Abstract
Purpose
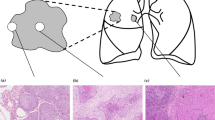
The expansion of micrometastatic tumors to macrometastatic ones is thought to be tightly regulated by several microenvironmental factors. The aim of this study was to elucidate the morphological and phenotypical differences between micrometastatic and macrometastatic tumors.
Method
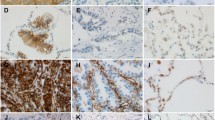
We first examined the morphological characteristics of 66 lymph node (LN) micrometastatic tumors (less than 2 mm in size) and 51 macrometastatic tumors (more than 10 mm in size) in 42 lung adenocarcinoma cases. Then, we evaluated the expression level of E-cadherin, S100A4, ALDH1, and Geminin in cancer cells and the number of smooth muscle actin (SMA), CD34, and CD204 (+) stromal cells in the primary tumors, matched micrometastatic tumors, and macrometastatic tumors (n = 34, each).
Results
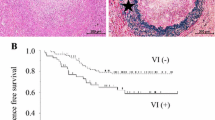
Tumor budding reflects the process of EMT, and stromal reactions were observed more frequently in macrometastatic tumors (P < 0.001). E-cadherin staining score for the micrometastatic tumors was significantly higher than that for the primary tumors (P < 0.001). In contrast, the E-cadherin staining score for the macrometastatic tumors was significantly lower than that for the micrometastatic tumors (P = 0.017). As for the stromal cells, the numbers of SMA (+) fibroblasts, CD34 (+) microvessels, and CD204 (+) macrophages were significantly higher for the macrometastatic tumors and primary tumors than for the micrometastatic tumors (P < 0.001, all).
Conclusion
The present study clearly showed that dynamic microenvironmental changes (e.g., EMT-related changes in cancer cells and structural changes in stromal cells) occur during the growth of micrometastases into macrometastases.





Similar content being viewed by others
Abbreviations
- LN:
-
Lymph node
- EMT:
-
Epithelial-mesenchymal transition
- MET:
-
Mesenchymal-epithelial transition
- PT:
-
Primary tumor
- Mic:
-
Micrometastatic tumor
- Mac:
-
Macrometastatic tumor
- CAF:
-
Cancer-associated fibroblast
- TAM:
-
Tumor-associated macrophage
- NSCLC:
-
Non-small cell lung cancer
- ALDH1:
-
Aldehyde dehydrogenase 1
- SMA:
-
Smooth muscle actin
References
Aokage K, Ishii G, Ohtaki Y, Yamaguchi Y, Hishida T et al (2011) Dynamic molecular changes associated with epithelial-mesenchymal transition and subsequent mesenchymal-epithelial transition in the early phase of metastatic tumor formation. Int J Cancer 128:1585–1595
Brabletz T, Jung A, Reu S, Porzner M, Hlubek F et al (2001) Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci U S A 98:10356–10361
Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW et al (2006) Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res 66:11271–11278
Chao YL, Shepard CR, Wells A (2010) Breast carcinoma cells re-express E-cadherin during mesenchymal to epithelial reverting transition. Mol Cancer 9:179
Condeelis J, Segall JE (2003) Intravital imaging of cell movement in tumours. Nat Rev Cancer 3:921–930
Dimou A, Neumeister V, Agarwal S, Anagnostou V, Syrigos K et al (2012) Measurement of aldehyde dehydrogenase 1 expression defines a group with better prognosis in patients with non-small cell lung cancer. Am J Pathol 181:1436–1442
Elzagheid A, Algars A, Bendardaf R, Lamlum H, Ristamaki R et al (2006) E-cadherin expression pattern in primary colorectal carcinomas and their metastases reflects disease outcome. World J Gastroenterol 12:4304–4309
Fidler IJ (2003) The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer 3:453–458
Gao D, Vahdat LT, Wong S, Chang JC, Mittal V (2012) Microenvironmental regulation of epithelial-mesenchymal transitions in cancer. Cancer Res 72:4883–4889
Goubran HA, Kotb RR, Stakiw J, Emara ME, Burnouf T (2014) Regulation of tumor growth and metastasis: the role of tumor microenvironment. Cancer Growth Metastasis 7:9–18
Hatanaka Y, Hashizume K, Nitta K, Kato T, Itoh I et al (2003) Cytometrical image analysis for immunohistochemical hormone receptor status in breast carcinomas. Pathol Int 53:693–699
Hudson LG, Zeineldin R, Stack MS (2008) Phenotypic plasticity of neoplastic ovarian epithelium: unique cadherin profiles in tumor progression. Clin Exp Metastasis 25:643–655
Kirita K, Ishii G, Matsuwaki R, Matsumura Y, Umemura S et al (2013) Identification of biological properties of intralymphatic tumor related to the development of lymph node metastasis in lung adenocarcinoma. PLoS ONE 8:e83537
Kojima M, Higuchi Y, Yokota M, Ishii G, Saito N et al (2014) Human subperitoneal fibroblast and cancer cell interaction creates microenvironment that enhances tumor progression and metastasis. PLoS ONE 9:e88018
Ksiazkiewicz M, Markiewicz A, Zaczek AJ (2012) Epithelial-mesenchymal transition: a hallmark in metastasis formation linking circulating tumor cells and cancer stem cells. Pathobiology 79:195–208
Luzzi KJ, MacDonald IC, Schmidt EE, Kerkvliet N, Morris VL et al (1998) Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol 153:865–873
MacDonald IC, Groom AC, Chambers AF (2002) Cancer spread and micrometastasis development: quantitative approaches for in vivo models. BioEssays 24:885–893
Markiewicz A, Ahrends T, Welnicka-Jaskiewicz M, Seroczynska B, Skokowski J et al (2012) Expression of epithelial to mesenchymal transition-related markers in lymph node metastases as a surrogate for primary tumor metastatic potential in breast cancer. J Transl Med 10:226
Mehlen P, Puisieux A (2006) Metastasis: a question of life or death. Nat Rev Cancer 6:449–458
Okudela K, Woo T, Mitsui H, Suzuki T, Tajiri M et al (2013) Downregulation of ALDH1A1 expression in non-small cell lung carcinomas–its clinicopathologic and biological significance. Int J Clin Exp Pathol 6:1–12
Otranto M, Sarrazy V, Bonte F, Hinz B, Gabbiani G et al (2012) The role of the myofibroblast in tumor stroma remodeling. Cell Adh Migr 6:203–219
Pollard JW (2004) Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 4:71–78
Ronnov-Jessen L, Petersen OW, Bissell MJ (1996) Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev 76:69–125
Samatov TR, Tonevitsky AG, Schumacher U (2013) Epithelial-mesenchymal transition: focus on metastatic cascade, alternative splicing, non-coding RNAs and modulating compounds. Mol Cancer 12:107
Shibue T, Weinberg RA (2009) Integrin beta1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc Natl Acad Sci U S A 106:10290–10295
Shibue T, Brooks MW, Inan MF, Reinhardt F, Weinberg RA (2012) The outgrowth of micrometastases is enabled by the formation of filopodium-like protrusions. Cancer Discov 2:706–721
Shibue T, Brooks MW, Weinberg RA (2013) An integrin-linked machinery of cytoskeletal regulation that enables experimental tumor initiation and metastatic colonization. Cancer Cell 24:481–498
Taddei ML, Giannoni E, Comito G, Chiarugi P (2013) Microenvironment and tumor cell plasticity: an easy way out. Cancer Lett 341:80–96
Thiery JP (2002) Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2:442–454
Troester MA, Lee MH, Carter M, Fan C, Cowan DW et al (2009) Activation of host wound responses in breast cancer microenvironment. Clin Cancer Res 15:7020–7028
Tsai JH, Yang J (2013) Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev 27:2192–2206
Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J (2012) Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell 22:725–736
Wan L, Pantel K, Kang Y (2013) Tumor metastasis: moving new biological insights into the clinic. Nat Med 19:1450–1464
Wong CW, Lee A, Shientag L, Yu J, Dong Y et al (2001) Apoptosis: an early event in metastatic inefficiency. Cancer Res 61:333–338
Yamaguchi Y, Ishii G, Kojima M, Yoh K, Otsuka H et al (2010) Histopathologic features of the tumor budding in adenocarcinoma of the lung: tumor budding as an index to predict the potential aggressiveness. J Thorac Oncol 5:1361–1368
Yao D, Dai C, Peng S (2011) Mechanism of the mesenchymal-epithelial transition and its relationship with metastatic tumor formation. Mol Cancer Res 9:1608–1620
Acknowledgments
All work reported herein was performed at the National Cancer Center Hospital East, Kashiwa, Chiba, Japan. The research was approved by the Internal Review Board of the institution. No patient consent was required as the research is a retrospective chart review, and no personally identifiable information was included in the manuscript.
Conflict of interest
The authors have no conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aramaki, N., Ishii, G., Yamada, E. et al. Drastic morphological and molecular differences between lymph node micrometastatic tumors and macrometastatic tumors of lung adenocarcinoma. J Cancer Res Clin Oncol 142, 37–46 (2016). https://doi.org/10.1007/s00432-015-1996-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-015-1996-0