Abstract
Background
The optimal treatment sequence of epidermal growth factor receptor tyrosine kinase inhibitors (EGFR TKIs) and pemetrexed in previously treated advanced lung adenocarcinoma patients is currently unknown.
Methods
This retrospective study explored two sequential regimens incorporating EGFR TKIs and pemetrexed in advanced lung adenocarcinoma patients who had failed standard first-line chemotherapy. The medical records of 83 patients were carefully reviewed. 45 patients who received second-line EGFR TKIs followed by third-line pemetrexed were grouped as cohort A. 38 patients who received a strategy with the reverse sequence were grouped as cohort B. Progression-free survival, disease control duration and survival time were compared between the two cohorts.
Results
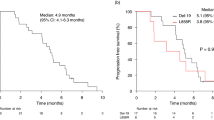
Median survival time is significantly longer in cohort A compared with cohort B (23.615 months vs. 16.269 months, HR: 0.549, 95% CI: 0.308–0.979, P = 0.042). Median disease control duration is 17.463 months in cohort A versus 11.587 months in cohort B (HR: 0.700, 95% CI: 0.409–1.196, P = 0.191). Median progression-free survival with second-line EGFR TKIs is significantly longer than second-line pemetrexed (8.056 months vs. 4.200 months, HR: 0.462, 95% CI: 0.281–0.758, P = 0.001). Median progression-free survival with third-line EGFR TKIs is 6.885 months versus 7.624 months with third-line pemetrexed (HR: 0.462, 95% CI: 0.605–1.767, P = 0.902). The rate of response to EGFR TKIs is higher in second-line setting than in third-line (44% vs. 34%).
Conclusions
We hypothesized that for EGFR-mutated patients, second-line EGFR TKIs followed by third-line pemetrexed is preferable to the reverse sequence.

Similar content being viewed by others
References
Ciuleanu T, Brodowicz T, Zielinski C et al (2009) Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 374:1432–1440
Gridelli C, Butts C, Ciardiello F et al (2008) An international, multicenter, randomized phase III study of first-line erlotinib followed by second-line cisplatin/gemcitabine versus first-line cisplatin/gemcitabine followed by second-line erlotinib in advanced non-small-cell lung cancer: treatment rationale and protocol dynamics of the TORCH trial. Clin Lung Cancer 9:235–238
Hanna N, Shepherd FA, Fossella FV et al (2004) Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 22:1589–1597
Huang SF, Liu HP, Li LH et al (2004) High frequency of epidermal growth factor receptor mutations with complex patterns in non-small cell lung cancers related to gefitinib responsiveness in Taiwan. Clin Cancer Res 10:8195–8203
Kim ES, Hirsh V, Mok T et al (2008) Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet 372:1809–1818
Maemondo M, Inoue A, Kobayashi K et al (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362:2380–2388
Miller VA, Kris MG, Shah N et al (2004) Bronchioloalveolar pathologic subtype and smoking history predict sensitivity to gefitinib in advanced non-small-cell lung cancer. J Clin Oncol 22:1103–1109
Miller KD, Chap LI, Holmes FA et al (2005) Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 23:792–799
Mok TS, Wu YL, Thongprasert S et al (2009) Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361:947–957
Pao W, Miller V, Zakowski M et al (2004) EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA 101:13306–13311
Scagliotti GV, Parikh P, von Pawel J et al (2008) Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 26:3543–3551
Schulz KF, Grimes DA (2005) Multiplicity in randomised trials II: subgroup and interim analyses. Lancet 365:1657–1661
Shepherd FA, Rodrigues Pereira J, Ciuleanu T et al (2005) Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353:123–132
Shigematsu H, Lin L, Takahashi T et al (2005) Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 97:339–346
Thatcher N, Chang A, Parikh P et al (2005) Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 366:1527–1537
Tiseo M, Gridelli C, Cascinu S et al (2009) An expanded access program of erlotinib (Tarceva) in patients with advanced non-small cell lung cancer (NSCLC): data report from Italy. Lung Cancer 64:199–206
Tsao MS, Sakurada A, Cutz JC et al (2005) Erlotinib in lung cancer—molecular and clinical predictors of outcome. N Engl J Med 353:133–144
Author information
Authors and Affiliations
Corresponding author
Additional information
Tingting Hong and Ruxia Zhang contributed equally to this manuscript and should be considered as co-first authors.
Rights and permissions
About this article
Cite this article
Hong, T., Zhang, R., Cai, D. et al. Second-line epidermal growth factor receptor inhibitors followed by third-line pemetrexed or the reverse sequence: a retrospective analysis of 83 Chinese patients with advanced lung adenocarcinoma. J Cancer Res Clin Oncol 138, 285–291 (2012). https://doi.org/10.1007/s00432-011-1084-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-011-1084-z