data used in the study are derived from a de-identified NCDB Participant User File (PUF) file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigators.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. The SUNY Upstate IRB has reviewed the project and has determined this project does not meet the definition of human subject research under the purview of the IRB according to federal regulations. IRB no: 1494553-1. The American College of Surgeons had reviewed and accepted the proposal for the study. NCDB PUF APPLICATION: 2016.985.
Consent to participate
Not applicable. Data were obtained from the NCDB PUF file. It is a Health Insurance Portability and Accountability Act (HIPAA) complaint data file where patient information is de-identified.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ashok Kumar, P., Paulraj, S., Wang, D. et al. Associated factors and outcomes of delaying adjuvant chemotherapy in breast cancer by biologic subtypes: a National Cancer Database study. J Cancer Res Clin Oncol 147, 2447–2458 (2021). https://doi.org/10.1007/s00432-021-03525-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-021-03525-6