Abstract
Purpose
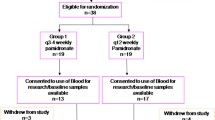
This trial is the first to compare directly the clinical response to and safety of oral and intravenous (IV) ibandronic acid for metastatic bone disease.
Methods
Patients ≥18 years with breast, prostate, lung, urogenital or colon cancer received IV ibandronic acid 6 mg infused over 15 min every 28 days or oral ibandronic acid 50 mg/day. Clinical response was determined using bone scintigraphy, radiography and serum C-terminal telopeptide of type I collagen (S-CTX) at months 3–6. Adverse events and biochemical safety measures were recorded.
Results
A total of 84.6 and 88.5% of patients had a complete/partial response to IV and oral ibandronic acid, respectively. Median percentage decreases in S-CTX were −39 and −35%, respectively. Bone pain scores decreased and analgesic use increased from month 0–3 and were stable from months 3–6. Both formulations improved physical and functioning scores.
Conclusion
Oral and IV ibandronic acid for bone metastases have similar efficacy and tolerability.




Similar content being viewed by others
References
Berenson JR, Rosen LS, Howell A et al (2001) Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer 91:1191–1200. doi:10.1002/1097-0142(20010401)91:7<1191::AID-CNCR1119>3.0.CO;2-0
Berruti A, Dogliotti L, Gorzegno G et al (1999) Differential patterns of bone turnover in relation to bone pain and disease extent in bone in cancer patients with skeletal metastases. Clin Chem 45:1240–1247
Body JJ, Diel IJ, Lichinitser MR, for the MF 4265 Study Group et al (2003) Intravenous ibandronate reduces the incidence of skeletal complications in patients with breast cancer and bone metastases. Ann Oncol 14:1399–1405. doi:10.1093/annonc/mdg367
Body JJ, Diel IJ, Lichinitzer M et al (2004) Oral ibandronate reduces the risk of skeletal complications in breast cancer patients with metastatic bone disease: results from two randomised, placebo-controlled phase III studies. Br J Cancer 90:1133–1137. doi:10.1038/sj.bjc.6601663
Body JJ, Lichinitser M, Tjulandin S, Garnero P, Bergstrom B (2007) Oral ibandronate is as active as intravenous zoledronic acid for reducing bone turnover markers in women with breast cancer and bone metastases. Ann Oncol 18:1165–1171. doi:10.1093/annonc/mdm119
Brown JE, Thomson CS, Ellis SP, Gutcher SA, Purohit OP, Coleman RE (2003) Bone resorption predicts for skeletal complications in metastatic bone disease. Br J Cancer 89:2031–2037. doi:10.1038/sj.bjc.6601437
Brown JE, Cook RJ, Major P et al (2005) Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumours. J Natl Cancer Inst 97:59–69
Chang JT, Green L, Beitz J (2003) Renal failure with the use of zoledronic acid. N Engl J Med 349:1676–1679. doi:10.1056/NEJM200310233491721
Coleman RE (2001) Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 27:165–176. doi:10.1053/ctrv.2000.0210
Coleman RE (2002) The clinical use of bone resorption markers in patients with malignant bone disease. Cancer 94:2521–2533. doi:10.1002/cncr.10522
Coleman RE (2006) Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 12(20 suppl):6243s–6249s. doi:10.1158/1078-0432.CCR-06-0931
Coleman RE, Major P, Lipton A et al (2005) Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol 23:4925–4935. doi:10.1200/JCO.2005.06.091
Diel IJ, Solomayer EF, Bastert G (2000) Treatment of metastatic bone disease in breast cancer: bisphosphonated. Clin Breast Cancer 1:43–51
Domchek SM, Younger J, Finkelstein DM, Seiden MV (2000) Predictors of skeletal complications in patients with metastatic breast carcinoma. Cancer 89:363–368. doi:10.1002/1097-0142(20000715)89:2<363::AID-CNCR22>3.0.CO;2-3
Garnero P (2001) Markers of bone turnover in prostate cancer. Cancer Treat Rev 27:187–192. doi:10.1053/ctrv.2000.0213
Heidenreich A, Ohlmann C, Olbert P, Hegele A (2003) High-dose ibandronate is effective and well tolerated in the treatment of pain and hypercalcaemia due to metastatic urologic cancer. Eur J Cancer 1(Suppl 5):S270
Jackson GH (2005) Renal safety of ibandronate. Oncologist 10(Suppl 1):14–18. doi:10.1634/theoncologist.10-90001-14
Leyland-Jones B (2004) Pharmacokinetic and clinical equivalence of oral and intravenous ibandronate for metastatic bone disease. Eur J Cancer suppl 2:9–12
Lipton A, Demers L, Curley E et al (1998) Markers of bone resorption in patients treated with pamidronate. Eur J Cancer 34:2021–2026. doi:10.1016/S0959-8049(98)00277-9
Lipton A, Hei Y, Coleman R, Major P, Cook R (2005) Suppression of bone turnover markers by zoledronic acid and correlation with clinical outcome. J Clin Oncol 23(Suppl 16 S):115 Abstract 532
Markowitz GS, Fine PL, D’agati VD (2002) Nephrotic syndrome after treatment with pamidronate. Am J Kidney Dis 39:1118–1122. doi:10.1053/ajkd.2002.32797
McPartland C, Grosjean L (2004) Treatment of painful bone metastases in Europe and Canada: the role of bisphosphonates. Ann Oncol 15(Suppl 3):iii50 abstract
Menssen HD, Sakalová A, Fontana A et al (2002) Effects of long-term intravenous ibandronate therapy on skeletal-related events, survival, and bone resorption markers in patients with advanced multiple myeloma. J Clin Oncol 20:2353–2359. doi:10.1200/JCO.2002.02.032
Mystakidou K, Katsouda E, Stathopoulou E, Vlahos L (2005) Approaches to managing bone metastases from breast cancer: the role of bisphosphonates. Cancer Treat Rev 31:303–311. doi:10.1016/j.ctrv.2005.03.005
Ohlmann C, Heidenreich A (2003) Efficacy of ibandronate in the management of painful osseous metastases due to hormone refractory prostate cancer. Support Care Cancer 11:396 Abstract A–38
Pecherstorfer M, Rivkin S, Body JJ, Diel I, Bergström B (2006) Long-term safety of intravenous ibandronic acid for up to 4 years in metastatic breast cancer: an open-label trial. Clin Drug Investig 26:315–322. doi:10.2165/00044011-200626060-00002
Roodman GD (2004) Mechanisms of bone metastasis. N Engl J Med 350:1655–1664. doi:10.1056/NEJMra030831
Vassiliou V, Kalogeropoulou C, Giannopoulou E, Leotsinidis M, Tsota I, Kardamakis D (2007) A novel study investigating the therapeutic outcome of patients with lytic, mixed and sclerotic bone metastases treated with combined radiotherapy and ibandronate. Clin Exp Metastasis 24:169–178. doi:10.1007/s10585-007-9066-x
Yahara J, Noguchi M, Noda S (2003) Quantitative evaluation of bone metastasis in patients with advanced prostate cancer during systemic treatment. BJU Int 92:379–384. doi:10.1046/j.1464-410X.2003.04362.x
Yau V, Chow E, Davis L, Holden L, Schueller T, Danjoux C (2004) Pain management in cancer patients with bone metastases remains a challenge. J Pain Symptom Manage 27:1–3. doi:D:10.1016/j.jpainsymman.2003.10.003
Acknowledgment
The authors received assistance from a medical communications agency in drafting the manuscript.
Conflict of interest
None of the authors has a conflict of interest.
Funding source
There is no sponsor or funding source to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mystakidou, K., Stathopoulou, E., Parpa, E. et al. Oral versus intravenous ibandronic acid: a comparison of treatment options for metastatic bone disease. J Cancer Res Clin Oncol 134, 1303–1310 (2008). https://doi.org/10.1007/s00432-008-0419-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-008-0419-x