Abstract
Purpose
Gefitinib is safe for the treatment of non-small cell lung cancer (NSCLC), but some patients experience toxicities and require dose reduction. The purpose of this study was to evaluate the effect of gefitinib dose reduction on survival.
Methods
We retrospectively analyzed 263 patients with NSCLC harboring sensitive epidermal growth factor receptor (EGFR) mutation. All patients had recurred or metastatic disease and received gefitinib 250 mg daily as palliative chemotherapy.
Results
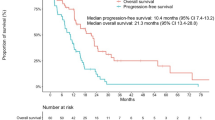
Of the 263 patients, 23 had gefitinib dose reduction due to toxicities (1 due to mucositis, 5 due to skin rash, 11 due to hepatotoxicity and 6 for both skin and hepatotoxicity). In the dose reduction group, the mean dose intensity was 0.84 (range 0.48–0.98). Patients with dose reduction showed significantly prolonged progression-free survival (PFS) and overall survival (OS) compared to those receiving the standard dose (median PFS: 14.0 vs. 10.6 months, P = 0.042, median OS: 54.5 vs. 29.6, P = 0.020). In multivariate analysis, the effect of dose reduction was not significantly associated with prolonged PFS [hazard ratio (HR) 0.619, 95 % confidence interval (CI) 0.357–1.073, P = 0.085], or OS (HR 0.625, 95 % CI 0.287–1.362, P = 0.237). However, patients receiving low-dose gefitinib tended to have superior survival outcomes compared to those receiving standard-dose gefitinib.
Conclusions
The patients experiencing gefitinib dose reduction or short-term treatment interruption due to toxicities did not show inferior survival, compared to those receiving full dose of gefitinib in NSCLC patients with EGFR mutation.

Similar content being viewed by others
References
Chen J, Gu R, Wang Q, Dassarath M, Yin Z, Yang K, Wu G (2012) Gefitinib-induced hepatotoxicity in patients treated for non-small cell lung cancer. Onkologie 35:509–513. doi:10.1159/000341828
Foo J, Michor F (2009) Evolution of resistance to targeted anti-cancer therapies during continuous and pulsed administration strategies. PLoS Comput Biol 5:e1000557. doi:10.1371/journal.pcbi.1000557
Giovannetti E et al (2010) Association of polymorphisms in AKT1 and EGFR with clinical outcome and toxicity in non-small cell lung cancer patients treated with gefitinib. Mol Cancer Ther 9:581–593. doi:10.1158/1535-7163.MCT-09-0665
Han JY et al (2012) First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung Journal of clinical oncology: official journal of the American Society of. Clin Oncol 30:1122–1128. doi:10.1200/JCO.2011.36.8456
Hayakawa H et al (2013) Lower gefitinib dose led to earlier resistance acquisition before emergence of T790M mutation in epidermal growth factor receptor-mutated lung cancer model. Cancer Sci. doi:10.1111/cas.12284
Keam B et al (2013a) How molecular understanding affects to prescribing patterns and clinical outcome of gefitinib in non-small cell lung cancer? 10 year experience of single institution cancer research and treatment: official. J Korean Cancer Assoc 45:178–185. doi:10.4143/crt.2013.45.3.178
Keam B et al (2013b) Rare and complex mutations of epidermal growth factor receptor, and efficacy of tyrosine kinase inhibitor in patients with non-small cell lung cancer. Int J Clin Oncol. doi:10.1007/s10147-013-0602-1
Kim YT et al (2008) Molecular changes of epidermal growth factor receptor (EGFR) and KRAS and their impact on the clinical outcomes in surgically resected adenocarcinoma of the lung. Lung Cancer 59:111–118. doi:10.1016/j.lungcan.2007.08.008
Liu HB, Wu Y, Lv TF, Yao YW, Xiao YY, Yuan DM, Song Y (2013) Skin rash could predict the response to EGFR tyrosine kinase inhibitor and the prognosis for patients with non-small cell lung cancer: a systematic review and meta-analysis. PloS One 8:e55128. doi:10.1371/journal.pone.0055128
Lyman GH (2009) Impact of chemotherapy dose intensity on cancer patient outcomes. J Natl Compr Canc Netw 7:99–108
Maemondo M et al (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362:2380–2388. doi:10.1056/NEJMoa0909530/362/25/2380
Mitsudomi T et al (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 11:121–128. doi:10.1016/S1470-2045(09)70364-XS1470-2045(09)70364-X
Mumenthaler SM et al (2011) Evolutionary modeling of combination treatment strategies to overcome resistance to tyrosine kinase inhibitors in non-small cell lung cancer. Mol Pharm 8:2069–2079. doi:10.1021/mp200270v
Park JH et al (2013) Tumor burden is predictive of survival in patients with non-small-cell lung cancer and with activating epidermal growth factor receptor mutations who receive gefitinib. Clin Lung Cancer 14:383–389. doi:10.1016/j.cllc.2012.10.007
Santos FP et al (2010) Clinical impact of dose reductions and interruptions of second-generation tyrosine kinase inhibitors in patients with chronic myeloid leukaemia. Br J Haematol 150:303–312. doi:10.1111/j.1365-2141.2010.08245.x
Satoh H et al (2011) Low-dose gefitinib treatment for patients with advanced non-small cell lung cancer harboring sensitive epidermal growth factor receptor mutations. J Thorac Oncol 6:1413–1417. doi:10.1097/JTO.0b013e31821d43a8
Shah NP et al (2010) Potent, transient inhibition of BCR-ABL with dasatinib 100 mg daily achieves rapid and durable cytogenetic responses and high transformation-free survival rates in chronic phase chronic myeloid leukemia patients with resistance, suboptimal response or intolerance to imatinib. Haematologica 95:232–240. doi:10.3324/haematol.2009.011452
Solit DB, She Y, Lobo J, Kris MG, Scher HI, Rosen N, Sirotnak FM (2005) Pulsatile administration of the epidermal growth factor receptor inhibitor gefitinib is significantly more effective than continuous dosing for sensitizing tumors to paclitaxel. Clin Cancer Res 11:1983–1989. doi:10.1158/1078-0432.CCR-04-1347
Suzumura T et al (2012) Reduced CYP2D6 function is associated with gefitinib-induced rash in patients with non-small cell lung cancer. BMC Cancer 12:568. doi:10.1186/1471-2407-12-568
Therasse P et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205-216
Wildiers H, Reiser M (2011) Relative dose intensity of chemotherapy and its impact on outcomes in patients with early breast cancer or aggressive lymphoma. Crit Rev Oncol Hematol 77:221–240. doi:10.1016/j.critrevonc.2010.02.002
Acknowledgments
This study was supported by grants from the Innovative Research Institute for Cell Therapy, Republic of Korea (A062260). This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korean Government (2010-0009563). We would especially like to thank our database manager, Ju Youn Kim, for data management. We also wish to thank the members of the Seoul National University Hospital Lung Study Group.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sim, S.H., Keam, B., Kim, DW. et al. The gefitinib dose reduction on survival outcomes in epidermal growth factor receptor mutant non-small cell lung cancer. J Cancer Res Clin Oncol 140, 2135–2142 (2014). https://doi.org/10.1007/s00432-014-1768-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-014-1768-2