Abstract
We analyzed plasma melatonin levels in different groups of preterm newborns without hypoxia and their relationship with several perinatal variables like gestational age or neonatal pain. Prospective cohort study of preterm newborns (PTNB) without perinatal hypoxia, Apgar > 6 at 5 min, and oxygen needs on the third day of life. We compared melatonin levels at day 3 of life in different groups of non-hypoxic preterm infants (Student’s t-tests, Mann-Whitney U, and chi2) and analyzed the relationship of melatonin with GA, birth weight, neonatal pain (Premature Infant Pain Profile (PIPP) scale), caffeine treatment, parenteral nutrition, or the development of free radical diseases (correlation study, linear regression) and factors associated with moderate/intense pain and free radical diseases (logistic regression analysis). Sixty-one preterm infants with gestational age (GA) of 30.7 ± 2.0 weeks with no oxygen requirements at day 3 of life were studied with plasma melatonin levels of 33.8 ± 12.01 pg/ml. Preterm infants weighing < 1250 g at birth had lower plasma melatonin levels (p = 0.05). Preterm infants with moderate or severe pain (PPIPP > 5) have lower melatonin levels (p = 0.01), and being preterm with PIPP > 5 is associated with lower plasma melatonin levels (p = 0.03). Being very preterm (GA < 32 GS), having low weight for gestational age (LWGA), receiving caffeine treatment, or requiring parenteral nutrition did not modify melatonin levels in non-hypoxic preterm infants (p = NS). Melatonin on day 3 of life in non-hypoxic preterm infants is not associated with later development of free radical diseases (BPD, sepsis, ROP, HIV, NEC).
Conclusion: We observed that preterm infants with moderate to severe pain have lower melatonin levels. These findings are relevant because they reinforce the findings of other authors that melatonin supplementation decreases pain and oxidative stress in painful procedures in premature infants. Further studies are needed to evaluate whether melatonin could be used as an analgesic in painful procedures in preterm infants.
Trial registration: Trial registration was not required since this was an observational study.
What Is Known: • Melatonin is a potent antioxidant and free radical scavenger in newborns under stress conditions: hypoxia, acidosis, hypotension, painful procedures, or parenteral nutrition. • Pain stimulates the production of melatonin. • Various studies conclude that melatonin administration decreases pain during the neonatal period. What Is New: • Non-hypoxic preterm infants with moderate to severe pain (PIPP>5) have lower levels of melatonin. • Administration of caffeine and treatment with parenteral nutrition do not modify melatonin levels in non-hypoxic preterm infants. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preterm newborns (PTNB) are highly exposed to oxidative stress in their first days of life because they have numerous factors that generate an overproduction of free radicals: transition from intrauterine to postnatal life going from a PO2 of 20–25 mm up to 100 mm Hg, hypoxia in labor or postpartum, occasional needs for mechanical ventilation, acidosis, infections, transfusions, exogenous inputs of free radicals in parenteral nutrition, and exposure to drugs, as well as the numerous painful procedures to which they are exposed in intensive care units. These factors lead to additional production of free radicals that generate oxidative damage [1,2,3,4,5,6,7]. Furthermore, newborns, especially premature ones, have a decreased antioxidant capacity [8, 9].
The imbalance between prooxidant factors and the antioxidant capacity of the organism causes tissue damage, leading to “oxygen radical diseases of the newborn”: respiratory distress syndrome (RDS), bronchopulmonary dysplasia (BPD), retinopathy of prematurity (ROP), hypoxic-ischemic disease (HID), intraventricular hemorrhage (IVH), or sepsis and necrotizing enterocolitis (NEC) [1, 4, 10,11,12,13]. Melatonin is a potent antioxidant and can effectively scavenge free radicals in newborns [9, 14].
When comparing the antioxidant capacity of melatonin with other antioxidants such as vitamin C, vitamin E, glutathione, and NADPH, melatonin proves to be more efficient in protecting against oxidative stress. One melatonin molecule can eliminate up to 10 reactive oxygen species (ROS) molecules, whereas classical antioxidants can eliminate one or fewer ROS molecules [14, 15]. The antioxidant properties of melatonin make it vital in situations of oxidative stress resulting from unfavorable environmental conditions, such as acidosis and hypotension [16].
During the early stages of pregnancy, the fetus relies on melatonin produced by the mother and placenta, which can cross barriers such as the placenta and blood-brain barrier [17]. The pineal gland produces negligible amounts of melatonin in newborns. Instead, it is primarily produced in organs like the intestine, skin, and bone marrow, where its synthesis and metabolism occur in the mitochondria [18,19,20].
PTNBs are at a higher risk of experiencing oxidative stress [2, 4] due to their low efficiency in the natural antioxidant system. This stress can result in diseases caused by oxidative damage, especially when the consumption and degradation of melatonin and antioxidants are higher than their synthesis and production capacity [14, 20]. It has been observed that even premature infants who do not have hypoxia can have elevated levels of oxygen radicals in their bodies. However, administering melatonin to such infants can help reduce lipid peroxidation. Studies have shown that melatonin can also help decrease oxidative damage and the frequency of diseases caused by free radicals, such as BPD, HIV, NEC, ROP, and sepsis [9, 10, 12].
Despite these contributions, few studies analyze melatonin blood levels in newborns, especially in PTNB [12, 21, 22]. Furthermore, almost all PTNBs experience perinatal asphyxia and hypoxia and often receive supplemental oxygen, which can significantly alter their melatonin levels [3, 23]. Therefore, we aimed to (1) analyze melatonin levels in PTNB without hypoxia-ischemia or oxygen supplementation; (2) study whether neonatal pain and other perinatal factors can influence circulating melatonin levels; and (3) analyze if these preterm infants without apparent oxidative damage and theoretically “healthy” (of which we do not know other references) develop oxygen radical diseases (BPD, ROP, HIV, NEC or sepsis) and to determine their relationship with neonatal plasma melatonin.
Materials and methods
Study design, eligible population, and inclusion criteria
A prospective cohort study was conducted on PTNB under 35 weeks of gestation who were admitted to the Neonatology Unit or Neonatal Intensive Care Unit (NICU) of the Torrecardenas University Hospital in Almeria, Spain. The neonatal ICU of this center provides services to a population of 741,000 people.
The inclusion criteria were preterm newborns < 35 weeks of gestation who did not require supplemental oxygen at 72 h of life and who maintained a SO2 > 92%. The exclusion criteria were (1) perinatal hypoxia, (2) Apgar test score < 7 at 5 min of life, (3) use of supplemental oxygen before 72 h of life with a FiO2 > 0.4, (4) presence of sepsis or arterial hypotension, (5) existence of confirmed metabolic disease, (6) evidence of congenital malformations, (7) need for surgical intervention, (8) diabetic mother, or (9) refusal of the parents to sign the informed consent or to be included in the study.
Data collection
The care protocols of the Neonatal Unit were adhered to. Therefore, all premature infants under 30 weeks of gestational age and those with apnea pauses received an initial dose of 20 mg/kg of caffeine citrate, followed by a maintenance dose of 5 mg per kilogram every 24 h.
Blood samples were taken on the third day after birth, between 8 and 9 AM, to minimize any impact on the body’s natural melatonin rhythm. None of the selected patients underwent any painful procedures during the 8 h prior to the blood collection.
The variables analyzed were (1) perinatal variables: gestational age at birth (GA), newborn weight (NBW), weight for the gestational age (WGA) [24], and presence of multiple gestation. Before the analytical extraction, the PTNB degree of pain was evaluated using the PIPP scale [25]. The respiratory assistance was also assessed on the third day of life: high flow, CPAP, or BIPAP. (2) Analytical variables: plasma melatonin (Elabscience competitive ELISA kit), human melatonin (MT) with ELISA Kit E-EL-H2016. After centrifugation, the samples were preserved at −20 °C until measurement, lactic acid, hemoglobin, urea, creatinine, GOT, and GPT. (3) Hemodynamic and respiratory variables: mean values of hemodynamic constants taken on the third day of life: heart rate (HR), respiratory rate (RR), and mean arterial blood pressure (MAP).
All preterm infants were followed up to record whether they presented any disease related to oxidative stress of prematurity: sepsis, BPD [26], HIV [27], NEC, and ROP [28].
We compared the different variables between the group of large preterm infants (GA < 32 weeks) and preterm infants 32–34 weeks to evaluate if there were differences between them.
Statistical study
Frequencies and percentages were used for qualitative variables and means, and standard deviation was used for quantitative variables. The chi-square test (χ2) was used to compare qualitative variables. The normality or non-normality of the variables was determined using the Kolgomorov-Smirnov test. Student’s t-test and the Mann-Whitney U test were used to compare the two groups of quantitative variables, and the Kruskal-Wallis test was used for quantitative variables. To compare ordinal variables, we used nonparametric tests. Linear regression analysis was used to evaluate the association of the different variables with melatonin levels, and logistic regression analysis was used to evaluate the variables associated with free radical diseases. All statistical analyses considered p-value < 0.05 statistically significant.
Ethical aspects
The mothers of the newborns gave their written consent before participating in the study. The study followed the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the Torrecardenas University Hospital, with reference code PI.DCC/MMC-2019.
Results
Sixty-seven PTNBs met the inclusion criteria. Six patients whose parents did not wish to participate in the study were excluded. Therefore, 61 PTNBs were included in the study. They were between 27 and 34 weeks of gestation, with a mean gestational age (GA) of 30.7 ± 2.0 OS (95% CI, 30.2–31.2 OS), and 29.5% had a GA < 30 OS. A total of 40 preterm infants (65.6%) were from single gestation and 21 (34.4%) from twin gestation. All preterm infants maintained StcO2 > 92% (97.5 ± 1.8%) without any supplemental oxygen supply, although 19 of them (31.1%) required some ventilatory support (4 high flow ventilation and 15 CPAP or BIPAP). All preterm infants had an Apgar at birth at 5 min greater than 6, and 88.5% had an Apgar test at 5 min of life of 9 or 10.
The mean melatonin on the third day of life in preterm infants without perinatal asphyxia and without oxygen needs was 33.8+/−12.01 pg/ml (95% CI, 30.5–37.2 pg/ml), with slightly lower values in preterm infants < 32 weeks without statistically significant differences (p = NS) (Table 1). When we compared three gestational age brackets: < 30 weeks (n = 18) vs. 30–31 weeks (n = 21) vs. 32–34 weeks (n = 22), the median melatonin and its minimum and maximum values were respectively 30.1 pg/ml (12.3–59.2 pg/ml) vs. 27.9 og/nk (18.0 pg/ml vs. 51.2 pg/ml) vs. 31.4 pg/ml (21.2–76.6 pg/ml). In the < 32 weeks group, there was a higher incidence of males; they more frequently had moderate or severe pain (PIPP > 5) (p = 0.02), more frequently required ventilatory support, were more frequently administered caffeine, had higher parenteral nutrition needs, and had a higher incidence of free radical diseases with low and identical capillary lactic acid levels in both study groups (Tables 1 and 2).
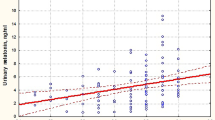
Preterm infants weighing < 1000 g (p = 0.002), preterm infants < 1250 g (p = 0.05), male preterm infants (p = 0.04), and preterm infants with moderate to severe pain (PIPP score > 5) (p = 0.01) had lower melatonin levels (Table 3). Figure 1 depicts the relationship between melatonin and the PIPP score.
Moderate or severe pain (PIPP score > 5) was found in 50.8% of preterm infants without asphyxia and oxygen needs. Presenting a PIPP score > 5 was associated with lower plasma melatonin levels (p = 0.03) (Table 4). Receiving caffeine treatment or parenteral nutrition was not associated with different plasma melatonin levels (p = NS), as seen in Tables 3 and 4.
23.7% of preterm infants between 27 and 34 OS without perinatal asphyxia and without oxygen needs presented some free radical disease (n = 14) (Table 4), 5 cases presented BPD, 11 cases of HIV (72% grade I), 11 cases clinical late sepsis, 2 cases NEC, and 2 ROP. We did not observe an association between plasma melatonin levels on the third day of life and the subsequent development of free radical disease. Table 4 describes the factors associated with the development of free radical disease.
Discussion
It is difficult to precisely determine the production capacity and circulating levels of melatonin in PTNB due to the need for studies on this group of children [12, 21, 22]. Melatonin levels can vary significantly due to factors that increase oxidative stress in PTEN. These factors include episodes of pain that increase free radicals, the requirement for supplemental oxygen, and an imbalance between melatonin consumption and production [3, 10, 14, 18, 29].
In our study, we found that preterm infants aged 27–34 weeks (GA, 30.7+/−2 weeks) on the third day of life had plasma melatonin levels of 33.8 ± 12.01 pg/ml (95% CI, 30.5–37.2 pg/ml). Our results agree with those observed by Marseglia et al. [12]. Table 1 shows that plasma melatonin levels are practically identical in preterm infants < 30 weeks and those born between 31 and 32 weeks. However, preterm infants < 32 weeks have lower plasma melatonin levels than those born between 32 and 34 weeks. Other authors have reported lower melatonin levels in preterm infants, with a more significant relationship between lower gestational age and melatonin levels in larger infants [21, 30].
The lower association of melatonin with gestational age and higher plasma melatonin levels in our study may be due to two aspects: (1) Our premature infants who did not have perinatal asphyxia and did not require oxygen had lower levels of oxidative stress and consumed less melatonin [1, 2, 10, 12, 14]. This aspect suggests that few preterm infants in our study had deficient melatonin levels. 9.8% had plasma melatonin levels < 10 pg/ml, whereas in the prospective multicenter study by Biran et al., 81% of their preterm infants < 34 weeks had plasma melatonin < 7 pg/ml on the third day of life. (2) In our study, there were very few preterm infants < 28 weeks or weighing < 1000 g (n = 7, 11.4%), which were those with the most significant oxidative damage and consequently would have a higher melatonin consumption [1, 10,11,12].
Concerning birth weight, we observed that preterm infants under 1250 g had lower melatonin levels than those over 1250 g (p = 0.05), and preterm infants < 1500 g have slightly lower melatonin levels (p = 0.06) (Table 2) with a decrease of 6.1 pg/ml of their melatonin levels (0.08). At lower weights, there were usually lower melatonin levels (r = 0.22, p = 0.07), as shown in Tables 3 and 4. Muñoz-Hoyos et al. [22] analyzed plasma melatonin levels in newborns with respiratory distress. They described newborns < 1500 g had significantly lower levels, probably because their study included term newborns, who usually have higher melatonin levels than PTNBs [31].
Preterm infants in the intensive care unit (ICU) undergo numerous painful procedures, increasing their oxidative stress levels (6.36). Although pain stimulates melatonin production in a physiological attempt to eliminate the free radicals generated, melatonin is often consumed to neutralize them [1, 32, 33]. Moderate or severe pain (PPIPP > 5) is present in 50.8% of preterm infants without asphyxia and oxygen needs, being especially frequent in preterm infants < 32 weeks (Table 2). We have observed that preterm infants with moderate–severe pain (PIPP > 5) have lower plasma melatonin levels (p = 0.01) (Table 3) and being preterm with PIPP > 5 was associated with lower plasma melatonin levels (p = 0.03) (Table 4).
The low levels of melatonin in PTNB with moderate to severe pain may be due to an imbalance between melatonin production and consumption. This imbalance results in higher consumption that cannot be counteracted by the immature antioxidant system of these children [6, 34]. This consideration may explain why melatonin administration has been shown to decrease pain and oxidative stress markers during painful procedures. Melatonin administration has also been observed to decrease the PIPP score in preterm infants [7, 33].
It has been reported that certain drugs, like caffeine, can elevate melatonin levels in the blood by competing for the same metabolic pathway [35]. Caffeine treatment was received by 43.5% of our preterm infants, and no differences in melatonin levels were observed between preterm infants who received caffeine and those who did not, likely due to the significant individual variability in the bioavailability of this drug and the hepatic metabolism in PTNB [29].
Parenteral nutrition is an exogenous source of free radicals, where oxidative stress can come from in vitro nutrient oxidation of solutions and in vivo reactions when intravenous prooxidant molecules are infused [5, 36]. Our study found that receiving parenteral nutrition did not impact melatonin levels in preterm infants who did not experience hypoxia. This finding may be explained because these infants are less exposed to free radicals, and their natural melatonin production is sufficient to counteract any exogenous free radicals. Additionally, protecting parenteral nutrition from light and adding mono- and polyunsaturated fatty acids (PUFA) minimizes free radical formation [37].
Low melatonin levels are one of the determinant factors in the development of free radical diseases (BPD, ROP, HIV, NEC, and sepsis) in PTNB [16, 30]. In preterm infants without hypoxia, free radical diseases also occur in 23.7% of cases [37]. We observed that most preterm infants can produce melatonin levels above 25 pg/ml at 72 h of life [12]. On the third day of life, there were no differences between the melatonin levels at third day of life of preterm infants who developed free radical disease (sepsis, ROP, BPD, HIV, or NEC) probably because many factors associated with free radical disease occurred after the third-day melatonin was measured (late sepsis in 18% of cases, enterocolitis, etc.). Melatonin was not associated with developing these diseases. These diseases were associated with lower gestational age, lower birth weight, lower Apgar test score at birth, and requiring mechanical ventilation or moderate–severe pain on the third day of life, as shown in Tables 3 and 4 [1, 8, 9, 37].
Deficiency of melatonin at the mitochondrial level may be the primary cause of free radical diseases, rather than plasma melatonin levels, as melatonin synthesis and metabolization occur significantly at the mitochondria [18,19,20]. Mitochondria contain much higher concentrations of melatonin in their granules compared to the melatonin levels found in the bloodstream [38]. It has been demonstrated that free radicals at the mitochondrial level can cause cellular injury implicated in the pathogenesis of free radical diseases due to oxidative self-injury and mitochondrial dysfunction [39, 40]. This reasoning is supported by the fact that premature infants treated with melatonin have a decreased incidence of free radical diseases [10, 12, 30, 41] when plasma melatonin levels 500–1000 times higher than physiological levels are reached. Since melatonin has a great facility to cross cell membranes [17], these doses may increase melatonin at the mitochondrial level, which could explain the clinical improvement and prevention of free radical disease seen with its administration.
Ongoing clinical trials, such as the one registered on Clinical Trial.gov (NCT04235773) [42], will help us better understand the effects of administering melatonin to preterm infants below 30 weeks of age. These trials will evaluate the pharmacokinetic and metabolic properties of melatonin at these ages and its effectiveness in preventing free radical diseases that commonly cause neurological morbidity and high mortality [43,44,45,46].
The study has some strengths: it followed a prospective longitudinal design, and the pain scale assessment was performed exclusively by two trained nurses, which reduced inter-subject variability. However, some limitations also exist. The most relevant is the presence of other factors that may affect melatonin levels. These factors include light stress due to light intensity in the neonatal ICU, acoustic stress, emotional stress from the kangaroo program, antibiotic treatment, free radicals present in parenteral nutrition, or other factors not accounted for in this study.
Conclusions
We observed that preterm infants with moderate to severe pain have lower melatonin levels. These findings are relevant because they reinforce the findings of other authors that melatonin supplementation decreases pain and oxidative stress in painful procedures in premature infants. To reduce the damage caused by oxidative stress, we should avoid unnecessary extractions and minimize pain in premature infants. Further studies are needed to evaluate whether melatonin could be used as an analgesic in painful procedures in preterm infants.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
Abbreviations
- BDP:
-
Bronchopulmonary dysplasia
- GA:
-
Gestational age at birth
- HID:
-
Hypoxic-ischemic disease
- IVH:
-
Intraventricular hemorrhage
- MAP:
-
Arterial blood pressure
- MT:
-
Human melatonin
- NEC:
-
Necrotizing enterocolitis
- NICU:
-
Neonatology Unit or Neonatal Intensive Care Unit
- PIPP:
-
Premature Infant Pain Profile
- PTNB:
-
Preterm newborns
- RDS:
-
Respiratory distress syndrome
- ROP:
-
Retinopathy of prematurity
- ROS:
-
Reactive oxygen species
- WGA:
-
Weight for the gestational age
References
Cannavò L, Perrone S, Viola V et al (2021) Oxidative stress and respiratory diseases in preterm newborns. Int J Mol Sci 22:12504. https://doi.org/10.3390/ijms222212504
Lembo C, Buonocore G, Perrone S (2021) Oxidative stress in preterm newborns. Antioxidants 10:1672. https://doi.org/10.3390/antiox10111672
Di Fiore JM, Vento M (2019) Intermittent hypoxemia and oxidative stress in preterm infants. Respir Physiol Neurobiol 266:121–129. https://doi.org/10.1016/j.resp.2019.05.006
Perrone S, Santacroce A, Longini M et al (2018) The free radical diseases of prematurity: from cellular mechanisms to bedside. Oxid Med Cell Longev 2018:1–14. https://doi.org/10.1155/2018/7483062
Karthigesu K, Bertolo RF, Brown RJ (2021) Parenteral nutrition and oxidant load in neonates. Nutrients 13:2631. https://doi.org/10.3390/nu13082631
Slater L, Asmerom Y, Boskovic DS et al (2012) Procedural pain and oxidative stress in premature neonates. J Pain 13:590–597. https://doi.org/10.1016/j.jpain.2012.03.010
Cannavò L, Perrone S, Marseglia L et al (2022) Potential benefits of melatonin to control pain in ventilated preterm newborns: an updated review. Pain Pract 22:248–254. https://doi.org/10.1111/papr.13069
Saugstad OD, Sejersted Y, Solberg R et al (2012) Oxygenation of the newborn: a molecular approach. Neonatology 101:315–325. https://doi.org/10.1159/000337345
Gitto E, Marseglia L, Manti S et al (2013) Protective role of melatonin in neonatal diseases. Oxid Med Cell Longev 2013:1–6. https://doi.org/10.1155/2013/980374
D’Angelo G, Chimenz R, Reiter RJ, Gitto E (2020) Use of melatonin in oxidative stress related neonatal diseases. Antioxidants 9:477. https://doi.org/10.3390/antiox9060477
de Almeida VO, Pereira RA, Amantéa SL et al (2022) Neonatal diseases and oxidative stress in premature infants: an integrative review. J Pediatr (Rio J) 98:455–462. https://doi.org/10.1016/j.jped.2021.11.008
Marseglia L, Gitto E, Laschi E et al (2021) Antioxidant effect of melatonin in preterm newborns. Oxid Med Cell Longev 2021:6308255. https://doi.org/10.1155/2021/6308255
D’angelo G, Cannavò L, Reiter RJ, Gitto E (2022) Melatonin administration from 2000 to 2020 to human newborns with hypoxic-ischemic encephalopathy. Am J Perinatol 39:824–829. https://doi.org/10.1055/s-0040-1719151
Tan D-X, Manchester LC, Esteban-Zubero E et al (2015) Melatonin as a potent and inducible endogenous antioxidant: synthesis and metabolism. Molecules 20:18886–18906. https://doi.org/10.3390/molecules201018886
Ortiz GG, Pacheco-Moisés FP, Gómez-Rodríguez VM et al (2013) Fish oil, melatonin and vitamin E attenuates midbrain cyclooxygenase-2 activity and oxidative stress after the administration of 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine. Metab Brain Dis 28:705–709. https://doi.org/10.1007/s11011-013-9416-0
Aly H, Elmahdy H, El-Dib M et al (2015) Melatonin use for neuroprotection in perinatal asphyxia: a randomized controlled pilot study. J Perinatol 35:186–191. https://doi.org/10.1038/jp.2014.186
Motta-Teixeira LC, Machado-Nils AV, Battagello DS et al (2018) The absence of maternal pineal melatonin rhythm during pregnancy and lactation impairs offspring physical growth, neurodevelopment, and behavior. Horm Behav 105:146–156. https://doi.org/10.1016/j.yhbeh.2018.08.006
Manchester LC, Coto-Montes A, Boga JA et al (2015) Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J Pineal Res 59:403–419. https://doi.org/10.1111/jpi.12267
Tan D-X, Manchester LC, Qin L, Reiter RJ (2016) Melatonin: a mitochondrial targeting molecule involving mitochondrial protection and dynamics. Int J Mol Sci 17. https://doi.org/10.3390/ijms17122124
Tan D-X, Reiter RJ (2019) Mitochondria: the birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Res 2:44–66
Biran V, Decobert F, Bednarek N et al (2019) Melatonin levels in preterm and term infants and their mothers. Int J Mol Sci 20. https://doi.org/10.3390/ijms20092077
Muñoz-Hoyos A, Bonillo-Perales A, Avila-Villegas R et al (2007) Melatonin levels during the first week of life and their relation with the antioxidant response in the perinatal period. Neonatology 92:209–216. https://doi.org/10.1159/000102957
Saugstad OD (1996) Mechanisms of tissue injury by oxygen radicals: implications for neonatal disease. Acta Paediatr 85:1–4. https://doi.org/10.1111/j.1651-2227.1996.tb13880.x
Stirnemann J, Villar J, Salomon LJ et al (2017) International estimated fetal weight standards of the INTERGROWTH-21st Project. Ultrasound Obstet Gynecol 49:478–486. https://doi.org/10.1002/uog.17347
Ballantyne M, Stevens B, McAllister M et al (1999) Validation of the premature infant pain profile in the clinical setting. Clin J Pain 15:297–303. https://doi.org/10.1097/00002508-199912000-00006
Higgins RD, Jobe AH, Koso-Thomas M et al (2018) Bronchopulmonary dysplasia: executive summary of a workshop. J Pediatr 197:300–308. https://doi.org/10.1016/j.jpeds.2018.01.043
Papile LA, Burstein J, Burstein R, Koffler H (1978) Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 92:529–534. https://doi.org/10.1016/s0022-3476(78)80282-0
Chiang MF, Quinn GE, Fielder AR et al (2021) International classification of retinopathy of prematurity. Third Ed Ophthalmol 128:e51–e68. https://doi.org/10.1016/j.ophtha.2021.05.031
Merchant NM, Azzopardi DV, Hawwa AF et al (2013) Pharmacokinetics of melatonin in preterm infants. Br J Clin Pharmacol 76:725–733. https://doi.org/10.1111/bcp.12092
Pavlyshyn Н, Sarapuk I, Kozak K (2023) The relationship of melatonin concentration in preterm infants and adverse outcomes in the late neonatal period. Biochem Med (Zagreb) 33:010706. https://doi.org/10.11613/BM.2023.010706
Pavlyshyn H, Sarapuk I, Kozak K (2022) Peculiarities of melatonin levels in preterm infants. Wien Klin Wochenschr. https://doi.org/10.1007/s00508-022-02109-9
Perrone S, Romeo C, Marseglia L et al (2023) Melatonin in newborn infants undergoing surgery: a pilot study on its effects on postoperative oxidative stress. Antioxidants 12:563. https://doi.org/10.3390/antiox12030563
Gitto E, Aversa S, Salpietro CD et al (2012) Pain in neonatal intensive care: role of melatonin as an analgesic antioxidant. J Pineal Res 52:291–295. https://doi.org/10.1111/j.1600-079X.2011.00941.x
Perrone S, Bellieni CV, Negro S et al (2017) Oxidative stress as a physiological pain response in full-term newborns. Oxid Med Cell Longev 2017:1–7. https://doi.org/10.1155/2017/3759287
Carloni S, Proietti F, Rocchi M et al (2017) Melatonin pharmacokinetics following oral administration in preterm neonates. Molecules 22:2115. https://doi.org/10.3390/molecules22122115
Perrone S, Salvi G, Bellieni CV, Buonocore G (2007) Oxidative stress and nutrition in the preterm newborn. J Pediatr Gastroenterol Nutr 45(Suppl 3):S178–S182. https://doi.org/10.1097/01.mpg.0000302968.83244.d2
Buonocore G, Perrone S, Longini M et al (2002) Oxidative stress in preterm neonates at birth and on the seventh day of life. Pediatr Res 52:46–49. https://doi.org/10.1203/00006450-200207000-00010
Tan D-X, Manchester LC, Liu X et al (2013) Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin’s primary function and evolution in eukaryotes. J Pineal Res 54:127–138. https://doi.org/10.1111/jpi.12026
Tarocco A, Caroccia N, Morciano G et al (2019) Melatonin as a master regulator of cell death and inflammation: molecular mechanisms and clinical implications for newborn care. Cell Death Dis 10:317. https://doi.org/10.1038/s41419-019-1556-7
Perez M, Robbins ME, Revhaug C, Saugstad OD (2019) Oxygen radical disease in the newborn, revisited: oxidative stress and disease in the newborn period. Free Radic Biol Med 142:61–72. https://doi.org/10.1016/j.freeradbiomed.2019.03.035
Häusler S, Robertson NJ, Golhen K et al (2023) Melatonin as a therapy for preterm brain injury: what is the evidence? Antioxidants 12:1630. https://doi.org/10.3390/antiox12081630
Garofoli F, Longo S, Pisoni C et al (2021) Oral melatonin as a new tool for neuroprotection in preterm newborns: study protocol for a randomized controlled trial. Trials 22:82. https://doi.org/10.1186/s13063-021-05034-w
Acuña-Castroviejo D, Escames G, Macías M et al (1995) Cell protective role of melatonin in the brain. J Pineal Res 19:57–63. https://doi.org/10.1111/j.1600-079x.1995.tb00171.x
Reiter R, Tang L, Garcia JJ, Muñoz-Hoyos A (1997) Pharmacological actions of melatonin in oxygen radical pathophysiology. Life Sci 60:2255–2271. https://doi.org/10.1016/s0024-3205(97)00030-1
Jerez-Calero A, Salvatierra-Cuenca MT, Benitez-Feliponi Á et al (2020) Hypothermia plus melatonin in asphyctic newborns: a randomized-controlled pilot study. Pediatr Crit Care Med 21:647–655. https://doi.org/10.1097/PCC.0000000000002346
Sánchez-Forte M, Moreno-Madrid F, Muñoz-Hoyos A et al (1997) The effect of melatonin as anti-convulsant and neuron protector. Rev Neurol 144:1229–1234
Funding
Funding for open access publishing: Universidad de Almería/CBUA.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by C.S.B., D.C.C., M.I.R.L., A.M.H., A.A., M.A.V.L., T.P.C., B.J.N.S., A.B.P, and J.C.B.P. The first draft of the manuscript was written by C.S.B., D.C.C., M.I.R.L., and A.B.P., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the Torrecardenas University Hospital, with reference code PI.DCC/MMC-2019.
Consent to participate
Informed consent was shown to all subjects involved in the study.
Consent for publication
All the participants of this study were informed about the research’s aims and nature. None of them objected to having their data published in a journal article.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Daniele De Luca
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sánchez-Borja, C., Cristóbal-Cañadas, D., Rodríguez-Lucenilla, M.I. et al. Lower plasma melatonin levels in non-hypoxic premature newborns associated with neonatal pain. Eur J Pediatr (2024). https://doi.org/10.1007/s00431-024-05632-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00431-024-05632-1