Abstract
Purpose
The combined small-cell lung cancer (c-SCLC) is rare and has unique clinicopathological futures. The aim of this study is to investigate 18F-FDG PET/CT parameters and clinicopathological factors that influence the prognosis of c-SCLC.
Methods
Between November 2005 and October 2014, surgical-resected tumor samples from c-SCLC patients who received preoperative 18F-FDG PET/CT examination were retrospectively reviewed. The maximum standardized uptake value (SUVmax), metabolic tumor volume (MTV) and total lesion glycolysis (TLG) were used to evaluate metabolic parameters in primary tumors. The survivals were evaluated with the Kaplan–Meier method. Univariate and multivariate analyses were used to evaluate potential prognostic factors.
Results
Thirty-one patients were enrolled, with a median age of 62 (range: 35 − 79) years. The most common mixed component was squamous cell carcinoma (SCC, n = 12), followed by large-cell carcinoma (LCC, n = 7), adenocarcinoma (AC, n = 6), spindle cell carcinoma (n = 4), adenosquamous carcinoma (n = 1) and atypical carcinoid (n = 1). The median follow-up period was 53.0 (11.0–142.0) months; the 5-year overall survival (OS) and progression-free survival(PFS) rate were 48.4% and 35.5%, respectively. Univariate survival analysis showed that gender, smoking history, tumor location were associated with PFS (P = 0.036, P = 0.043, P = 0.048), SUVmax and TNM stage were closely related to PFS in both Mixed SCC and non-SCC component groups (P = 0.007, P = 0.048). SUVmax, smoking history, tumor size and mixed SCC component were influencing factors of OS in patients (P = 0.040, P = 0.041, P = 0.046, P = 0.029). Multivariate survival analysis confirmed that TNM stage (HR = 2.885, 95%CI: 1.323–6.289, P = 0.008) was the most significantly influential factor for PFS. High SUVmax value (HR = 9.338, 95%CI: 2.426–35.938, P = 0.001) and mixed SCC component (HR = 0.155, 95%CI: 0.045–0.530, P = 0.003) were poor predictors for OS.
Conclusion
Surgical-resected c-SCLCs have a relatively good prognosis. TNM stage is the most significant factor influencing disease progression in surgical-resected c-SCLCs. SUVmax and mixed NSCLC components within c-SCLCs had a considerable influence on the survival. Both high SUVmax and mixed SCC component are poor predictors for patients with c-SCLCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Combined small-cell lung carcinoma (c-SCLC) is defined as small-cell lung cancer (SCLC) combined with an additional component that consists of any of the histological types of non-small-cell lung cancer (NSCLC), including adenocarcinoma (AC), squamous cell carcinoma (SCC), large-cell carcinoma (LCC), or spindle cell, carcinoid and other rare types (Travis 2014). C-SCLC is comparatively uncommon and accounts for only 1−3% of all SCLCs (Moon et al. 2019).
18F-fluorodeoxyglucose-positron emission tomography/computed tomography (18F-FDG PET/CT) imaging using the tracer 18F-FDG has emerged as an essential imaging tool for diagnosis and staging of lung cancer. The National Comprehensive Cancer Network guidelines have recommended the application of 18F-FDG PET/CT for SCLC patients (Johnson 2001). SUVmax measured on 18F-FDG PET/CT is used to quantify FDG uptake of tumor cells; the degree of tumor uptake of 18F-FDG on PET/CT is shown to be an valuable prognostic gauge in malignant tumors (Bai et al. 2017; Hsieh et al. 2018; Hsu et al. 2016; Kwon et al. 2016; Lee et al. 2015, 2018; Park et al. 2014, 2016; Zhu et al. 2018). While, volumetric parameters such as metabolic tumor volume (MTV) and total lesion glycolysis (TLG) are investigated for independent prognostic parameters in NSCLC and some other cancers (Albano et al. 2018; Burger et al. 2016; Hasbek et al. 2019; Lemarignier et al. 2017; Tsujikawa et al. 2017). 18F-FDG PET/CT is the main imaging tool for initial staging and influences patient management and early assessment of tumor response (Kim et al. 2018; Zer et al. 2016). With the development of 18F-FDG PET/CT technology, lung cancers are diagnosed earlier and more SCLC patients undergo surgery and have pathological examinations, which have led to more c-SCLC diagnoses recently (Qin and Lu 2018; Zhang et al. 2017). However, influence of primary tumor metabolic parameters and mixed NSCLC components on survival of c-SCLC, and whether they are associated with prognosis are unclear.
The present study was performed to examine whether preoperative metabolic parameters of primary tumors measured on 18F-FDG PET/CT and mixed NSCLC components are correlated with overall survival in surgical-resected c-SCLC.
Materials and methods
Patients and diagnosis
The Ethics Committee of Tianjin medical university cancer institute and hospital (TMUCIH) approved this study, which was carried out in accordance with the Declaration of Helsinki. The requirement for informed consent was waived as the study was retrospective.
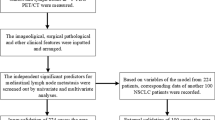
A retrospective review of postoperative lung cancer patients who had 18F-FDG PET/CT examination before surgery in TMUCIH between November 2005 and October 2014 was conducted. During this period, 1035 patients underwent 18F-FDG PET/CT examinations and surgical resection of primary lung cancers at the Department of Thoracic Surgery of our institution. Thirty-seven (3.6%) patients were diagnosed with c-SCLC, six patients with incomplete clinical and follow-up data were excluded. Thirty-one (3.0%) consecutive patients with pathologically confirmed c-SCLC were retrospectively reviewed, based on the diagnostic criteria proposed by the 2015 edition of the WHO classification system. Each surgically resected tumor was systematically sampled according to standard principles. Paraffin-embedded tumor specimens, which included the widest cross sections, were reassessed by two senior clinical pathologist. Immunohistochemistry staining of surgically resected c-SCLC was used to for the modification of the classification of SCLC and non-SCLC components within c-SCLC.
Neoadjuvant and adjuvant treatment
Two cycles of neoadjuvant chemotherapy (EP regimen) was performed in 3 patients who underwent pneumonectomy. Twenty-five patients accepted adjuvant chemotherapy with EP regimen or EP combined with TP, GP or AP regimen, and one patient with EGFR mutation in mixed adenocarcinoma component was given gefitinib as adjuvant therapy. Five patients with stage I and II A did not accept adjuvant chemotherapy treatment. The EP regimen was etoposide 100 mg/m2 (days 1–3) and cisplatin or carboplatin (cisplatin 75 mg/m2, carboplatin AUC = 5–6; day 1). The TP regimen was paclitaxel 135–175 mg/m2 (days 1) and cisplatin or carboplatin (cisplatin 75 mg/m2, carboplatin AUC = 5–6; day 1). The GP regimen was gemcitabine 1000–1250 mg/m2 (days 1 and 8) and cisplatin or carboplatin (cisplatin 75 mg/m2, carboplatin AUC = 5–6; day 1). The AP regimen was pemetrexed 500 mg/m2 (days 1) and cisplatin or carboplatin (cisplatin 75 mg/m2, carboplatin AUC = 5–6; day 1). Chemotherapy was administered at 3-week intervals for total 4–6 cycles. Local radiotherapy and prophylactic brain irradiation (PCI) were given in 11 patients. 3D conformal radiotherapy or intensity-modulated radiotherapy (PTV, 54 Gy/30f) was administered concurrent or followed chemotherapy. The radiotherapy fields covered primary lesions, hilar and ipsilateral mediastinal lymph nodes. Finally, PCI (25 Gy/10f) was performed.
18F-FDG PET/CT imaging and interpretation
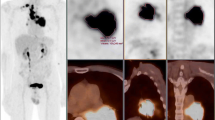
In our study, all patients (n = 31) underwent preoperative 18F-FDG PET/CT examination to confirm clinical stage and exclude distant metastasis. PET/CT scans were performed using a GE Discovery Elite PET/CT scanner (GE Medical Systems, Waukesha, WI, USA). All patients were requested to fast for at least 6 h prior to the 18F-FDG PET/CT scan. Serum glucose levels were measured before the 18F-FDG injection; no patient had a glucose level that exceeded 6.8 mmol/L. FDG was administered intravenously at a dose of 4.2 MBq 18F-FDG/kg body weight. After an hour, a spiral CT scan with ~ 25 effective mAs, 130 kVp, and a 5-mm slice thickness was taken, followed by a PET emission scan from the distal femur to the top of the skull (Yu et al. 2017a, b).
Two board-certified nuclear medicine physicians reviewed the PET/CT images side by side and calculated the area SUVmax, MTV, and TLG using line attenuation correction and iterative reconstruction of the image in the manually constructed radionuclide focal volume of interest (VOI). SUVmax was defined as the highest pixel value. The tumor size was expressed by the maximum diameter measured on the lung window in CT.
Follow-up
Patients were followed-up every 3 months for the first year and then every 3–6 months thereafter. Methods to obtain follow-up information include: communication with physicians, looking up to inpatient or outpatient records, death certificates, and communication with patient or patient’s family. Progression-free survival (PFS) was defined as the interval from the date of resection to the date of proven detection of local recurrence or metastasis. The duration of overall survival (OS) was defined as the interval between the day of surgery and the date of death by any cause or the last follow-up date. The primary end-point of the study was OS.
Statistical analysis
Pearson correlation analysis and Spearman rank correlation analysis were used, respectively, according to whether the variables were normally distributed or not. Kaplan–Meier analysis was used for univariate survival analysis and compared using the log-rank test. Cox risk regression model was used for multivariate analysis affecting prognosis. Significant predictors of univariate analysis (P < 0.05) supported by clinical evidence were included in the Cox’s multivariate analysis. Backward stepwise (Likelihood Ratio) was used to estimate the association between the predictors and outcomes, using hazard ratio (HR) and its 95% confidence interval (95% CI) as the indicators. P value < 0.05 (two sided) was considered statistically significant. Statistical analyses were performed using SPSS software (version 23.0; IBM-SPSS, Inc., Chicago, IL, USA).
Results
Patient characteristics
A total of 31 patients diagnosed with c-SCLC were included in the present study. Patient clinical characteristics are listed in Table 2. The median age was 62 (range: 35 − 79) years. Most patients had a history of smoking (n = 22, 71.0%) and patients were overwhelmingly male (5.2:1). C-SCLC developed predominantly in peripheral sites (n = 23, 74.2%). Of these patients, a lobectomy was performed in 21 patients, while bilobectomy was performed in 3, pneumonectomy in 3, and 4 patients underwent a wedge resection. Radical mediastinal lymph node dissection was performed in 29 patients. Two IA patients with wedge resection did not receive lymph node dissection. In our study, squamous cell carcinoma (SCC, n = 12) was the most common mixed component (Men et al. 2016), followed by large-cell carcinoma (LCC, n = 7), adenocarcinoma (AC, n = 6), spindle cell carcinoma (n = 4), adenosquamous carcinoma (n = 1) and atypical carcinoid (n = 1). The final pathologic lung cancer stages in the patients were as follows: stage IA in 8 patients, IB in 5, IIA in 8, IIIA in 9 and IIIB in 1.
The relationship between tumor metabolic status and clinicopathological characteristics
Pearson or Spearman rank correlation analysis was used to analyze the relationship between primary tumor metabolic parameters (SUVmax, MTV, TLG) and clinicopathological features, including gender, age, smoking history, tumor location, tumor size, Lymph node metastasis, mixed NSCLC components, TNM stage, SCC, NSE, CEA, white blood cell (WBC) count, neutrophil, lymphocyte, neutrophil–lymphocyte ratio (NLR), platelet–lymphocyte ratio (PLR) and hemoglobin (HGB). Primary tumor SUVmax measured on 18F-FDG PET/CT has no significant correlation with clinicopathological factors. Both MTV and TLG were significant correlated with tumor size, WBC and lymphocyte count (MTV: P < 0.001, P = 0.023, P < 0.001; TLG: P < 0.001, P = 0.009, P < 0.001, Table 1).
Survival analysis
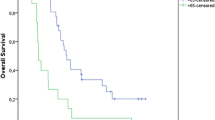
There was no treatment-related death in this cohort and all deaths were due to the primary disease. The median follow-up period was 53.0 (11.0–142.0) months. At the end of follow-up, nine patients had local recurrence and seven developed distant metastasis to bone, brain and liver. The 3-year and 5-year overall survival(OS) rate was 67.7% and 48.4%, corresponding progression-free survival(PFS) rate were 51.6% and 35.5%, respectively. Enrolled patients had the minimum SUVmax value of 3.3 and the maximum SUVmax value of 20.1. The minimum value of MTV and TLG was 1.65 cm3 and 6.17 g/ml × cm3, maximum value was 2179.0 cm3 and 3919.3 g/ml × cm3, respectively. Tumor metabolic parameters distribution and inter-group comparison of common mixed NSCLC components are shown in Fig. 1 (One-way ANOVA, P > 0.05). There was no significant difference between the common mixed NSCLC component groups. We used RStudio (R version 3.6.1) to draw the time-dependence ROC curve and get the optimal cutoff value (SUVmax = 9.0, MTV = 10.35 cm3, TLG = 128.23 g/ml × cm3). Univariate survival analysis showed that gender, smoking history, tumor location were prognostic factors of PFS (P = 0.036, P = 0.043, P = 0.048, Table 2). Male, non-smoking and peripheral c-SCLCs had a relatively longer PFS. SUVmax, mixed NSCLC component, tumor size, TNM stage and chemotherapy were associated with PFS, but not statistically significant (0.05 < P < 0.1, Table 2). We further analyzed the predictive value of these factors in the mixed SCC and non-SCC component groups separately and found that SUVmax and TNM stage were closely related to disease progression in both SCC and non-SCC component groups (P = 0.007, P = 0.048, Fig. 2). Kaplan–Meier survival analysis showed that SUVmax, smoking history, tumor size and mixed SCC component were influencing factors of OS in patients (P = 0.040, P = 0.041, P = 0.046, P = 0.029, Table 2). Moreover, stratified analysis showed that the SUVmax of mixed SCC group and non-SCC group were significantly correlated with OS (P = 0.004, Fig. 3).
In Cox’s multivariate analysis, TNM stage (HR = 2.885, 95%CI: 1.323–6.289, P = 0.008) was the most significantly influential factor for PFS. High SUVmax value (HR = 9.338, 95%CI: 2.426–35.938, P = 0.001) and mixed SCC component (HR = 0.155, 95%CI: 0.045–0.530, P = 0.003) were poor prognostic factors for OS. The final analysis showed that besides TNM stage, SUVmax and mixed NSCLC components were important predictors of c-SCLC patients.
Discussion
C-SCLC is a rare tumor with independent biological characteristics (Babakoohi et al. 2013; Qin and Lu 2018). Previous reports showed that up to 28% of SCLC patients who underwent surgical resection were c-SCLC (Nicholson et al. 2002). Fushimi et al. (1996) also reported that the frequency of c-SCLC in the primary sites was statistically higher in autopsy specimens (14.3%) than in biopsy or cytology specimens (8.6%). A retrospective study conducted by zhao et al. showed that 5.9% of surgically excised SCLC patients were c-SCLCs (Zhao et al. 2019). For patients diagnosed based on limited biopsy material, such as bronchial biopsy or needle aspiration, the possibility of detecting a combined histology is lower due to the limited amount of biopsy specimens. Our research subjects were all the pathological diagnosis after surgical resection, which ensured the accuracy and reliability of the diagnosis.
Conventionally, the treatment of c-SCLC refers to the guidelines for SCLC, and multimodality therapy is often recommended. Surgery plays an increasing role in limited-stage SCLCs, especially in c-SCLCs. A retrospective study conducted by Zhao et al. (2019) showed that 5-year survival rates of surgical-resected SCLC were 63.8%, 65.5%, and 34.9% for pathologic stages I, II and III, respectively, and suggested that surgery may also have potential benefit for stage II and some stage IIIA SCLC patients. In our study cohort, c-SCLCs are mainly peripheral located (74.2%) and earlier stage. All patients undergo radical resection, the most common mixed component is SCC, which is consistent with previous studies (Fraire et al. 1992; Hage et al. 1998; Men et al. 2016). In our study cohort, the 5-year overall survival and progression-free survival rate were 48.4% and 35.5%, suggesting that surgery is critical for c-SCLC because it not only provides an accurate diagnosis but also improves treatment outcomes (Stinchcombe 2017; Veronesi et al. 2015).
All our patients had a SUVmax value of > 2.5, the optimum cutoff value of SUVmax, MTV and TLG was 9.0, 10.35 and 128.23, respectively. Pearson and spearman correlation analysis showed that MTV and TLG were significant correlated to tumor size, WBC and lymphocyte count. The WBC count before treatment was an indicator of systemic inflammation. We found that volumetric parameters MTV and TLG were closely related to hematological WBC count. A recent study conducted in 73 advanced HNSCC patients (Ohashi et al. 2020) proved that WBC count was significantly correlated with 18F-FDG PET/CT parameters, and speculated that tumor with upregulated aerobic glycolysis produce large amounts of lactic acid and cytokines and might mediate systemic inflammation via the lactic acid-induced IL-23/IL-17 pathway. Several studies also confirmed the relationship between PET-CT volumetric metabolic parameters and NLR/PLR in SCLC, NSCLC, cervical carcinoma and colorectal cancer (Du et al. 2019; McSorley et al. 2018; Mirili et al. 2019; Wang et al. 2020).
We included 31 surgical-resected c-SCLC patients with preoperative 18F-FDG PET/CT examination in our study and demonstrate that TNM stage was the most significantly influential factor for PFS, high SUVmax and mixed SCC component of the primary lesions were poor predictors of OS in c-SCLCs. A cohort study of 5002 patients (Nicholson et al. 2016) has confirmed the prognostic value of both clinical and pathologic TNM staging in SCLC patients with limited-stage disease. Several studies have reported that high SUVmax values in 18F-FDG PET/CT as a prognostic factor are associated with a poorer clinical outcome in patients with various malignancies, such as head and neck cancer, renal cell carcinoma, cervical cancer, gastric cancer and NSCLC (Bille et al. 2013; Brunette et al. 2018; Chon et al. 2019; Ha et al. 2017; Pankowska et al. 2019). Kwon et al. (2016) conducted a retrospective study and enrolled 59 limited-stage SCLC patients who underwent pretreatment 18F-FDG PET/CT and found that highest SUVmax is an independent prognostic factor for survival in limited-stage SCLC patients. Chang et al. (2019) analyzed the prognostic implication of 18F-FDG PET/CT in 30 LD-SCLC patients who underwent standard chemotherapy after radiotherapy and confirmed that SUVmax measured on pretreatment 18F-FDG PET/CT were independent and significant prognostic factors in LD-SCLC patients after chemoradiotherapy with curative intent. The percentile (%) change in SUVmax during and after treatment might be a better surrogate marker of clinical efficacy of chemotherapy compared to a single pre-treatment SUVmax value. A group of Korean investigators (Kim et al. 2018) compared 18F-FDG PET/CT parameters obtained from two consecutive PET/CT scans performed before and after treatment in 59 SCLC patients to predict prognosis. The results showed a significant reduction in SUVmax following treatment was an important independent prognostic factor for overall survival.
Mixed SCC component is another important prognostic indicator. Although most patients in the SCC component group were early-stage patients with lower SUVmax value, their prognosis was still poor, suggesting that mixed NSCLC components had independent and significant prognostic value for c-SCLC. Consistent with our study, Men et al. (2016) confirmed that the most common mixed component was SCC in 114 c-SCLCs, but survival analysis showed no significant difference between the SCC and non-SCC component group (P = 0.198), perhaps due to the different TNM stages of enrolled patients and only half of their patients had surgery. Small case series suggest that EGFR-TKI could also be used in c-SCLC with EGFR mutations (Okamoto et al. 2006; Tatematsu et al. 2008; Zakowski et al. 2006). EGFR mutations are more likely found in c-SCLC with adenocarcinoma component. In this study, there was one case of c-SCLC mixed with 60% adenocarcinoma accompanied by chest wall invasion and EGFR21 mutation. After surgery, gefitinib was given as adjuvant therapy, with a total survival of 18 months. Previous published studies were also scattered case reports, so it is difficult to accurately evaluate their efficacy because of data sparsity (Lu et al. 2012; Okamoto et al. 2006; Takagi et al. 2013; Zakowski et al. 2006).
There are some deficiencies in this study: first, it is a retrospective study; second, the sample size is small; third, the lack of uniform adjuvant treatment. In addition, because it was a single-center retrospective study, the results may be biased.
Conclusion
We conducted a retrospective study of surgical-resected c-SCLC patients with preoperative 18F-FDG PET/CT examination. Primary tumor SUVmax measured on 18F-FDG PET/CT has no significant correlation with clinicopathological factors. Volumetric parameters MTV and TLG are significantly correlated with tumor size, WBC and lymphocyte count. TNM stage is the most significant factor influencing disease progression in surgical-resected c-SCLCs. Both high SUVmax value and mixed SCC component are poor prognostic factors in patients with c-SCLC. Surgical-resected c-SCLCs have a relatively good prognosis; multidisciplinary combination therapy is the main treatment mode for c-SCLC, especially for limited-stage disease. Due to the limitations of our research, these observations should be confirmed by further large-scale studies.
References
Albano D, Bosio G, Treglia G, Giubbini R, Bertagna F (2018) 18F-FDG PET/CT in solitary plasmacytoma: metabolic behavior and progression to multiple myeloma. Eur J Nucl Med Mol Imaging 45:77–84
Babakoohi S, Fu P, Yang M, Linden PA, Dowlati A (2013) Combined SCLC clinical and pathologic characteristics. Clin Lung Cancer 14:113–119
Bai L et al (2017) SUVmax of 18F-FDG PET/CT correlates to expression of major chemotherapy-related tumor markers and serum tumor markers in gastric adenocarcinoma patients. Oncol Rep 37:3433–3440
Bille A et al (2013) The prognostic significance of maximum standardized uptake value of primary tumor in surgically treated non-small-cell lung cancer patients: analysis of 413 cases. Clin Lung Cancer 14:149–156
Brunette LL et al (2018) Predictive value of FDG PET/CT to detect lymph node metastases in cervical cancer. Clin Nucl Med 43:793–801
Burger IA et al (2016) 18F-FDG PET/CT of non-small cell lung carcinoma under neoadjuvant chemotherapy: background-based adaptive-volume metrics outperform TLG and MTV in predicting histopathologic response. J Nucl Med 57:849–854
Chang H, Lee SJ, Lim J, Lee JS, Kim YJ, Lee WW (2019) Prognostic significance of metabolic parameters measured by (18)F-FDG PET/CT in limited-stage small-cell lung carcinoma. J Cancer Res Clin Oncol 145:1361–1367
Chon HJ et al (2019) The clinical implications of FDG-PET/CT differ according to histology in advanced gastric cancer. Gastric Cancer 22:113–122
Du S, Sun H, Gao S, Xin J, Lu Z (2019) Metabolic parameters with different thresholds for evaluating tumor recurrence and their correlations with hematological parameters in locally advanced squamous cell cervical carcinoma: an observational (18)F-FDG PET/CT study. Quant Imaging Med Surg 9:440–452
Fraire AE, Johnson EH, Yesner R, Zhang XB, Spjut HJ, Greenberg SD (1992) Prognostic significance of histopathologic subtype and stage in small cell lung cancer. Hum Pathol 23:520–528
Fushimi H et al (1996) Histologic changes in small cell lung carcinoma after treatment. Cancer 77:278–283
Ha SC et al (2017) Pretreatment tumor SUVmax predicts disease-specific and overall survival in patients with head and neck soft tissue sarcoma. Eur J Nucl Med Mol Imaging 44:33–40
Hage R, Elbers JR, de Brutel RA, van den Bosch JM (1998) Surgery for combined type small cell lung carcinoma. Thorax 53:450–453
Hasbek Z, Ozer H, Erturk SA, Erdiş E, Yucel B, Çiftçi E, Çakmakcilar A (2019) Relationships between hypoxia induced factor-1α and (18)F-FDG PET/CT parameters in colorectal cancer. Rev Esp Med Nucl Imagen Mol 38:355–361
Hsieh CE et al (2018) Pretreatment primary tumor and nodal SUVmax values on 18F-FDG PET/CT images predict prognosis in patients with salivary gland carcinoma. Clin Nucl Med 43:869–879
Hsu HH et al (2016) SUVmax and tumor size predict surgical outcome of synchronous multiple primary lung cancers. Medicine 95:0000000000002351
Johnson BE (2001) NCCN: small cell lung cancer. Cancer Control 8:32–43
Kim H, Yoo IR, Boo SH, Park HL, Jh O, Kim SH (2018) Prognostic value of pre- and post-treatment FDG PET/CT parameters in small cell lung cancer patients. Nucl Med Mol Imaging 52:31–38
Kwon SH et al (2016) The Highest Metabolic Activity on FDG PET Is Associated With Overall Survival in Limited-Stage Small-Cell Lung Cancer. Medicine 95:0000000000002772
Lee HY et al (2015) Role of CT and PET imaging in predicting tumor recurrence and survival in patients with lung adenocarcinoma: a comparison with the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society Classification of Lung Adenocarcinoma. J Thorac Oncol 10:1785–1794
Lee SJ, Chung MS, Shin SJ, Choi YY (2018) Correlation of tumor uptake on breast-specific gamma imaging and fluorodeoxyglucose PET/CT with molecular subtypes of breast cancer. Medicine 97:0000000000012840
Lemarignier C, Martineau A, Teixeira L, Vercellino L, Espié M, Merlet P, Groheux D (2017) Correlation between tumour characteristics, SUV measurements, metabolic tumour volume, TLG and textural features assessed with (18)F-FDG PET in a large cohort of oestrogen receptor-positive breast cancer patients. Eur J Nucl Med Mol Imaging 44:1145–1154
Lu HY et al (2012) Mutation status of epidermal growth factor receptor and clinical features of patients with combined small cell lung cancer who received surgical treatment. Oncol Lett 3:1288–1292
McSorley ST, Khor BY, Tsang K, Colville D, Han S, Horgan PG, McMillan DC (2018) The relationship between (18) F-FDG-PETCT-derived markers of tumour metabolism and systemic inflammation in patients with recurrent disease following surgery for colorectal cancer. Colorectal Dis 20:407–415
Men Y et al (2016) Further understanding of an uncommon disease of combined small cell lung cancer: clinical features and prognostic factors of 114 cases. Chin J Cancer Res 28:486–494
Mirili C et al (2019) Prognostic significance of neutrophil/lymphocyte ratio (NLR) and correlation with PET-CT metabolic parameters in small cell lung cancer (SCLC). Int J Clin Oncol 24:168–178
Moon SW, Seo JH, Jeon HW, Moon MH (2019) Effect of histological subtype and treatment modalities on T1–2 N0–1 small cell lung cancer: a population-based study. Thorac Cancer 10:1229–1240
Nicholson SA et al (2002) Small cell lung carcinoma (SCLC): a clinicopathologic study of 100 cases with surgical specimens. Am J Surg Pathol 26:1184–1197
Nicholson AG et al (2016) The International Association for the Study of Lung cancer lung cancer staging project: proposals for the revision of the clinical and pathologic staging of small cell lung cancer in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol 11:300–311
Ohashi T et al (2020) The importance of FDG-PET/CT parameters for the assessment of the immune status in advanced HNSCC. Auris Nasus Larynx 20:30022–30025
Okamoto I, Araki J, Suto R, Shimada M, Nakagawa K, Fukuoka M (2006a) EGFR mutation in gefitinib-responsive small-cell lung cancer. Ann Oncol 17:1028–1029
Pankowska V, Malkowski B, Wedrowski M, Wedrowska E, Roszkowski K (2019) FDG PET/CT as a survival prognostic factor in patients with advanced renal cell carcinoma. Clin Exp Med 19:143–148
Park SB et al (2014) Prognostic value of volumetric metabolic parameters measured by [18F]fluorodeoxyglucose-positron emission tomography/computed tomography in patients with small cell lung cancer. Cancer Imaging 14:1470–7330
Park S et al (2016) Correlation between semi-quantitative (18)F-FDG PET/CT Parameters and Ki-67 expression in small cell lung cancer. Nucl Med Mol Imaging 50:24–30
Qin J, Lu H (2018) Combined small-cell lung carcinoma. Onco Targets Ther 11:3505–3511
Stinchcombe TE (2017) Current treatments for surgically resectable, limited-stage, and extensive-stage small cell lung cancer. Oncologist 22:1510–1517
Takagi Y, Nakahara Y, Hosomi Y, Hishima T (2013) Small-cell lung cancer with a rare epidermal growth factor receptor gene mutation showing "wax-and-wane" transformation. BMC Cancer 13:1471–2407
Tatematsu A et al (2008) Epidermal growth factor receptor mutations in small cell lung cancer. Clin Cancer Res 14:6092–6096
Travis WD (2014) The 2015 WHO classification of lung tumors. Pathologe 2:014–1974
Tsujikawa T et al (2017) (18)F-FDG PET radiomics approaches: comparing and clustering features in cervical cancer. Ann Nucl Med 31:678–685
Veronesi G, Bottoni E, Finocchiaro G, Alloisio M (2015) When is surgery indicated for small-cell lung cancer? Lung Cancer 90:582–589
Wang Y et al (2020) New insight on the correlation of metabolic status on (18)F-FDG PET/CT with immune marker expression in patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging 47:1127–1136
Yu X, Song X, Zhu L, Chen W, Dai D, Li X, Xu W (2017a) Role of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in the diagnosis of newly found suspected malignant solitary pulmonary lesions in patients who have received curative treatment for colorectal cancer. Gastroenterol Res Pract 3458739:12
Yu X et al (2017b) Pretreatment metabolic parameters measured by 18F-FDG-PET to predict the outcome of first-line chemotherapy in extensive-stage small-cell lung cancer. Nucl Med Commun 38:193–200
Zakowski MF, Ladanyi M, Kris MG (2006) EGFR mutations in small-cell lung cancers in patients who have never smoked. N Engl J Med 355:213–215
Zer A et al (2016) The Role of 18F-FDG PET/CT on staging and prognosis in patients with small cell lung. Cancer Eur Radiol 26:3155–3161
Zhang C, Yang H, Zhao H, Lang B, Yu X, Xiao P, Zhang X (2017) Clinical outcomes of surgically resected combined small cell lung cancer: a two-institutional experience. J Thorac Dis 9:151–158
Zhao X et al (2019) Surgical resection of SCLC: prognostic factors and the tumor microenvironment. J Thorac Oncol 14:914–923
Zhu D, Wang Y, Wang L, Chen J, Byanju S, Zhang H, Liao M (2018) Prognostic value of the maximum standardized uptake value of pre-treatment primary lesions in small-cell lung cancer on 18F-FDG PET/CT: a meta-analysis. Acta Radiol 59:1082–1090
Acknowledgements
This work was supported by supported by grants from the National Natural Science Foundation of China (2018ZX09201015), the National Key Technology R&D Program (Grants No. 2018YFC1313400), and National Natural Science Funds of China (Grants No. 81972772).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hui, Z., Wei, F., Ren, H. et al. Primary tumor standardized uptake value (SUVmax) measured on 18F-FDG PET/CT and mixed NSCLC components predict survival in surgical-resected combined small-cell lung cancer. J Cancer Res Clin Oncol 146, 2595–2605 (2020). https://doi.org/10.1007/s00432-020-03240-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-020-03240-8