Abstract
Thrombocytopenia is common in preterm neonates and can be associated with hemorrhage. Most platelet transfusions are prophylactic. Previously, higher platelet-count thresholds were recommended for neonates, but this recommendation has been questioned in recent studies. In the PlaNeT2 trial, mortality and serious bleeding were more frequent in neonates with the highest platelet-count threshold than in others. Following this trial, we changed our platelet transfusion practice by lowering the platelet-count threshold for prophylactic transfusion from 50,000 to 25,000/mm3. We conducted a before–after retrospective cohort study to quantify the frequency of platelet transfusions and assess the new protocol by analyzing death and serious hemorrhage events. This retrospective monocentric study included neonates born before 37 weeks of gestation with platelet count < 150,000/mm3 during the 2 years preceding the new platelet transfusion protocol (high prophylactic transfusion threshold, 50,000/mm3) and during the 2 years after the new platelet transfusion protocol (low prophylactic transfusion threshold, 25,000/mm3). The primary outcome was the proportion of neonates receiving at least one platelet transfusion in both groups. We also compared the proportion of deaths and severe hemorrhage events. A total of 707 neonates with thrombocytopenia were identified. In the high-threshold group, 99/360 (27.5%) received at least one platelet transfusion as compared with 56/347 (16.1%) in the low-threshold group (p < 0.001). The groups did not differ in proportion of deaths or severe hemorrhage events.
Conclusions: A reduced platelet-count threshold for transfusion allowed for a significant reduction in the number of platelet transfusions without increasing severe hemorrhage events.
What is Known: • A recent randomized trial suggested that restrictive platelet-count thresholds for platelet transfusion could be beneficial for preterm neonates. |
What is New: • On lowering the platelet-count threshold for transfusion from 50,000 to 25,000/mm3, the number of transfusions significantly decreased without increasing severe hemorrhage events in a neonatal intensive care unit. |
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The management of thrombocytopenia (platelet count < 150,000/mm3) in neonatal intensive care units (NICUs) involves platelet transfusion and aims at preventing hemorrhage, specifically in very preterm neonates who are at risk of high-grade intraventricular and intra-parenchymal hemorrhage [1,2,3]. Nearly 10% of the neonates admitted to NICUs receive a platelet transfusion [4, 5]. However, evidence guiding platelet transfusion practices in preterm neonates is sparse and is mainly based on expert opinion. Hence, the platelet-count threshold for transfusion varies considerably among countries, from 20,000 to 100,000/mm3 [6,7,8]. In France, recommendations were updated in 2015 and advocated a threshold of < 30,000/mm3 for neonates with non-immune thrombocytopenia and 50,000/mm3 for neonates with risk factors [9].
Overall, 90 to 98% of platelet transfusions in NICUs are prophylactic (i.e., prior to any hemorrhage) [10]. However, there is insufficient evidence to show a causal association between platelet count and risk of hemorrhage. Recent studies support lowering the platelet-count threshold for transfusion without increasing the risk of hemorrhage [10,11,12]. Among these studies, the PlaNeT2 randomized trial compared platelet transfusion thresholds of 25,000 and 50,000/mm3 in preterm neonates. Mortality, serious hemorrhage events, and significant neurodevelopmental impairment at 2 years of corrected age were more frequent in neonates with the highest threshold than others [13,14,15]. Potential complications of platelet transfusion should also be considered [6, 14, 15]. Multiple studies have shown an association between platelet transfusions and neonatal mortality [16,17,18,19,20].
The platelet-count threshold for transfusion was modified in our NICU after the publication of the PlaNeT2 results [13], from a prophylactic platelet-count threshold of 50,000 to one of 25,000/mm3 in most cases. We conducted a before–after retrospective cohort study to quantify the frequency of platelet transfusions and assess the new protocol’s tolerance by analyzing death and serious hemorrhage events.
Methods
Setting
Our department is a NICU within a type 3 maternity hospital with 5500 births per year. About 1250 neonates per year are admitted to the NICU. All neonates delivered before 34 weeks of gestation (WG) undergo neurosensory investigations, in particular cerebral ultrasonography twice in the first 10 days and at 3 weeks of life. Ultrasonography is performed more often in cases of clinical instability.
Platelet-count thresholds for transfusion and protocols
The results of PlaNeT2 were presented in a weekly literature review. After that, we updated the platelet transfusion protocol after a discussion with the medical team. Until December 2020, the prophylactic platelet-count threshold for transfusion was 50,000/mm3. In cases of surgery or active hemorrhage, the threshold was set at 80,000 to 100,000/mm3.
Since December 2020, our local protocol has recommended a platelet-count threshold for transfusion of 25,000/mm3. In cases of active hemorrhage or serious hemorrhage in the previous 72 h, lumbar puncture, hemodynamic instability, or gestational age < 26 WG in the first 72 h of life, the threshold is 50,000/mm3. Serious hemorrhage was defined as high-grade intraventricular hemorrhage or pulmonary or digestive hemorrhage.
Transfusion modalities are in line with the 2015 French recommendations, that is, apheresis platelet concentrates from a single donor with pediatric processing, deplasmatized in case of alloimmune thrombocytopenia, and treated with amotosalen, respecting the ABO system whenever possible. The volume transfused is 15 to 20 ml/kg or 0.1 to 0.2 × 1011 platelets/kg over 60 min. The transfusion is administered by a peripheral venous catheter or the second line of an umbilical venous catheter.
Study design and population
In this retrospective monocentric study, we included all neonates born at < 37 WG and admitted to the NICU from 1 January 2018 to 31 December 2019 (representing the high-threshold (HT) group) and from 1 January 2021 to 31 December 2022 (representing the low-threshold (LT) group). Neonates were eligible for the study if they presented at least one episode of thrombocytopenia (platelet count < 150,000/mm3) during their neonatal stay. Exclusion criteria were false severe thrombocytopenia defined as platelet count < 50,000/mm3 and controlled at > 150,000/mm3 in the following 12 h without any transfusion.
Outcomes
The primary outcome was the number of neonates receiving at least one platelet transfusion. Secondary outcomes were the number of transfusions per neonate who received a platelet transfusion and rates of death and severe hemorrhage events within 28 days after a diagnosis of severe thrombocytopenia < 50,000/mm3. Severe hemorrhage was defined as rectal hemorrhage, grade 3 or 4 intraventricular hemorrhage according to Papile’s classification (23), pulmonary hemorrhage (fresh bleeding through an endotracheal tube with increased ventilatory requirements), or hemorrhage associated with shock.
Data collection
The study was declared to the institution’s registry (no. 20220701152308). Parents were informed that their newborn’s data could be used to evaluate medical practices and that they had the right to object. Data were obtained retrospectively from health records and included data on the antenatal period and thrombocytopenia and platelet transfusion. The main neonatal characteristics and outcomes were also recorded. For neonates with severe thrombocytopenia (< 50,000/mm3), additional data collected were red blood cell transfusion, complications occurring in the 28 days after the diagnosis of severe thrombocytopenia (digestive hemorrhage, high-grade intraventricular hemorrhage, death) and occurrence of bronchopulmonary dysplasia.
Statistical analysis
Descriptive statistics were used to summarize the data. Categorical variables are described with number (percentage) and were compared by chi-squared test. Quantitative data are described with mean (SD) and were compared by the Student t-test. We compared the perinatal characteristics of infants and biological results concerning platelet counts in both periods, the 2 years preceding the new platelet transfusion protocol (HT threshold) and the 2 years after the new platelet transfusion protocol (LT threshold). We also compared characteristics of neonates receiving at least one platelet transfusion in both periods. We describe the outcomes of infants with severe thrombocytopenia. We compared the characteristics of infants with severe (< 50,000/mm3) and moderate (50–150,000/mm3) thrombocytopenia. All analyses involved using SAS 9.4 (SAS Institute Inc., Cary, NC, USA) with significance set at p < 0.05.
Results
We identified 707 patients with thrombocytopenia: 360 in the HT group and 347 in the LT group. Baseline characteristics are presented in Table 1. The groups were comparable in characteristics, except for fewer cesarean deliveries and fewer neonates with intra-uterine growth restriction in the HT than in the LT group.
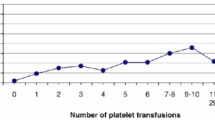
The LT and HT groups had similar mean platelet counts at birth and evolution during the first postnatal days (Table 2). In the HT group, 99/360 (27.5%) patients received at least one platelet transfusion as compared with 56/347 (16.1%) in the LT group (p < 0.001), for a relative risk reduction of 41%. The mean number of platelet transfusions per patient was 1.8 (SD 1.6) in the LT group and 2.4 (SD 2.4) in the HT group (p < 0.01). This reduction was accompanied by a reduction in volume transfused: mean 27.5 (SD 24.7) ml/kg in the LT group and 35.0 (SD 35.3) ml/kg in the HT group. Additional investigations were conducted for about 40% of the neonates in cases of persistent thrombocytopenia that was not explained by growth retardation or sepsis. The most frequently investigated condition was cytomegalovirus infection, investigated in nearly 40% of all neonates included but positive for only 3.1%.
Overall, 98 neonates in the HT group and 90 in the LT group presented severe thrombocytopenia (platelet count < 50,000/mm3); for these infants, the mean gestational age was 28.1 (SD 3.3) WG and mean birthweight 957 (SD 489) g. Their characteristics and outcomes, in particular death or digestive or intraventricular hemorrhage, are in Table 3. Fewer infants in the LT than in the HT group had complications in the 28 days after the diagnosis of severe thrombocytopenia (28% vs 42%, p = 0.04). Mortality rates were high in both groups: 33.7% in the HT group and 25.6% in the LT group (p = 0.22) (i.e., 29.8% of neonates with severe thrombocytopenia). In comparison, the mortality rate was 9.3% for neonates with moderate thrombocytopenia (platelet count 50 to 150,000/mm3). No pulmonary hemorrhage event was recorded, and only one neonate from the HT group had a retinal hemorrhage. The occurrence of bronchopulmonary dysplasia, defined as the need for ventilatory support (oxygen or invasive and non-invasive ventilation at 36 WG), was quite high, at 63% among neonates with thrombocytopenia < 50 G/l and comparable in the two groups. However, Table 3 presents data on neonates who had severe thrombocytopenia < 50,000 platelets/mm3, which is a marker of overall severity. Among thrombocytopenic children with > 50,000 platelets/mm3, the occurrence of bronchopulmonary dysplasia is lower (161/351 or 45.9%).
We also compared the baseline characteristics of neonates with severe versus moderate thrombocytopenia (platelet count 50,000 to 150,000/mm3); the results are in Table S1, supplementary data. Neonates with severe thrombocytopenia (platelet count < 50,000/mm3) were more premature and had a lower birthweight than those with moderate thrombocytopenia.
In most cases, the indication for transfusion was asymptomatic thrombocytopenia under the threshold (Table 4). The two groups were similar in clinical condition at first transfusion, with many neonates having sepsis and requiring invasive ventilation (Table 4). Active hemorrhage occurred in 20.2% of the neonates in the HT group and 35.7% in the LT group. Prior hemodynamic instability requiring inotropic support was more frequent but not significantly in the HT than in the LT group.
Some neonates (14.4% in the HT group and 20.6% in the LT group) did not meet the platelet-count threshold for transfusion. The mean minimum platelet count was 58,000 (SD 10,000) per cubic millimeter in the HT group and 33,000 (SD 8000) per cubic millimeter in the LT group. Moreover, 12 (3%) neonates in the HT group did not receive a transfusion even though their minimum platelet count was < 50,000/mm3. Seven (60%) had a platelet count very close to the threshold, and the platelet count was above the threshold when it was checked a second time. Two neonates had severe neurological damage, and withholding or withdrawing life-sustaining therapies had been decided. Medical records were incomplete for three neonates of the HT group, and we could not record any information about transfusion. In the LT group, one neonate had a minimum platelet count < 25,000/mm3 and did not receive transfusion. The minimum platelet count occurred in a sepsis context and was close to the threshold (24,000/mm3), with a count of 60,000/mm3 12 h later.
Discussion
In this monocentric retrospective cohort study, a lower platelet-count threshold for transfusion in preterm neonates allowed for a significant reduction in the number of transfusions without increasing severe hemorrhage events. Rates of platelet transfusions in preterm neonates with thrombocytopenia < 150,000/mm3 decreased from 27% in the HT group to 16% in the LT group (i.e., a relative risk reduction of 41%). In both periods, the 2 years preceding the new platelet transfusion protocol (HT threshold) and the 2 years after the new platelet transfusion protocol (LT threshold), severe thrombocytopenia < 50,000/mm3 was associated with high mortality.
However, the change in platelet-count threshold for transfusion was not associated with increased rates of death or severe hemorrhage. This finding in our real-world study agrees with the PlaNeT2 trial results. In this trial, which included infants with platelet count < 100,000/mm3, 90% of the neonates in the HT group and 53% in the LT group received at least one platelet transfusion (i.e., a relative risk reduction of 41% as well). Moreover, morbi-mortality was less frequent in the LT than in the HT group [13]. A recent study, also inspired by the PlaNeT2 trial, did not find a reduction in the number of platelet transfusions after a change in platelet transfusion guidelines [22]. However, the study maintained a threshold of 50,000/mm3 for neonates at risk of intra-ventricular hemorrhage, which concerns many neonates in a NICU. In contrast, in another study, restrictive local transfusion thresholds in a NICU decreased the proportion of platelet-transfused neonates by 46% [23].
Although the protocol with a lower threshold was generally well applied, 36% of the transfusions were performed out of protocol, more in the LT than in the HT group. The new protocol was implemented in December 2020, and our study began in January 2021 so that medical staff could get used to it. To raise awareness among the medical team about the change in the platelet transfusion protocol, we requested that each indication for platelet transfusion be documented in the patient’s record. This gave us the opportunity to reiterate the importance of restrictive transfusion practices. However, staff may have been concerned that the platelet count would quickly fall below the threshold, or they may have wished to avoid repeated blood samples, therefore performing more transfusions. This observation is consistent with the previous study of transfusions [23], finding an adherence to the new protocol of about 70%. To reduce non-indicated transfusions, the Boston Children’s Hospital NICU set up a key driver diagram to improve the staff’s educational effort and encourage staff buy-in [24]. This program included strengthening the education of providers and nursing staff about the need to use restrictive platelet transfusion thresholds, reminding them daily of the protocol change and discussing situations that required clarification. In our case, reviewing evidence about the potential harm of the use of the liberal transfusion threshold and the poor evidence of an association between low platelet count and intra-ventricular hemorrhage would improve adherence to the new protocol.
Recent studies question the association between thrombocytopenia and hemorrhage in neonates, pointing to the limited effectiveness of platelet transfusions in reducing the risk of hemorrhage and even suggesting that platelet transfusions may be harmful [14, 16,17,18,19,20,21, 26]. One hypothesis is the potential induction of an inflammatory process when neonates are transfused with adult platelets [25]. Platelets may also promote excessive angiogenic signals during a vulnerable period of brain development [27]. Transfusion-related harm may involve hemodynamic parameters, and fast volume expansion after platelet transfusion administration may disrupt blood flow in the brain and increase the risk of hemorrhage [28]. As a result, platelet count may not be the only factor to consider when prescribing transfusion, and some authors suggest that platelet mass and time of occlusion may better predict the risk of hemorrhage [29, 30].
Our study has some limitations. First, it was a single-center retrospective study, which limits the generalization of our results. We also had missing data, for example, the rate of missing data for bronchopulmonary dysplasia is significant (24.5% in the overall population, 21.2% in the population with thrombocytopenia < 50,000/mm3) due to transfers before 36 WG to other hospitals. In these cases, the precise respiratory status at 36 WG was not available. Additionally, the occurrence of bronchopulmonary dysplasia is likely overestimated because transferred neonates generally have a more favorable respiratory outcome than non-transferred neonates. We did not record other aspects of clinical practice that could have changed between the two periods. However, if changes of clinical practice occurred, they probably had a low impact on the risk of thrombocytopenia or major bleeding. Although our study is one of the largest real-world studies of neonates with thrombocytopenia, our sample size might still be too small to draw conclusions about rare side effects.
Our study highlights that lowering the platelet-count threshold for transfusion may result in transfusion savings without increasing complications. This is also the conclusion of a recent meta-analysis of platelet transfusion that recommended the use of restrictive platelet-count thresholds for transfusion in neonates [28]. Platelet transfusion modalities such as volume and duration are still being actively studied and may evolve in the coming years [31].
Conclusion
The findings of this retrospective study suggest that lowering the platelet-count threshold for transfusion in neonates born < 37 WG is associated with fewer transfusions and seems safe. These real-life results are in line with the current trend to reduce the number of platelet transfusions.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- CMV:
-
Cytomegalovirus
- IQR:
-
Interquartile range
- HT:
-
High threshold
- LT:
-
Low threshold
- NICU:
-
Neonatal intensive care unit
- PCR:
-
Polymerase chain reaction
- WG:
-
Weeks of gestation
References
Chevallier M, Debillon T, Pierrat V, Delorme P, Kayem G, Durox M et al (2017) Leading causes of preterm delivery as risk factors for intraventricular hemorrhage in very preterm infants: results of the EPIPAGE 2 cohort study. Am J Obstet Gynecol 216:518.e1-518.e12
Lai GY, Shlobin N, Garcia RM et al (2022) Global incidence proportion of intraventricular haemorrhage of prematurity: a meta-analysis of studies published 2010–2020. Arch Dis Child Fetal Neonatal Ed 107:513–519
Bolisetty S, Dhawan A, Abdel-Latif M, Bajuk B, Stack J, Oei JL et al (2014) Intraventricular hemorrhage and neurodevelopmental outcomes in extreme preterm infants. Pediatrics 133:55–62
Christensen RD, Henry E, Wiedmeier SE, Stoddard RA, Sola-Visner MC, Lambert DK et al (2006) Thrombocytopenia among extremely low birth weight neonates: data from a multihospital healthcare system. J Perinatol 26:348–353
Del Vecchio A, Sola MC, Theriaque DW, Hutson AD, Kao KJ, Wright D et al (2001) Platelet transfusions in the neonatal intensive care unit: factors predicting which patients will require multiple transfusions. Transfusion 41:803–8
Cremer M, Sallmon H, Kling PJ, Bührer C, Dame C (2016) Thrombocytopenia and platelet transfusion in the neonate. Semin Fetal Neonatal Med 21:10–18
Del Vecchio A (2014) Evaluation and management of thrombocytopenic neonates in the intensive care unit. Early Hum Dev 90:S51–S55
Gunnink SF, Vlug R, Fijnvandraat K, van der Bom JG, Stanworth SJ, Lopriore E (2014) Neonatal thrombocytopenia: etiology, management and outcome. Expert Rev Hematol 7:387–395
HAS. Recommandations de bonnes pratiques - Transfusion de plaquettes : produits, indications. French guidelines for platelet transfusions. Available online: https://www.has-sante.fr/upload/docs/application/pdf/2015-11/recommandations_-_transfusion_de_plaquettes.pdf. Accessed 6 Aug 2024
Stanworth SJ, Clarke P, Watts T, Ballard S, Choo L, Morris T et al (2009) Prospective, observational study of outcomes in neonates with severe thrombocytopenia. Pediatrics 124:e826–e834
Lopriore E (2019) Updates in red blood cell and platelet transfusions in preterm neonates. Am J Perinatol 36:S37-40
Andrew M, Vegh P, Caco C, Kirpalani H, Jefferies A, Ohlsson A et al (1993) A randomized, controlled trial of platelet transfusions in thrombocytopenic premature infants. J Pediatr 123:285–291
Curley A, Stanworth SJ, Willoughby K, Fustolo-Gunnink SF, Venkatesh V, Hudson C et al (2019) Randomized trial of platelet-transfusion thresholds in neonates. N Engl J Med 380:242–251
Fustolo-Gunnink SF, Fijnvandraat K, van Klaveren D, Stanworth SJ, Curley A, Onland W et al (2019) Preterm neonates benefit from low prophylactic platelet transfusion threshold despite varying risk of bleeding or death. Blood 134:2354–2360
Benjamin RJ, Dy B, Perez J, Eder AF, Wagner SJ (2014) Bacterial culture of apheresis platelets: a mathematical model of the residual rate of contamination based on unconfirmed positive results. Vox Sang 106:23–30
Baer VL, Lambert DK, Henry E, Christensen RD (2009) Severe thrombocytopenia in the NICU. Pediatrics 124:e1095-1100
Baer VL, Lambert DK, Henry E, Snow GL, Sola-Visner MC, Christensen RD (2007) Do platelet transfusions in the NICU adversely affect survival? Analysis of 1600 thrombocytopenic neonates in a multihospital healthcare system. J Perinatol 27:790–796
Garcia MG, Duenas E, Sola MC, Hutson AD, Theriaque D, Christensen RD (2001) Epidemiologic and outcome studies of patients who received platelet transfusions in the neonatal intensive care unit. J Perinatol 21:415–420
Bolat F, Kılıç SÇ, Oflaz MB, Gülhan E, Kaya A, Güven AS et al (2012) The prevalence and outcomes of thrombocytopenia in a neonatal intensive care unit: a three-year report. Pediatr Hematol Oncol 29:710–720
Davenport PE, Wood TR, Heagerty PJ, Sola-Visner MC, Juul SE, Patel RM (2024) Platelet transfusion and death or neurodevelopmental impairment in children born extremely preterm. JAMA Netw Open 7:e2352394
Burstein J, Papile L, Burstein R (1979) Intraventricular hemorrhage and hydrocephalus in premature newborns: a prospective study with CT. Am J Roentgenol 132:631–635
Bahr TM, Christensen TR, Henry E, Astin M, Ilstrup SJ, Ohls RK et al (2023) Platelet transfusions in a multi-neonatal intensive care unit health care organization before and after publication of the PlaNeT-2 clinical trial. J Pediatr 257:113388
Zabeida A, Chartrand L, Lacroix J, Villeneuve A (2023) Platelet transfusion practice pattern before and after implementation of a local restrictive transfusion protocol in a neonatal intensive care unit. Transfusion 63:134–142
Davenport PE, Chan Yuen J, Briere J et al (2021) Implementation of a neonatal platelet transfusion guideline to reduce non-indicated transfusions using a quality improvement framework. J Perinatol 41:1487–1494
Nguyen KA, Hamzeh-Cognasse H, Sebban M, Fromont E, Chavarin P, Absi L et al (2014) A Computerized prediction model of hazardous inflammatory platelet transfusion outcomes. PLoS ONE 9:e97082
Ribeiro HS, Assunção A, Vieira RJ, Soares P, Guimarães H, Flor-de-Lima F (2023) Platelet transfusions in preterm infants: current concepts and controversies—a systematic review and meta-analysis. Eur J Pediatr 182:3433–3443
Ferrer-Marin F, Chavda C, Lampa M, Michelson AD, Frelinger AL, Sola-Visner M (2011) Effects of in vitro adult platelet transfusions on neonatal hemostasis. J Thromb Haemost 9:1020–1028
van der Staaij H, Stanworth SJ, Fustolo-Gunnink SF (2023) Prophylactic platelet transfusions: why less is more. Clin Perinatol 50:775–792
Gerday E, Baer VL, Lambert DK, Paul DA, Sola-Visner MC, Pysher TJ et al (2009) Testing platelet mass versus platelet count to guide platelet transfusions in the neonatal intensive care unit. Transfusion 49:2034–2039
Davenport P, Sola-Visner M (2022) Platelets in the neonate: not just a small adult. Res Pract Thromb Haemost 6(3):e12719
Moore CM, Curley AE (2021) Neonatal platelet transfusions: starting again. Transfus Med Rev 35:29–35
Acknowledgements
The authors thank Laura Smales for correcting the English.
Funding
Open access funding provided by Hospices Civils de Lyon.
Author information
Authors and Affiliations
Contributions
E.Z.T., H.T., S.G. and E.B. designed the study; S.G. and E.B prepared the data for analysis; H.T. analyzed the data; E.Z.T., H.T., and E.B. interpreted the data; E.B., E.Z.T. and H.T. wrote the report, all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This is a single-center retrospective observational study. In accordance with the Declaration of Helsinki, the study was declared to APHP as required by RGPD rules (registration number 20220701152308). Parents were informed that their newborn’s data could be used to evaluate medical practices unless they stated their opposition.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Daniele De Luca
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Billion, E., Ghattas, S., Jarreau, PH. et al. Lowering platelet-count threshold for transfusion in preterm neonates decreases the number of transfusions without increasing severe hemorrhage events. Eur J Pediatr (2024). https://doi.org/10.1007/s00431-024-05709-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00431-024-05709-x