Abstract
Purpose
The aim of this study was to examine whether decitabine priming prior to low-dose chemotherapeutic regimens could improve outcomes in patients with myelodysplastic syndromes—refractory anemia with excess of blasts (MDS-RAEB).
Methods
The current retrospective analysis included all MDS-RAEB patients receiving idarubicin/cytarabine (IA) or aclacinomycin/cytarabine (AA), with or without decitabine priming during a period from February 2010 to May 2015. Treatment response and toxicity were compared between patients receiving decitabine priming and those who did not. A panel of 6 MDS-related genes was examined using bone marrow specimens.
Results
A total of 81 patients were included in the analysis: 40 received decitabine priming prior to chemotherapy (decitabine priming group). The median follow-up was 10.9 months (IQR: 6.2–21.9). The rate of overall response (OR) and complete remission (CR) was significantly higher in the decitabine priming group than in the chemotherapy group (OR: 75.0 vs. 51.2%, p = 0.027; CR: 55.0 vs. 29.3%, p = 0.019). Overall survival (OS) did not differ significantly between the two groups (19.5 vs. 14.7 months, p = 0.082). In a subgroup analysis that included only patients at < 60 years of age, the CR rate in the decitabine priming group was significantly higher than in the chemotherapy group (65.5 vs. 31.0%, p = 0.009). Survival benefit of decitabine priming was apparent in patients at < 60 years of age (22.4 months with 95% CI of 6.7–38.1 vs. 14.7 months with 95% CI of 11.4–18.0 months in the chemotherapy group, p = 0.028), patients with intermediate and unfavorable karyotypes (22.4 months with 95% CI of 15.1–29.7 vs. 11.9 months with 95% CI of 4.0–19.8 months in the chemotherapy group, p = 0.042), and patients with mutated splicing factor genes (35.3 months with 95% CI of 21.4–49.2 vs. 17.8 months with 95% CI of 13.8–21.8 months in the chemotherapy group, p = 0.039). Grade 3–4 hematological and non-hematological toxicities were not significantly different between the two groups.
Conclusions
Decitabine priming prior to low-dose chemotherapy could improve treatment responses in patients with MDS-RAEB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myelodysplastic syndromes (MDS) are a group of clonal hematopoietic cells disorders characterized by persistent cytopenias and propensity to progression to acute myeloid leukemia (AML) (Ades et al. 2014). According to the International Prognostic Scoring System (IPSS), MDS is classified into low, intermediate-1, intermediate-2 and high-risk groups. Hematopoietic stem cell transplantation (HSCT) is the preferred treatment in intermediate-2- and high-risk MDS patients (Greenberg et al. 2011; Malcovati et al. 2013). For patients not eligible for transplantation, chemotherapeutic regimens similar to that used for AML remains an important approach, with approximately 50% complete remission (CR) rate (Beran et al. 2001; Kantarjian et al. 2007b; Knipp et al. 2007). However, high-intensity chemotherapy is associated with high early stage mortality (around 5–20%) and short survival (6–12 months) in MDS patients (Beran et al. 2001; Kantarjian et al. 2007b; Knipp et al. 2007).
An important advance in the treatment of intermediate- and high-risk MDS is the use of DNA methyltransferase inhibitors. Decitabine (2′-deoxy-5-azacytidine) is a representative demethylating agent that reactivates tumor suppressor genes by demethylating these genes (Kantarjian et al. 2006). In patients receiving decitabine monotherapy, the rate of CR and overall response (OR) has been reported to be 13–39 and 32–70%, respectively (Iastrebner et al. 2010; Kantarjian et al. 2007a, c; Lee et al. 2011; Oki et al. 2012; Steensma et al. 2009). Decitabine in combination with a variety of agents, including histone deacetylase inhibitors, thalidomide, and conventional chemotherapeutics, has been developed to treat intermediate- and high-risk MDS and AML (Blum et al. 2007; Daver et al. 2016; Gao et al. 2015; Garcia-Manero et al. 2006; Geng et al. 2016; Jiang et al. 2015; Kirschbaum et al. 2014; Li et al. 2015; Song et al. 2012; Yang et al. 2005; Zhao et al. 2015). Several studies showed that decitabine in combination with chemotherapy improved the outcomes in patients with relapsed/refractory AML or AML transformed from MDS (MDS/AML) (Leonard et al. 2014; Li et al. 2015; Scandura et al. 2011; Song et al. 2012). Studies using MDS/AML and AML cell lines suggested synergistic effects when decitabine exposure was followed by chemotherapeutic drugs (e.g. idarubicin, daunomycin, clarubicin, homoharringtonine and thalidomide) (Li et al. 2014).
Based on these observations, we adopted a regimen of decitabine priming followed by low-dose idarubicin/cytarabine (IA). Though the preliminary trial suggested promising anti-leukemic effects (Ye et al. 2016), it had limitations with varying diseases (MDS, MDS/AML, and AML with no MDS background) and small sample size. In the current study, we examined whether decitabine priming prior to low-dose chemotherapy is superior to chemotherapy alone for MDS with refractory anemia with excess of blasts (MDS-RAEB). Subgroup analyses were carried out based on patient age, WHO classification, karyotypes and mutation status of six genes related to MDS (DNMT3A, IDH1, IDH2, SF3B1, SRSF2 and U2AF1).
Patients and methods
Patients
This study was approved by the Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University. The study included all patients with MDS-RAEB based on the 2008 WHO classification (Vardiman et al. 2009), receiving low-dose chemotherapy regimen, including IA and aclacinomycin/cytarabine (AA), with or without decitabine priming during a period from February 2010 to May 2015. Cases with one or more of the following conditions were excluded from data analysis: (1) secondary MDS; (2) having previously received chemotherapy or any demethylating agent; (3) severe comorbid cardiac, pulmonary, neurologic, or metabolic diseases; (4) malignant tumors; (5) impaired hepatic (serum total bilirubin level ≥ 2 × upper normal limit) or renal (serum creatinine ≥ 2 × upper normal limit) function prior to treatment.
Treatment regimens
The IA regimen consisted of intravenous infusion of idarubicin (6–8 mg/m2/day, d1-3) and cytarabine (100 mg/m2/day, d1-7). The AA regimen consisted of intravenous infusion of aclacinomycin (20 mg/day, d1-4) and cytarabine (10 mg/m2, q12h, d1-14). Decitabine was delivered at a dose of 20 mg/m2/day via intravenous infusion over 1 h for three consecutive days followed by IA or AA regimen. In the low-dose IA regimen, daily idarubicin dosage was reduced to 3 mg/m2/day, and lasted for 4–6 days; daily cytarabine dosage was reduced to 10 mg/m2, q12h, and lasted for 14 days. In the low-dose AA regimen, daily aclacinomycin dosage was reduced to 10 mg/day, and lasted for 6–8 days; cytarabine was given at a dose of 10 mg/m2, q12h, and lasted for 7–14 days. G-CSF was administered (150 µg twice a day) when neutrophil count was lower than 1 × 109/L, and discontinued when neutrophil count elevated to 2 × 109/L. Treatment cycle was repeated every 4 weeks unless upon myelosuppression. Supportive care, including standard antiemetic, blood transfusion and antimicrobial therapy, were given at the physician’s discretion.
Follow-up
The last follow-up was conducted on February 2016. The median follow-up was 10.9 months (IQR: 6.2–21.9). The overall survival (OS) was defined as the period from the day of diagnosis to the day of death regardless of the cause or the day of HSCT. Data were censored at the last follow-up.
Evaluation of treatment response and toxicity
Treatment response was assessed using modified International Working Group (IWG 2006) response criteria (Cheson et al. 2006), and categorized to CR, partial remission (PR), marrow CR (mCR), hematologic improvement (HI), stable disease (SD), and treatment failure. OR included CR, PR, mCR and HI. The extent and duration of severe bone marrow suppression was evaluated using the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Event version 3.0 (CTCAE v3.0) (Trotti et al. 2003). Given the fact that the majority of the patients had pre-treatment neutropenia or thrombocytopenia, we documented duration of grade 3–4 hematologic toxicity in the CR patients during treatment. Grade 3–4 non-hematological toxicities were also evaluated.
DNA sequencing
Bone marrow mononuclear cells were used to sequence six MDS-related genes, including three epigenetic regulatory genes (DNMT3A, IDH1, IDH2) and three splicing factor genes (SF3B1, SRSF2, and U2AF1). DNA segments that were sequenced were: exon 17/18 of DNMT3A (NM_175629.2) (Ahmad et al. 2014), exon 4 of IDH1 (NM_001282387.1) (Yan et al. 2009), exon 11 of IDH2 (NM_002168.3) (Ahmad et al. 2014), exon 13–16 of SF3B1 (NG_032903.2) (Brecqueville et al. 2012; Rossi et al. 2011), exon 1 of SRSF2 (NM_003016.4) (Patnaik et al. 2013), and exon 2/6 of U2AF1 (NM_001025203.1) (Patnaik et al. 2013).
Statistical analysis
Statistical analysis was conducted using the SPSS 22.0 software (SPSS Inc.; Chicago, IL, USA). The baseline characteristics and toxicities were compared using the Mann–Whitney U test for two independent samples. Categorical variables were analyzed with the Chi-square test or the Fisher’s exact test. Survival curves were constructed by the Kaplan–Meier method and compared by the log-rank test. Statistical significance was set at p < 0.05 (2-sided). Factors associated with CR and OS were analyzed using a stepwise approach: first with univariate analysis, followed by multivariate COX or logistic regression if p was < 0.10 in the univariate analysis. The factors entered into the initial regression model as independent variables included: sex, age, blood cell count, WHO classification, cytogenetic risk, treatment allocation, and splicing factor and epigenetic regulatory gene mutations.
Results
Patient characteristics
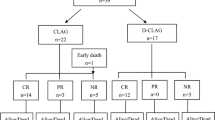
A total of 81 patients were included in data analyses. Among them 41 patients received low-dose chemotherapy (n = 17 for IA; n = 24 for AA), and 40 received decitabine priming prior to chemotherapy (n = 23 for IA; n = 17 for AA). Patient baseline characteristics, including age, sex, blood cell count, cytogenetic and IPSS risk classifications, were generally comparable between the two groups (Table 1). The percentage of RAEB-2 was not significantly different between the chemotherapy group (65.9%, 27/41) and the decitabine priming group (80%, 32/40) (p = 0.152). Mutation status of splicing factor or epigenetic regulatory genes was also comparable (Table 2).
Treatment response
In the overall analysis that included all 81 subjects, the rate of OR and CR was 64.2 and 42.0%, respectively. Patients treated with decitabine priming achieved higher OR (75 vs. 51.2% in the chemotherapy group, p = 0.027) and CR (55.0 vs. 29.3%, p = 0.019) (Table 3).
In the univariate analysis, CR was associated with age (p = 0.018), neutrophil count (p = 0.034) and treatment (decitabine priming or not, p = 0.019). After adjusting age and neutrophil count, decitabine priming remained to be associated with higher CR (OR: 3.214, 95%CI 1.125–9.183; p = 0.029).
Patient survival
The median follow-up was 10.9 months (IQR: 6.2–21.9). Of the 81 subjects, six were lost to follow-up (3 cases in each group). OS was not significantly different between the two groups (19.5 months with 95% CI of 9.4–29.6 months in the decitabine priming group vs. 14.7 months with 95% CI of 11.0–18.4 months in the chemotherapy group, p = 0.082) (Fig. 1). Patients who achieved CR had prolonged OS regardless of the treatment: 23.1 months (95% CI 9.9–36.3) vs. 10.2 months (95% CI 6.0–14.4) in those not achieving CR (p = 0.038) in patients receiving chemotherapy alone (Fig. 2a); 35.5 months (95% CI 12.3–58.3) vs. 12.2 months (95% CI 6.9–17.5) (p = 0.014) in patients receiving decitabine priming (Fig. 2b).
In the univariate analysis, OS was associated with sex (p = 0.028), cytogenetic risk (p = 0.013), treatment (p = 0.086) and splicing factor gene mutation status (p = 0.089). After adjustment for sex, cytogenetic risk and treatment, mutated splicing factor genes remained to be associated with shorter OS (HR 0.406, 95% CI 0.166–0.990; p = 0.048).
Subgroup analysis
A subgroup analysis based on age revealed an association of decitabine priming with higher CR rate (65.5% in the decitabine priming group vs. 31.0% in the chemotherapy group, p = 0.009) as well as longer OS (22.4 months with 95% CI of 6.7–38.1 vs. 14.7 months with 95% CI of 11.4–18.0 months, p = 0.028) in subjects at <60 years of age (Table 4; Fig. 3a). A Subgroup analysis based on karyotypes revealed an association of decitabine priming with prolonged OS (22.4 months with 95% CI of 15.1–29.7 vs. 9 months with 95% CI of 4.0–19.8 months, p = 0.042) (Fig. 3f), but not higher CR (Table 4) in patients with intermediate and unfavorable (non-favorable) karyotypes. A subgroup analysis based on splicing factor genes revealed an association of decitabine priming with prolonged survival (35.3 months with 95% CI of 21.4–49.2 months vs. 17.8 months with 95% CI of 13.8–21.8 months, p = 0.039) (Fig. 4a), but not higher CR in patients with mutated splicing factor genes.
Toxicities
The rate of grade 3/4 neutropenia (61% in the chemotherapy group vs. 52.5% in the decitabine priming group, p = 0.441) and thrombocytopenia (82.9 vs. 75%, p = 0.381) was comparable between the two groups. Also, the duration of grade 3/4 neutropenia and thrombocytopenia did not differ significantly between the two groups (Table 5). There was no significant difference in non-hematological toxicities between the two groups (Table 5). One patient in each group died within 4 weeks from the beginning of treatment. The cause of death was cerebral hemorrhage in the chemotherapy group and severe pulmonary infection in the decitabine priming group.
Discussion
Potential benefits and risks of decitabine in combination with conventional chemotherapy in patients with myeloid neoplasms have been extensively investigated. In a previous in vitro study with pediatric AML cells, combination of decitabine and cytarabine produced synergistic anti-leukemia effect (Leonard et al. 2014). In a previous study from our research group, decitabine followed by idarubicin produced synergistic anti-leukemia effects in both cultured cells and xenograft animal models (Li et al. 2014). Clinical studies that examined the combination of decitabine and chemotherapeutics, such as standard DA (daunomycin and cytarabine), low-dose AA, and CAG (G-CSF and low-dose AA) suggested CR rate at 50–60% and OR rate at 60–90% in AML and MDS/AML (Li et al. 2015; Scandura et al. 2011; Song et al. 2012). In a previous study (Ye et al. 2016), we reported a CR rate of 43% in MDS, 75% in MDS/AML and 29% in relapsed/refractory AML. The advances in mechanistic and clinical studies (Leonard et al. 2014; Li et al. 2014; Scandura et al. 2011; Song et al. 2012) have led to the use of epigenetic priming in high-risk myeloid neoplasms.
In the current study, we examined whether decitabine priming prior to low-dose chemotherapy (IA or AA) could improve outcomes in intermediate- and high-risk MDS patients. The results revealed increased response rate and prolonged survival in patients treated with decitabine priming prior to low-dose chemotherapy compared with those treated with chemotherapy alone. Consistent with the results of previous clinical trials (Lee et al. 2011; Li et al. 2015; Song et al. 2012), the median OS of patients achieving CR in the current study was significantly longer than that of patients with non-CR regardless of the treatment (decitabine priming or chemotherapy alone). A subgroup analysis in the current study showed a higher CR (65.5%) with a longer OS (22.4 months) in patients at <60 years of age in the decitabine priming group. This finding suggested that patients at <60 years of age could benefit more from decitabine priming treatment. Previous studies suggested that decitabine monotherapy is a better choice for MDS patients with poor karyotypes (Li et al. 2013; Lubbert et al. 2001; Wu et al. 2016). Several studies showed that decitabine in combination with CAG could achieve 50–70% CR in AML and MDS patients with complex karyotypes (Gao et al. 2015; Li et al. 2015). Gao et al. also noted an association of treatment response with the number of courses in AML and MDS patients with complex karyotypes (Gao et al. 2015). Patients with poor karyotypes who received decitabine in combination with CAG tended to have a longer OS (Li et al. 2015). The current study showed longer OS with decitabine priming (22.4 months) in MDS-RAEB patients with non-favorable karyotypes. The CR rate was 66.7% with decitabine priming vs. 21.4% in subjects receiving chemotherapy alone. We believe that such a difference is clinically meaningful despite the lack of statistical significance, presumably due to small sample size.
Mutations of about 40 genes have been identified in MDS. The most frequently mutated genes include SF3B1, U2AF1, SRSF2, ZRSR2, TET2, DNMT3A, EZH2, ASXL1, RUNX1, TP53, STAG2, CBL, and NRAS. Mutated SF3B1 gene is highly enriched in patients with refractory anemia with ringed sideroblasts, and rarely detected in MDS-RAEB patients (Malcovati et al. 2014, 2011; Papaemmanuil et al. 2011). Single SF3B1 mutation has been associated with more favorable prognosis, but may not represent an independent risk factor (Malcovati et al. 2014; Patnaik et al. 2012). Other mutated genes including U2AF1, SRSF2, DNMT3A, IDH1/2, SETBP1 and CBL have been associated with poor survival and progression to AML (Bejar et al. 2011; Graubert et al. 2011, 2012; Haferlach et al. 2014; Kosmider et al. 2010; Makishima et al. 2013; Pardanani et al. 2010; Thol et al. 2012; Walter et al. 2011). In the current study, we examined the mutational status of the three epigenetic regulatory genes (IDH1/2, DNMT3A) and the three splicing factor genes (SF3B1, SRSF2, and U2AF1) in 81 MDS-RAEB patients. The results suggested mutations of splicing factor genes correlated with decreased OS but did not affect the CR. Among patients harboring mutated splicing factor genes, OS was significantly prolonged in the decitabine priming group. These results suggested that patients with mutated splicing factor genes may be suitable for decitabine priming.
AML-type chemotherapy increases early phase mortality (5–20%) and decreases long-term survival (Beran et al. 2001; Kantarjian et al. 2007b). In addition, most of intermediate- and high-risk MDS patients are elderly with diminished function reserve. Based on the above facts, the chemotherapy regimens in the current study were modified (decreased dosage). The low-dose chemotherapy resulted in a lower 4-week mortality (2.5%). Also, grade 3/4 hematological and non-hematological toxicities were tolerated in the current study. These results suggested that decitabine priming did not increase the toxicities of chemotherapy in MDS-RAEB patients.
In summary, the current study suggested that decitabine priming prior to low-dose chemotherapy could improve treatment response and prolong survival in patients with MDS-RAEB. The benefits were most apparent in patients at <60 years of age, with non-favorable karyotypes, and with mutated splicing factor genes. The results of this retrospective study require verification with prospective clinical trials.
References
Ades L, Itzykson R, Fenaux P (2014) Myelodysplastic syndromes. Lancet 383:2239–2252. doi:10.1016/S0140-6736(13)61901-7
Ahmad F, Mohota R, Sanap S, Mandava S, Das BR (2014) Molecular evaluation of DNMT3A and IDH1/2 gene mutation: frequency, distribution pattern and associations with additional molecular markers in normal karyotype Indian acute myeloid leukemia patients. Asian Pac J Cancer Prev 15:1247–1253
Bejar R et al (2011) Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med 364:2496–2506. doi:10.1056/NEJMoa1013343
Beran M et al (2001) High-dose chemotherapy in high-risk myelodysplastic syndrome: covariate-adjusted comparison of five regimens. Cancer 92:1999–2015
Blum W et al (2007) Phase I study of decitabine alone or in combination with valproic acid in acute myeloid leukemia. J Clin Oncol 25:3884–3891. doi:10.1200/JCO.2006.09.4169
Brecqueville M et al (2012) Mutation analysis of ASXL1, CBL, DNMT3A, IDH1, IDH2, JAK2, MPL, NF1, SF3B1, SUZ12, and TET2 in myeloproliferative neoplasms. Genes Chromosomes Cancer 51:743–755. doi:10.1002/gcc.21960
Cheson BD et al (2006) Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 108:419–425. doi:10.1182/blood-2005-10-4149
Daver N et al (2016) A phase II study of decitabine and gemtuzumab ozogamicin in newly diagnosed and relapsed acute myeloid leukemia and high-risk myelodysplastic syndrome. Leukemia 30:268–273. doi:10.1038/leu.2015.244
Gao S et al (2015) Decitabine in the treatment of acute myeloid leukemia and myelodysplastic syndromes, which combined with complex Karyotype respectively. Asian Pac J Cancer Prev 16:6627–6632
Garcia-Manero G et al (2006) Phase 1/2 study of the combination of 5-aza-2′-deoxycytidine with valproic acid in patients with leukemia. Blood 108:3271–3279. doi:10.1182/blood-2006-03-009142
Geng S et al. (2016) Effects of the combination of decitabine and homoharringtonine in SKM-1 and Kg-1a cells. Leuk Res 44:17–24. doi:10.1016/j.leukres.2016.02.002
Graubert TA et al (2011) Recurrent mutations in the U2AF1 splicing factor in myelodysplastic syndromes. Nat Genet 44:53–57. doi:10.1038/ng.1031
Graubert TA et al (2012) Recurrent mutations in the U2AF1 splicing factor in myelodysplastic syndromes. Nat Genet 44:53–57. doi:10.1038/ng.1031
Greenberg PL et al. (2011) NCCN Clinical Practice Guidelines in Oncology: myelodysplastic syndromes J Natl Compr Cancer Netw JNCCN 9:30–56
Haferlach T et al (2014) Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia 28:241–247. doi:10.1038/leu.2013.336
Iastrebner M et al. (2010) Decitabine in myelodysplastic syndromes and chronic myelomonocytic leukemia: Argentinian/South Korean multi-institutional clinical experience. Leuk Lymphoma 51:2250–2257. doi:10.3109/10428194.2010.524324
Jiang X et al (2015) The hypomethylating agent decitabine prior to chemotherapy improves the therapy efficacy in refractory/relapsed acute myeloid leukemia patients. Oncotarget 6:33612–33622. doi:10.18632/oncotarget.5600
Kantarjian H et al (2006) Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer 106:1794–1803. doi:10.1002/cncr.21792
Kantarjian H et al (2007a) Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood 109:52–57. doi:10.1182/blood-2006-05-021162
Kantarjian HM et al (2007b) Survival advantage with decitabine versus intensive chemotherapy in patients with higher risk myelodysplastic syndrome: comparison with historical experience. Cancer 109:1133–1137. doi:10.1002/cncr.22508
Kantarjian HM et al (2007c) Update of the decitabine experience in higher risk myelodysplastic syndrome and analysis of prognostic factors associated with outcome. Cancer 109:265–273. doi:10.1002/cncr.22376
Kirschbaum M et al (2014) A phase 1 clinical trial of vorinostat in combination with decitabine in patients with acute myeloid leukaemia or myelodysplastic syndrome. Br J Haematol 167:185–193. doi:10.1111/bjh.13016
Knipp S et al (2007) Intensive chemotherapy is not recommended for patients aged >60 years who have myelodysplastic syndromes or acute myeloid leukemia with high-risk karyotypes. Cancer 110:345–352. doi:10.1002/cncr.22779
Kosmider O et al (2010) Mutations of IDH1 and IDH2 genes in early and accelerated phases of myelodysplastic syndromes and MDS/myeloproliferative neoplasms. Leukemia 24:1094–1096. doi:10.1038/leu.2010.52
Lee JH et al (2011) A prospective multicenter observational study of decitabine treatment in Korean patients with myelodysplastic syndrome. Haematologica 96:1441–1447. doi:10.3324/haematol.2011.046078
Leonard SM, Perry T, Woodman CB, Kearns P (2014) Sequential treatment with cytarabine and decitabine has an increased anti-leukemia effect compared to cytarabine alone in xenograft models of childhood acute myeloid leukemia. PloS One 9:e87475. doi:10.1371/journal.pone.0087475
Li X et al. (2013) Cytogenetic response based on revised IPSS cytogenetic risk stratification and minimal residual disease monitoring by FISH in MDS patients treated with low-dose decitabine. Leuk Res 37:1516–1521. doi:10.1016/j.leukres.2013.09.006
Li K et al. (2014) Sequential combination of decitabine and idarubicin synergistically enhances anti-leukemia effect followed by demethylating Wnt pathway inhibitor promoters and downregulating Wnt pathway nuclear target. J Transl Med 12:167. doi:10.1186/1479-5876-12-167
Li J et al (2015) Efficacy and safety of decitabine in combination with G-CSF, low-dose cytarabine and aclarubicin in newly diagnosed elderly patients with acute myeloid leukemia. Oncotarget 6:6448–6458
Lubbert M et al (2001) Cytogenetic responses in high-risk myelodysplastic syndrome following low-dose treatment with the DNA methylation inhibitor 5-aza-2′-deoxycytidine. Br J Haematol 114:349–357
Makishima H et al (2013) Somatic SETBP1 mutations in myeloid malignancies. Nat Genet 45:942–946. doi:10.1038/ng.2696
Malcovati L et al (2011) Clinical significance of SF3B1 mutations in myelodysplastic syndromes and myelodysplastic/myeloproliferative neoplasms. Blood 118:6239–6246. doi:10.1182/blood-2011-09-377275
Malcovati L et al (2013) Diagnosis and treatment of primary myelodysplastic syndromes in adults: recommendations from the European LeukemiaNet. Blood 122:2943–2964. doi:10.1182/blood-2013-03-492884
Malcovati L et al (2014) Driver somatic mutations identify distinct disease entities within myeloid neoplasms with myelodysplasia. Blood 124:1513–1521. doi:10.1182/blood-2014-03-560227
Oki Y et al (2012) Phase I/II study of decitabine in patients with myelodysplastic syndrome: a multi-center study in Japan. Cancer Sci 103:1839–1847. doi:10.1111/j.1349-7006.2012.02386.x
Papaemmanuil E et al (2011) Somatic SF3B1 mutation in myelodysplasia with ring sideroblasts. N Engl J Med 365:1384–1395. doi:10.1056/NEJMoa1103283
Pardanani A et al (2010) Recurrent IDH mutations in high-risk myelodysplastic syndrome or acute myeloid leukemia with isolated del(5q). Leukemia 24:1370–1372. doi:10.1038/leu.2010.98
Patnaik MM et al (2012) SF3B1 mutations are prevalent in myelodysplastic syndromes with ring sideroblasts but do not hold independent prognostic value. Blood 119:569–572. doi:10.1182/blood-2011-09-377994
Patnaik MM et al (2013) Spliceosome mutations involving SRSF2, SF3B1, and U2AF35 in chronic myelomonocytic leukemia: prevalence, clinical correlates, and prognostic relevance. Am J Hematol 88:201–206. doi:10.1002/ajh.23373
Rossi D et al (2011) Mutations of the SF3B1 splicing factor in chronic lymphocytic leukemia: association with progression and fludarabine-refractoriness. Blood 118:6904–6908. doi:10.1182/blood-2011-08-373159
Scandura JM et al (2011) Phase 1 study of epigenetic priming with decitabine prior to standard induction chemotherapy for patients with AML. Blood 118:1472–1480. doi:10.1182/blood-2010-11-320093
Song LX et al (2012) Clinical outcome of treatment with a combined regimen of decitabine and aclacinomycin/cytarabine for patients with refractory acute myeloid leukemia. Ann Hematol 91:1879–1886. doi:10.1007/s00277-012-1550-y
Steensma DP et al (2009) Multicenter study of decitabine administered daily for 5 days every 4 weeks to adults with myelodysplastic syndromes: the alternative dosing for outpatient treatment (ADOPT) trial. J Clin Oncol 27:3842–3848. doi:10.1200/JCO.2008.19.6550
Thol F et al (2012) Frequency and prognostic impact of mutations in SRSF2, U2AF1, and ZRSR2 in patients with myelodysplastic syndromes. Blood 119:3578–3584. doi:10.1182/blood-2011-12-399337
Trotti A et al (2003) CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 13:176–181. doi:10.1016/S1053-4296(03)00031-6
Vardiman JW et al (2009) The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 114:937–951. doi:10.1182/blood-2009-03-209262
Walter MJ et al (2011) Recurrent DNMT3A mutations in patients with myelodysplastic syndromes. Leukemia 25:1153–1158. doi:10.1038/leu.2011.44
Wu L et al. (2016) Efficacy and toxicity of decitabine versus CHG regimen (low-dose cytarabine, homoharringtonine and granulocyte colony-stimulating factor) in patients with higher risk myelodysplastic syndrome: a retrospective study. Leuk Lymphoma 57:1367–1374. doi:10.3109/10428194.2015.1096351
Yan H et al (2009) IDH1 and IDH2 mutations in gliomas. N Engl J Med 360:765–773. doi:10.1056/NEJMoa0808710
Yang H, Hoshino K, Sanchez-Gonzalez B, Kantarjian H, Garcia-Manero G (2005) Antileukemia activity of the combination of 5-aza-2′-deoxycytidine with valproic acid. Leuk Res 29:739–748. doi:10.1016/j.leukres.2004.11.022
Ye XN et al. (2016) Epigenetic priming with decitabine followed by low-dose idarubicin/cytarabine has an increased anti-leukemic effect compared to traditional chemotherapy in high-risk myeloid neoplasms. Leuk Lymphoma 57:1311–1318. doi:10.3109/10428194.2015.1091931
Zhao WH, Zeng QC, Huang BT, Li BS, Chen RL (2015) Decitabine plus thalidomide yields more sustained survival rates than decitabine monotherapy for risk-tailored elderly patients with myelodysplastic syndrome. Leuk Res 39:424–428. doi:10.1016/j.leukres.2015.01.014
Acknowledgements
We thank Dr. Kehong Zhang at the Ivy Medical Consulting for language help in drafting this manuscript. He had no role in study design, data collection and analysis, and decision to publish.
Funding
This work was supported by The National Key Technology R&D Program (2014BAI09B13), National Natural Science Foundation of China Grants (30870914, 81270582, 81470290), National Public Health Grand Research Foundation (201202017), Major Program Fund of the Science Technology Department of Zhejiang Province (2013c03043-2), the Foundation of Innovation Team for Basic and Clinical Research of Zhejiang Province (2011R50015) and Zhejiang Province Fund for Distinguished Young Scholars (LR12H08001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors claim no conflict of interest.
Ethical approval
All procedures in the current study were in accordance with the ethical standards of the Institutional Research Committee and with the 1964 Helsinki declaration and its later amendments. For this type of study (retrospective data analysis), formal consent is not required.
Additional information
L. Ye and Y. Ren contributed equally.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ye, L., Ren, Y., Zhou, X. et al. Decitabine priming prior to low-dose chemotherapy improves patient outcomes in myelodysplastic syndromes-RAEB: a retrospective analysis vs. chemotherapy alone. J Cancer Res Clin Oncol 143, 873–882 (2017). https://doi.org/10.1007/s00432-016-2331-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-016-2331-0