Abstract
Purpose
The present study aimed to explore the influence of diet and physical activity (PA) changes on bone mineral content (BMC) and density (BMD) alterations in adolescents with obesity undergoing a weight loss program.
Methods
Six-month longitudinal data from 71 adolescents (aged 15.1 [± 1.6] years; 57.7% girls) with a BMI z-score of 3.03 (± 0.78), previously recruited for the PAC-MAnO trial, were analyzed using Generalized Estimation Equations for over time changes and linear regressions with BMC, BMD and BMD z-score as dependent variables, adjusting for confounders (including type of exercise- aerobic vs. combined).
Results
Adjusting for confounders, changes in carbohydrate (CH) and protein content showed to positively and negatively predict BMD z-score variance, respectively (β = 0.44, 95%CI: 0.01, 0.04, p < .001); β = -0.57, 95%CI: -0.06, -0.03, p < .001), yet no associations were found between PA and bone-related parameters. Combined exercise showed better results on BMC compared to aerobic exercise (β = 0.09, 95%CI: 0.05 to 0.13, p < .001).
Conclusions
Increased CH content, instead of protein, may be associated with BMD improvements in adolescents with obesity. Type of exercise may moderate the impact of PA on bone health.
Trial registration
Clinicaltrials.gov NCT02941770.
What is Known • Adolescents with obesity may be at a higher risk of osteopenia/osteoporosis • Obesity and inadequate diet and physical activity (PA) may have an adverse effect on bone metabolism | |
What is New • Improvements in adiposity and muscle mass and increased diet carbohydrate content are associated with bone mineral density (BMD) improvements • Type of exercise (i.e., combined training vs. aerobic) may moderate the impact of PA on BMD, and calcium intake may mediate this impact |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early and sustained exposure to a positive energy balance due to high consumption of energy-dense/low-quality foods and drinks [1], low levels of physical activity (PA) [2], and high levels of sedentary behaviors [3], together with a genetic component and psychosocial contributors may have driven the rising global prevalence and severity of pediatric obesity [4], anticipating the incidence of cardiometabolic comorbidities [5].
The likelihood of adolescents with obesity experiencing neuromusculoskeletal impairments, including injuries and fractures, is high [6], and may compromise their ability to function, move, and in their capacity to engage in a physically active lifestyle [7].
It has been suggested that adolescents with overweight/obesity have higher bone mineral content (BMC) and bone mineral density (BMD) compared to those with healthy weight [8]. However, other studies pointed to obesity as deleterious for bone health [9, 10], with adipokines playing a key role [11]. Moreover, obesity’s negative impact on bone health seems to be worsened by the presence of metabolic syndrome (MS) [12]. Obesity may eventually compromise peak bone mass accrual, impairing skeletal development during adolescence, increasing osteoporotic risk later in life [13].
The impact of obesity and MS on bone health, is not fully understood, with no consensus on how obesity affects BMC and BMD in adolescents [9, 12]. Environmental, behavioral, and genetic factors influence this complex relationship. Although 60–80% of peak bone mass is genetically determined [14], diet and PA behaviors (especially calcium and vitamin D intake and weight-bearing/muscle-strengthening activities) [15, 16] may account for the remaining 20- 40%.
Emerging evidence linking obesity to sub-optimal bone health in adolescents has been translated into clinical practice [17]. Yet, further research is needed in the context of weight loss programs, which, once focusing on total energy intake (TEI) restriction, may compromise calcium and vitamin D intake, consequently impacting bone health [18].
Few longitudinal studies exist on the influence of diet and PA changes on BMC and BMD in adolescents with obesity following a specialized weight management program. Thus, this study aimed to explore the influence of diet and PA changes on BMC and BMD alterations in adolescents with obesity undergoing a weight loss program, while controlling for potential confounders.
Materials and Methods
Study design and outcomes are reported according to CONSORT-Outcomes 2022 Extension [19].
Trial design
Relevant data from the PAC-MAnO trial (Clinicaltrials.gov NCT02941770) were analyzed in this study [20]. In brief, the PAC-MAnO trial consisted of a 3-arm non-randomized controlled trial testing the efficacy of a PA consultation-only (EGI) and a PA consultation plus weekly supervised physical exercise sessions (EGII), compared to standard care (CG) in the treatment of obesity in adolescents over 6 months, with a 6-month follow-up [20, 21]. Additional information regarding EGII exercise sessions can be found elsewhere [22].
The trial was approved by the Ethics Committee of the Faculty of Medicine of the University of Lisbon, Portugal (271/2016) and is under the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Participants
The PAC-MAnO enrolled 165 adolescents (12–17 years old) with overweight or obesity (BMI ≥ 85th percentile [23]) attending for the first time the Pediatric Obesity Clinic of Hospital de Santa Maria, Lisbon, a Public Central Hospital (CG, n = 55; EGI n = 55; EGII n = 55) [24]. Exclusion criteria included: (i) major non-obesity related pathologies, (ii) inability to perform regular PA, (iii) mental disorders, (iv) smoking, (v) prescriptions affecting weight or bone metabolism. All participants and caregivers provided informed assent/consent.
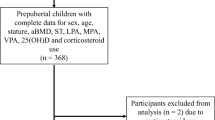
Of the 165 adolescents recruited, 132 (80.0%) attended 6-month assessments [24]. From these, 71 (54.0%) participants with obesity (BMI ≥ 97th percentile [23]) completed all the assessments, being included in this exploratory study (CG, n = 6; EGI n = 30; EGII n = 35). Included participants showed a higher age, BMI, and pubertal status than those excluded. No other differences were found between included and excluded participants, including in bone-related parameters (Supplemental Table 1).
Measurements
Height, body weight, and waist circumference (WC) were measured according to standard procedures. BMI (BMI = weight (kg)/height2 (m)) and waist-to-height ratio (WHtR = WC/Height) were calculated. Height z-score and BMI z-score were additionally computed using the World Health Organization (WHO) AnthroPlus calculator.
Body composition was assessed using dual-energy x-ray absorptiometry (DXA), using QDR 12.4 software, according to the National Health and Nutrition Examination Survey (NHANES) protocol [25]. Bone-related key measures variables included areal BMC, BMD, and BMD z-score, derived from total body less head assessment. BMD z-score was determined using DXA reference values from NHANES [26]. Total body fat mass (TBFM), trunk fat mass (Trunk FM), and fat-and-bone-free mass (FBFM) were assessed. Relative body fat mass (BFM) and muscle mass (MM) were computed as percentages of TBFM and FBFM divided by body weight, respectively. Central fat mass (Central FM) was calculated as a percentage of Trunk FM divided by TBFM.
Pubertal status was objectively assessed by a pediatrician according to Tanner stages [27].
MS was defined as the presence of at least two of the following: high resting systolic blood pressure (SBP)/diastolic blood pressure (DBP), high triglycerides (TG), low high-density lipoprotein cholesterol (HDL-C) or high glucose levels. Biochemical analyses were conducted according to standard procedures. Cut-off values for MS components are provided in Supplemental Table 2, according to previously published values [28].
Cardiorespiratory fitness (CRF), i.e., VO2 peak, was measured directly using a gas analyzer during a submaximal exercise test using a cycle ergometer. CRF assessment is extensively described elsewhere [22]. VO2 peak (ml/min) was additionally adjusted for body weight (ml/kg/min) and used in the analyses.
PA was assessed with triaxial accelerometers, worn above the right hip, programmed to use a 5-s cycle, during at least one weekend day and two weekdays, except during sleep, bathing, or swimming. Only days with more than 480 min registered, were included in the analysis. Activities between 0 and 149 counts/minute were considered as stationary time [29]; between 150 and 499, light PA (LPA); between 500 and 3999, moderate PA (MPA); and more than 4000 counts/minute vigorous PA (VPA) [30]. The daily average of stationary time, LPA, MPA, VPA, and MVPA (MPA + VPA) were calculated and used in the analysis.
Additional information about the exercise performed, and the frequency of regular (structured or unstructured) PA (times per week), was collected by recall at the time of the PA assessments. Due to high heterogeneity in the PAs performed, PAs were further categorized by exercise type (i.e., aerobic, strength, or combined) based on the main characteristics of the activity.
Dietary intake was assessed using three-day food records (2 weekdays and 1 weekend day) with semi-quantitative scaling (e.g., number of spoons or scoops), according to the 4th edition of Weight and Food portions [31]. Each food record underwent independent analysis by three trained nutritionists. Final data were included in the analyses after a group discussion. TEI, protein, carbohydrate (CH), fat, calcium, and vitamin D3 daily intake, as well as relative content of saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA), and trans fatty acids (Trans FA), were considered as variables of interest. Macronutrient content was calculated by dividing its equivalent in kcal by TEI (kcal/day).
Further information on measures and instruments is available elsewhere [20].
Statistical analysis
A Per Protocol Analysis (PPA) was performed using IBM SPSS statistics software (version 29.0, IBM, New York, USA). The Shapiro–Wilk test assessed normality for continuous variables. Mean ± SD and median (interquartile range, IR) were calculated for normal and skewed data. Baseline differences between girls and boys were analyzed with independent t-tests/Mann–Whitney U tests and Chi-squared for continuous and categorical variables, respectively. Generalized Estimating Equations (GEE), adjusted for age, pubertal status, and group (i.e., trial allocation group), were used to analyze over time changes and sex-by-time interaction in anthropometric, body composition, and bone-related parameters, and also cardiorespiratory fitness, PA, and diet. The associations between changes in these variables were performed with partial correlations controlling for sex, ethnicity, age, pubertal status, group, height, and presence of MS.
The variables significantly associated with over time changes in BMC, BMD, and BMD z-score were further included as independent variables in predictive models, using Linear regressions, adjusting for possible confounders [32].
Additional analyses investigating differences in body composition, diet, and PA behaviors changes by type of exercise were performed using GEE (adjusted for age, sex, pubertal status, and group) and Chi-squared (for categorical variables). Since only one participant performed strength training, data on this participant was gathered to the combined training group. Participants who changed the type of exercise performed during the 6 months were excluded from this specific analysis (n = 2, both boys from the combined training group).
A p-value of ≤ 0.05 was considered statistically significant.
Results
Post-hoc power calculation using BMC difference as endpoint, compared to previously published values in a similar population (29.4 ± 5.3) [33], and an alpha of 0.05 yielded a statistical power of 100%.
Seventy-one participants (93.0% Caucasian; 57.7% girls), with a mean age of 15.1 (± 1.6) years and a mean BMI of 34.44 (± 5.00) kg/m2, followed for 6 months, were included. The presence of MS components is presented in Supplemental Table 3. At baseline, the majority of participants presented a normal BMD z-score, yet 2 participants (2.8%) presented a BMD z-score of -2 SD; only 1 participant (1.4%) showed to meet the recommendations for calcium intake (i.e., 960 mg/day) [34]; 6 (8.5%) reported the minimum calcium intake needed to observe a beneficial impact of PA on BMD (i.e., 700 mg/day) [35]; and 19 (26.8%) met PA guidelines (≥ 60 min of MVPA [36]).
Sex differences in height, height z-score, BMI z-score, WC, FBFM (Table 1), TEI (Table 2), and pubertal status (Supplemental Table 3) but not in bone-related variables were found at baseline (Table 1).
Cross-sectional (baseline) correlations are presented in Supplemental Table 4.
Changes in bone-related parameters, anthropometrics, and body composition
Overall, participants showed a significant increase in BMC (β = 0.14; 95%CI: 0.10, 0.18), BMD (β = 0.04; 95%CI: 0.03, 0.05), and BMD z-score (β = 0.31; 95%CI: 0.10, 0.54), as well as in FBFM (β = 3.40, 95%CI: 1.94, 4.85) and MM (β = 2.45, 95%CI: 1.33, 3.57); and a decrease in height z-score (β = -0.12; 95%CI: -0.19, -0.04), BMI z-score (β = -0.13; 95%CI: -0.25, -0.02;), WHtR (β = -0.02; 95%CI: -0.03, -0.01), BFM (β = -2.10; 95%CI: -3.10, -1.09), Trunk FM (β = -1.77; 95%CI: -2.67, -0.87), and Central FM (β = -4.09; 95%CI: -5.72, -2.46). Among all, 13 (18.3%) and 9 (12.7%) participants showed an impairment in BMC and BMD, respectively (Table 1).
A sex-by-time interaction was found, with girls showing a smaller increase in BMC (β = -0.10; 95%CI: -0.15, -0.05), BMD (β = -0.02; 95%CI: -0.04, -0.01), BMD z-score (β = -0.26; 95%CI: -0.49, -0.02), FBFM (β = -2.78; 95%CI: -4.32, -1.23), and MM (β = -1.49; 95%CI: -2.92, -0.07), compared to boys; as well as a smaller decrease in Height z-score (β = -0.10; 95%CI: -0.15, -0.05), BFM (β = -1.35; 95%CI: -2.57, -0.13), Trunk FM (β = -1.10; 95%CI: -2.17, -0.02), Central FM (β = -2.83; 95%CI: -4.68, -0.97). More girls showed an impairment in BMC and BMD than boys (Table 1).
Changes in diet, physical activity, and cardiorespiratory fitness
Participants showed an significant increase in MUFA (β = 2.56; 95%CI:, 0.59, 4.54), MPA (β = 38.93; 95%CI: 27.23, 50.64), VPA (β = 7.62; 95%CI: 4.99, 10.24), MVPA (β = 46.55; 95%CI: 33.75, 59.35), and CRF (β = 1.10; 95%CI: 0.58, 1.62); as well as a decrease in Trans FA (β = -0.57; 95%CI:, -0.85, -0.29) and stationary time (β = 53.64; 95%CI:, 92.26, 15.01) (Table 2).
A sex-by-time interaction was found, with girls showing a higher increase in time spent stationary, compared to a decrease in boys (β = 65.52; 95%CI: 11.24, 119.80; p = 0.020); and a smaller increase in MPA (β = -24.96; 95%CI: -40.99, -8.93) and MVPA (β = -28.55; 95%CI: -46.89, -10.21) (Table 2).
Association between changes in bone parameters, anthropometrics, diet, and physical activity
BMC changes showed a significant correlation with WHtR (r = -0.322) and VO2 peak (r = 0.306) (Table 3). Changes in BMD were positively correlated with changes in CH intake (r = 0.432). BMD z-score changes were negatively correlated with changes in BMI z-score (r = -0.409), WHtR (r = -0.374), BFM% (r = -0.353), LPA (r = -0.345), and protein content (%) (r = -0.462); and positively with MM% (r = 0.412), VO2 peak (r = 0.436), and CH content (r = 0.317). No correlations were found between BMC/BMD/BMD z-score and changes in SFA, MUFA, PUFA, or Trans FA intake (data not shown).
Bone mineral content and bone mineral density variance
BMC variance was not explained by LPA, protein, or CH changes (Table 4).
BMD variance was explained by changes in protein and CH content but not by LPA. Changes in protein content were negatively associated with BMD even when adjusting for all the confounders (Model 4: β = -0.34, 95%CI: -0.03 to 0.00). Conversely, changes in CH content were positively associated with BMD (Model 4: β = 0.42, 95%CI: 0.01 to 0.03).
Similar results were found regarding BMD z-score variance. Adjusting for all the confounding variables (Model 4), changes in protein and CH content were shown to negatively and positively predict BMD z-score variance, respectively (β = -0.41, 95%CI: -0.04, -0.01; β = 0.29, 95%CI: 0.01, 0.03) (Table 4).
Changes in body composition, bone parameters, diet, and physical activity behaviors by exercise type
Participants exposed to combined training showed a higher decrease in BMI z-score (β = -0.21, 95%CI: -0.35 to -0.07), WHtR (β = -0.02, 95%CI: -0.04 to -0.01), Trunk FM (β = -1.75, 95%CI: -2.77 to -0.73), and stationary time (β = -65,17, 95%CI: -122.79 to -7.55); and a higher increase in MM (β = 1.95, 95%CI: 0.53 to 3.39), BMC (β = 0.09, 95%CI: 0.05 to 0.13), MVPA (β = 35.58, 95%CI: 16.67 to 54.48), and VO2 peak (β = 1.71, 95%CI: 0.76 to 2.66) compared to those exposed to aerobic exercise. No differences were found in BMD or BMD z-score between aerobic and combined exercise training groups (Supplemental Table 4).
Discussion
This study sought to explore the influence of diet and PA changes on BMC and BMD alterations in adolescents with obesity under a weight loss program, which assumes particular importance as this population may be at a higher risk of early development of osteopenia/osteoporosis, not only due to an adverse effect of obesity and MS on bone metabolism [9, 12], but also due to an inadequate diet, including low calcium intake [37], and low levels of PA [38] (usually observed in this population).
In fact, despite an overall 6-month increase in BMC, BMD and BMD z-score, 2 participants (2.8%) presented an impairment in BMD z-score of -2 SD at baseline and 9 (12.7%) showed an over time decrease in BMD. Changes in MM (%) were positively associated with BMD z-score, and changes in WHtR were inversely associated with both BMC and BMD z-score, suggesting that abdominal adiposity and proinflammatory adipokines have a deleterious effect on bone health, as previously reported by other authors [11, 39, 40].
Regarding the potential influence of diet on bone-related parameters, it is noteworthy that only macronutrient intake was associated with BMD/BMD z-score. Specifically, increases in CH content were associated with increases in BMD and BMD z-score; and increases in protein content were associated with decreases in BMD and BMD z-score. These associations persisted after adjusting for confounders. Though unexpected, these findings may be associated with a high animal protein consumption and (relatively) low CH intake leading to a possible hypercalcinuria, increasing urinary calcium excretion in these participants [41]. Others have suggested a deleterious effect of high CH intake on calcium metabolism, yet this effect depends on food sources [42]. High CH intake from natural food sources, rich in complex digestible CH, also providing dietary fiber, phytic acid, and oxalic acid – elements that can bind to calcium, may decrease gastrointestinal calcium absorption and bioavailability [42]. However, this mechanism may not apply to our participants, whose CH intake primarily comes from processed sources, poor in complex CH [1].
No associations were found between changes in PA and bone-related parameters when controlling for changes in body composition and diet. It has been suggested that higher PA intensities are positively associated with bone health in adolescents [43]. The lack of association between MVPA and bone-related parameters in this study may be explained by the fact that the impact of PA on bone metabolism may depend on calcium intake and availability and/or exercise characteristics (i.e., type of exercise). Indeed, only 6 (8.5%) participants reported the minimum intake suggested as needed to observe a beneficial impact of PA on BMD [35], with longitudinal data showing a significant overall increase in MVPA but not in calcium intake. In turn, a time-by-type of exercise (i.e., aerobic vs. combined) effect was observed on adiposity and bone parameters, with combined training showing better outcomes in BMI z-score, WHtR, Trunk FM, MM, and BMC compared to the aerobic one. This evidence aligns with the literature, especially concerning aerobic non-weight-bearing activities, such as swimming [44]. Combined training, particularly resistance exercise, exerts an increased mechanical load on bone, improving bone strength, compared to aerobic non-weight-bearing activities [44, 45].
The limited number of participants and the use of self-reported diet data are the two major limitations of the present study. As it has been previously suggested, adolescents with obesity tend to underreport food intake [46], which could lead to an underestimation of calcium intake. Yet, a possible underestimation of calcium intake would not be significative in our sample since adolescents with obesity tend to underreport energy-dense/low-quality foods and drinks, poor in calcium [1, 46]. The difference found between included and excluded participants regarding variables that are known to be associated with bone development (i.e., age, BMI, and pubertal status) can also be considered as a limitation, which was addressed by controlling data analysis for numerous confounding factors, including the ones mentioned, avoiding interpretation bias. Although the aim of the present study was not to investigate in depth the impact of exercise (but daily PA) on bone-related parameters, the high heterogeneity in the PAs reported by the participants did not allow further analysis, conditioning, for example, its categorization in just type of exercise (aerobic vs. combined), which may also be considered as a limitation.
Despite the acknowledged limitations, this study adds to the previous literature by exploring the influence of diet and PA changes on BMC/BMD of adolescents with obesity under a weight loss program using objectively measured PA and controlling for important potential confounding factors.
This study highlights the complex interplay between adiposity, diet, PA, and bone health in adolescents. Improvements in adiposity, MM, and higher CH diet showed associations with BMD/BMD z-score enhancement. Moreover, while exercise type (i.e., combined training vs. aerobic) may moderate the impact of PA on bone health, low calcium intake may compromise the possible effect of exercise on bone health, emphasizing the need for nutritional monitoring. Comprehensive monitoring of diet and PA behaviors, and not only energy balance, is essential for effective intervention strategies in this population.
Data availability
The data that support the findings of this study are available from the corresponding author, AVS, upon reasonable request.
Abbreviations
- BFM:
-
Body fat mass
- BMC:
-
Bone mineral content
- BMD:
-
Bone mineral density
- BMI:
-
Body mass index
- CH:
-
Carbohydrate
- FBFM:
-
Fat-and-bone-free mass
- MVPA:
-
Moderate-vigorous physical activity
- PA:
-
Physical activity
- TBFM:
-
Total body fat mass
- TEI:
-
Total energy intake
- WC:
-
Waist circumference
- WHtR:
-
Waist-to-height ratio
References
Ludwig DS, Peterson KE, Gortmaker SL (2001) “Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis,” (in eng). Lancet 357(9255):505–508. https://doi.org/10.1016/S0140-6736(00)04041-1
Farpour-Lambert NJ, Aggoun Y, Marchand LM, Martin XE, Herrmann FR, Beghetti M (2009) “Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children,” (in eng). J Am Coll Cardiol 54(25):2396–2406. https://doi.org/10.1016/j.jacc.2009.08.030
Tremblay MS et al (2011) “Systematic review of sedentary behaviour and health indicators in school-aged children and youth,” (in eng). Int J Behav Nutr Phys Act 8:98. https://doi.org/10.1186/1479-5868-8-98
Deckelbaum RJ, Williams CL (2001) “Childhood obesity: the health issue,” (in eng). Obes Res 9(Suppl 4):239S-243S. https://doi.org/10.1038/oby.2001.125
Steinbeck KS, Lister NB, Gow ML, Baur LA (2018) “Treatment of adolescent obesity,” (in eng). Nat Rev Endocrinol 14(6):331–344. https://doi.org/10.1038/s41574-018-0002-8
O’Malley GC, Shultz SP, Thivel D, Tsiros MD (2021) Neuromusculoskeletal Health in Pediatric Obesity: Incorporating Evidence into Clinical Examination. Current Obesity Reports 10(4):467–477. https://doi.org/10.1007/s13679-021-00463-9
Tsiros MD et al (2020) “Obesity, the new childhood disability? An umbrella review on the association between adiposity and physical function,” (in eng). Obes Rev 21(12):e13121. https://doi.org/10.1111/obr.13121
van Leeuwen J, Koes BW, Paulis WD, van Middelkoop M (2017) Differences in bone mineral density between normal-weight children and children with overweight and obesity: a systematic review and meta-analysis. Obesity Reviews 18(5):526–546. https://doi.org/10.1111/obr.12515
Dimitri P (2019) “The Impact of Childhood Obesity on Skeletal Health and Development,” (in eng). J Obes Metab Syndr 28(1):4–17. https://doi.org/10.7570/jomes.2019.28.1.4
Júnior IFF, Cardoso JR, Christofaro DGD, Codogno JS, de Moraes ACF, Fernandes RA (2013) The relationship between visceral fat thickness and bone mineral density in sedentary obese children and adolescents. BMC Pediatrics 13(1):37. https://doi.org/10.1186/1471-2431-13-37
Gil-Cosano JJ et al (2022) Leptin levels were negatively associated with lumbar spine bone mineral content in children with overweight or obesity. Acta Paediatrica 111(10):1966–1973. https://doi.org/10.1111/apa.16456
da Silva VN, Fiorelli LN, da Silva CC, Kurokawa CS, Goldberg TB (2017) “Do metabolic syndrome and its components have an impact on bone mineral density in adolescents?,” (in eng). Nutr Metab (Lond) 14:1. https://doi.org/10.1186/s12986-016-0156-0
Bachrach LK (2005) “Osteoporosis and measurement of bone mass in children and adolescents,” (in eng). Endocrinol Metab Clin North Am 34(3):521–35, vii. https://doi.org/10.1016/j.ecl.2005.04.001
Heaney RP et al (2000) “Peak bone mass,” (in eng). Osteoporos Int 11(12):985–1009. https://doi.org/10.1007/s001980070020
Demay MB, Sabbagh Y, Carpenter TO (2007) “Calcium and vitamin D: what is known about the effects on growing bone,” (in eng). Pediatrics 119(Suppl 2):S141–S144. https://doi.org/10.1542/peds.2006-2023F
Bailey DA, McKay HA, Mirwald RL, Crocker PR, Faulkner RA (1999) “A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: the university of Saskatchewan bone mineral accrual study,” (in eng). J Bone Miner Res 14(10):1672–1679. https://doi.org/10.1359/jbmr.1999.14.10.1672
Calleja M et al (2020) “Increased dairy product consumption as part of a diet and exercise weight management program improves body composition in adolescent females with overweight and obesity-A randomized controlled trial,” (in eng). Pediatr Obes 15(12):e12690. https://doi.org/10.1111/ijpo.12690
Shapses SA, Sukumar D (2012) “Bone metabolism in obesity and weight loss,” (in eng). Annu Rev Nutr 32:287–309. https://doi.org/10.1146/annurev.nutr.012809.104655
Butcher NJ et al (2022) “Guidelines for Reporting Outcomes in Trial Reports: The CONSORT-Outcomes 2022 Extension,” (in eng). JAMA 328(22):2252–2264. https://doi.org/10.1001/jama.2022.21022
Videira-Silva A, Sardinha L, Fonseca H (2018) “Effect of a Physical Activity Consultation in the Management of Adolescent Overweight (the PAC-MAnO project): study rationale, design and methods,” (in eng). BMJ Paediatr Open 2(1):e000214. https://doi.org/10.1136/bmjpo-2017-000214
Videira-Silva A, Manco L, Sardinha LB, Fonseca H (2023) Vigorous physical activity: A potential ally in adolescent obesity management. Eur J Sport Sci 23(4):607–616. https://doi.org/10.1080/17461391.2022.2035437
Videira-Silva A, Hetherington-Rauth M, Sardinha LB, Fonseca H (2023) “Combined high-intensity interval training as an obesity-management strategy for adolescents,” (in eng). Eur J Sport Sci 23(1):109–120. https://doi.org/10.1080/17461391.2021.1995508
de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J (2007) “Development of a WHO growth reference for school-aged children and adolescents,” (in eng). Bull World Health Organ 85(9):660–667. https://doi.org/10.2471/blt.07.043497
Videira-Silva A, Hetherington-Rauth M, Sardinha LB, Fonseca H (2021) The effect of a physical activity consultation in the management of adolescent excess weight: Results from a non-randomized controlled trial, (in eng), Clin Obes, p. e12484, https://doi.org/10.1111/cob.12484
NHANES (2007) Dual Energy X-ray Absorptiometry (DXA) Procedures Manual http://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_dexa.pdf
Kelly TL, Wilson KE, Heymsfield SB (2009) “Dual energy X-Ray absorptiometry body composition reference values from NHANES,” (in eng). PLoS One 4(9):e7038. https://doi.org/10.1371/journal.pone.0007038
Tanner JM (1981) “Growth and maturation during adolescence,” (in eng). Nutr Rev 39(2):43–55. https://doi.org/10.1111/j.1753-4887.1981.tb06734.x
Videira-Silva A, Freira S, Fonseca H (2020) “Metabolically healthy overweight adolescents: definition and components,” (in eng). Ann Pediatr Endocrinol Metab 25(4):256–264. https://doi.org/10.6065/apem.2040052.026
Tremblay MS et al (2017) “Sedentary Behavior Research Network (SBRN) - Terminology Consensus Project process and outcome,” (in eng). Int J Behav Nutr Phys Act 14(1):75. https://doi.org/10.1186/s12966-017-0525-8
Freedson P, Pober D, Janz KF (2005) Calibration of accelerometer output for children, (in eng), Med Sci Sports Exerc, vol. 37, no. 11 Suppl, pp. S523–30, Nov 2005. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/16294115
Goios A, Oliveira A, Afonso C, Amaral T, Martins M (2021) Pesos e Porções de Alimentos (Weights and Portions of Foods). (3rd edn, p 255). U. Porto Press
Golden NH et al (2014) Optimizing Bone Health in Children and Adolescents. Pediatrics 134(4):e1229–e1243. https://doi.org/10.1542/peds.2014-2173
Johannsen N, Binkley T, Englert V, Neiderauer G, Specker B (2003) “Bone response to jumping is site-specific in children: a randomized trial,” (in eng). Bone 33(4):533–539. https://doi.org/10.1016/s8756-3282(03)00220-5
N. Efsa Panel on Dietetic Products and Allergies (2015) Scientific Opinion on Dietary Reference Values for calcium. EFSA J 13(5):4101. https://doi.org/10.2903/j.efsa.2015.4101
Rowlands AV, Ingledew DK, Powell SM, Eston RG (2004) Interactive effects of habitual physical activity and calcium intake on bone density in boys and girls. J Appl Physiol 97(4):1203–1208. https://doi.org/10.1152/japplphysiol.00182.2004
World Health O (2020) WHO guidelines on physical activity and sedentary behaviour. Geneva: World Health Organization
Ruiz LD, Zuelch ML, Dimitratos SM, Scherr RE (2019) Adolescent Obesity: Diet Quality, Psychosocial Health, and Cardiometabolic Risk Factors, (in eng), Nutrients, vol. 12, no. 1, https://doi.org/10.3390/nu12010043
van Sluijs EMF et al (2021) Physical activity behaviours in adolescence: current evidence and opportunities for intervention. The Lancet 398(10298):429–442. https://doi.org/10.1016/S0140-6736(21)01259-9
Yao W et al (2023) “Association of total body fat and fat distribution with bone mineral density among children and adolescents aged 6–17 years from Guangzhou, China,” (in eng). Eur J Pediatr 182(3):1115–1126. https://doi.org/10.1007/s00431-022-04727-x
Mengel E et al (2018) “The associations between the changes in serum inflammatory markers and bone mineral accrual in boys with overweight and obesity during pubertal maturation: a 3-year longitudinal study in Estonian boys,” (in eng). Osteoporos Int 29(9):2069–2078. https://doi.org/10.1007/s00198-018-4580-z
Adam-Perrot A, Clifton P, Brouns F (2006) “Low-carbohydrate diets: nutritional and physiological aspects,” (in eng). Obes Rev 7(1):49–58. https://doi.org/10.1111/j.1467-789X.2006.00222.x
Kelsay JL (Oct1987) “Effects of fiber, phytic acid, and oxalic acid in the diet on mineral bioavailability,” (in eng). Am J Gastroenterol 82(10):983–986
Sayers A, Mattocks C, Deere K, Ness A, Riddoch C, Tobias JH (2011) Habitual Levels of Vigorous, But Not Moderate or Light, Physical Activity Is Positively Related to Cortical Bone Mass in Adolescents. J Clin Endocrinol Metab 96(5):E793–E802. https://doi.org/10.1210/jc.2010-2550
Min SK et al (2019) “Position Statement: Exercise Guidelines to Increase Peak Bone Mass in Adolescents,” (in eng). J Bone Metab 26(4):225–239. https://doi.org/10.11005/jbm.2019.26.4.225
Hong AR, Kim SW (2018) “Effects of Resistance Exercise on Bone Health,” (in eng). Endocrinol Metab (Seoul) 33(4):435–444. https://doi.org/10.3803/EnM.2018.33.4.435
Kant AK (2002) Association of Self-perceived Body Weight Status with Dietary Reporting by U.S. Teens*. Obes Res 10:1259–1269
Acknowledgements
AVS was supported by the Portuguese Foundation for Science and Technology (SFRH/BD/130193/2017). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. AVS funding organization played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Funding
Open access funding provided by FCT|FCCN (b-on). AVS was supported by the Portuguese Foundation for Science and Technology (SFRH/BD/130193/2017). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. AVS funding organization played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Author information
Authors and Affiliations
Contributions
This research was designed by AVS and directed by LBS and HF. AVS was responsible for data collection and analysis. AVS, IS, DF, and MG were responsible for literature search and data interpretation. AVS, IS, DF, MG, LBS, and HF contributed to the writing and reviewing of the manuscript. All authors meet the standard criteria for authorship and approved the final version of the manuscript as submitted.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
The trial was approved by the Ethics Committee of the Faculty of Medicine of the University of Lisbon, Portugal (271/2016) and is under the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed assent/consent was obtained from all individual participants included in the study and their respective caregivers.
Consent to publish
Not applicable.
Additional information
Communicated by Gregorio Milani
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Videira-Silva, A., Santos, I., Freaza, D. et al. Dietary content and combined training, but not daily physical activity, are associated with 6-month bone mineral changes in adolescents with obesity: A Secondary analysis of the PAC-MAnO trial. Eur J Pediatr 183, 3969–3978 (2024). https://doi.org/10.1007/s00431-024-05659-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-024-05659-4