Abstract
Purpose
Endostatin can normalize the tumor vasculature to some extent. However, exact length of its time window and corresponding markers for tumor vascular normalization are needed to be explored.
Methods
The A549 lung adenocarcinoma xenograft murine model was treated with recombinant human endostatin (rh-endostatin) for 14 days. Cisplatin was combined in different schedules. The effects of rh-endostatin on circulating endothelial cells (CECs) by flow cytometry, tumor vasculature and angiogenesis-related factors by confocal microscope and immunohistochemistry, and anti-tumor efficacy of cytotoxic drugs were observed.
Results
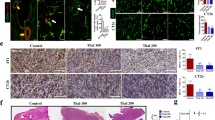
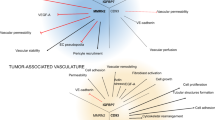
The activated CECs (aCECs) were increased on day 7 and decreased on day 10, and apoptotic CECs were increased on day 10. Tumor vasculature was transiently normalized with increased collagen coverage, decreased vessel permeability, intratumoral hypoxia, and microvascular density from day 7 to 10 after rh-endostatin administration. Extracellular matrix metalloproteinase inducer, vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 were transiently decreased by rh-endostatin from day 4 to 10, whereas the opposite effects were observed with tissue inhibitors of matrix metalloproteinase (TIMP)-1 and TIMP-2. The maximal anti-tumor effects of cisplatin were observed on administration from day 5 to 9 after rh-endostatin initial administration.
Conclusions
Rh-endostatin could transiently normalize tumor vasculature, probably via regulation of both pro- and anti-angiogenesis factors. The synergistic efficacy of anti-angiogenesis and chemotherapy was found during “the normalization window”. CEC could be a feasible blood biomarker for defining “vascular normalization window” and providing the evidence to make an optimizing combination therapeutic schedule in human tumor.






Similar content being viewed by others
Abbreviations
- Rh-endostatin:
-
Recombinant human endostatin
- NSCLC:
-
Non-small-cell lung cancer
- VEGF:
-
Vascular endothelial growth factor
- MMP:
-
Matrix metalloproteinase
- TIMP:
-
Tissue inhibitors of matrix metalloproteinase
- EMMPRIN:
-
Extracellular matrix metalloproteinase inducer
- PB:
-
Peripheral blood
- FC:
-
Flow cytometry
- CECs:
-
Circulating endothelial cells
- aCECs:
-
Activated circulating endothelial cells
- HIF-1α:
-
Hypoxia-inducible factor-1α
- MVD:
-
Microvascular density
- IFP:
-
Interstitial fluid pressure
- EPCs:
-
Endothelial precursor cells
- IOD:
-
Integrated optical density
- ECM:
-
Extracellular matrix
- TAF:
-
Tumor angiogenesis factor
References
Al-Dissi AN, Haines DM, Singh B et al (2010) Immunohistochemical expression of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 in canine simple mammary gland adenocarcinomas. Can Vet J 51(10):1109–1114
Asahara T, Masuda H, Takahashi T et al (1999a) Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 85:221–228
Asahara T, Takahashi T, Masuda H et al (1999b) VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J 18(14):3964–3972
Batchelor TT, Sorensen AG, di Tomaso E et al (2007) AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11(1):83–95
Beaudry P, Force J, Naumov GN et al (2005) Differential effects of vascular endothelial growth factor receptor-2 inhibitor ZD6474 on circulating endothelial progenitors and mature circulating endothelial cells: implications for use as a surrogate marker of antiangiogenic activity. Clin Cancer Res 11(9):3514–3522
Bertolini F (2008) Chemotherapy and the tumor microenvironment: the contribution of circulating endothelial cells. Cancer Metastasis Rev 27(1):95–101
Bertolini F, Paul S, Mancuso P et al (2003) Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res 63(15):4342–4346
Blann AD, Woywodt A, Bertolini F et al (2005) Circulating endothelial cells: Biomarker of vascular disease. Thromb Haemost 93(2):228–235
Carmeliet P, Jain RK (2000) Angiogenesis in cancer and other diseases. Nature 407(6801):249–257
Chang YS, di Tomaso E, McDonald DM et al (2000) Munn LL. Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci USA 97(26):14608–14613
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285(21):1182–1186
Furstenberger G, von Moos R, Lucas R et al (2006) Circulating endothelial cells and angiogenic serum factors during neoadjuvant chemotherapy of primary breast cancer. Br J Cancer 94(4):524–531
Goon PK, Lip GY, Boos CJ et al (2006) Circulating endothelial cells, endothelial progenitor cells, and endothelial microparticles in cancer. Neoplasia 8(2):79–88
Huang G, Chen L (2010) Recombinant human endostatin improves anti-tumor efficacy of paclitaxel by normalizing tumor vasculature in Lewis lung carcinoma. J Cancer Res Clin Oncol 136(8):1201–1211
Huang Y, Song N, Ding Y et al (2009) Pulmonary vascular destabilization in the premetastatic phase facilitates lung metastasis. Cancer Res 69(19):7529–7537
Jain RK (2005) Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307(5706):58–62
Khan SS, Solomon MA, McCoy JP Jr (2005) Detection of circulating endothelial cells and endothelial progenitor cells by flow cytometry. Cytometry B Clin Cytom 64(1):1–8
Li H, Raia V, Bertolini F et al (2008) Circulating endothelial cells as a therapeutic marker for thalidomide in combined therapy with chemotherapy drugs in a human prostate cancer model. BJU Int 101(7):884–888
Lyden D, Hattori K, Dias S et al (2001) Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med 7(11):1194–1201
Mancuso P, Burlini A, Pruneri G et al (2001) Resting and activated endothelial cells are increased in the peripheral blood of cancer patients. Blood 97(11):3658–3661
Mancuso P, Calleri A, Cassi C et al (2003) Circulating endothelial cells as a novel marker of angiogenesis. Adv Exp Med Biol 522:83–97
Ramalingam RR, Dahlberg SE, Langer CJ et al (2008) Outcomes for elderly, advanced-stage non small-cell lung cancer patients treated with bevacizumab in combination with carboplatin and paclitaxel: analysis of Eastern Cooperative Oncology Group Trial 4599. J Clin Oncol 26(1):60–65
Reimers N, Zafrakas K, Assmann V et al (2004) Expression of extracellular matrix metalloproteases inducer on micrometastatic and primary mammary carcinoma cells. Clin Cancer Res 10(10):3422–3428
Schuch G, Heymach JV, Nomi M et al (2003) Endostatin inhibits the vascular endothelial growth factor-induced mobilization of endothelial progenitor cells. Cancer Res 63(23):8345–8350
Shaked Y, Henke E, Roodhart JM et al (2008) Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell 14(3):263–273
Tong RT, Boucher Y, Kozin SV et al (2004) Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res 64(11):3731–3736
Wagner S, Fueller T, Hummel V et al (2003) Influence of VEGFR2 inhibition on MMP secretion and motility of microvascular human cerebral endothelial cells (HCEC). J Neurooncol 62(3):221–231
Wang J, Sun Y, Liu Y et al (2005) Results of randomized, multicenter, double-blind phase III trial of rh-endostatin (YH-16) in treatment of advanced non-small cell lung cancer patients. Zhongguo Fei Ai Za Zhi 8(4):283–290
Weichselbaum RR (2005) How does antiangiogenic therapy affect brain tumor response to radiation? Nat Clin Pract Oncol 2(5):232–233
Weidner N (1995) Intratumor microvessel density as a prognostic factor in cancer. Am J Pathol 147(1):9–19
Yuan J, Wu CW, Liu ZJ et al (2010) Observation of the antitumor effect of endostar combined with docetaxel under different administration sequences. Chin J Oncol 32(8):580–585
Zhen FS, Wang J, Liang J et al (2010) Low-dose metronomic chemotherapy with cisplatin: can it suppress angiogenesis in H22 hepatocarcinoma cells? Int J Exp Path 91:10–16
Zucker S, Vacirca J (2004) Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev 23(1–2):101–117
Acknowledgments
This work was supported by grants from Tianjin Science & Technology Project (No. 09ZCZDSF04400) and CSCO (Y-X2011-001).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, N., Zheng, D., Wei, X. et al. Effects of recombinant human endostatin and its synergy with cisplatin on circulating endothelial cells and tumor vascular normalization in A549 xenograft murine model. J Cancer Res Clin Oncol 138, 1131–1144 (2012). https://doi.org/10.1007/s00432-012-1189-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-012-1189-z