Abstract
This study compared short-term effectiveness of proton pump inhibitors (PPI), swallowed topical corticosteroids (STC), and dietary therapies in reversing clinical and histological features in pediatric patients with eosinophilic esophagitits (EoE). Determinants for treatment choice and PPI therapy effectiveness were also assessed. A cross-sectional study analysis of patients under 18 years old recruited onto the multicenter EoE CONNECT registry was performed. Clinico-histological response was defined as symptomatic improvement plus a peak eosinophil count below 15 per high-power field after treatment. Effectiveness of first-line options used in monotherapy was compared. Overall, 393 patients (64% adolescents) receiving PPI, STC, or dietary monotherapy to induce EoE remission were identified. PPI was the preferred option (71.5%), despite STC providing the highest clinico-histological response rates (66%) compared to PPI (44%) and diet (42%). Logistic regression identified fibrotic features and recruitment at Italian sites independently associated to first-line STC treatment; age under 12 associated to dietary therapy over other options. Analysis of 262 patients in whom PPI effectiveness was evaluated after median (IQR) 96 (70–145) days showed that this effectiveness was significantly associated with management at pediatric facilities and use of high PPI doses. Among PPI responders, decrease in rings and structures in endoscopy from baseline was documented, with EREFS fibrotic subscore for rings also decreasing among responders (0.27 ± 0.63 vs. 0.05 ± 0.22, p < 0.001).
Conclusion: Initial therapy choice for EoE depends on endoscopic phenotype, patient’s age, and patients’ origin. High PPI doses and treatment in pediatric facilities significantly determined effectiveness, and reversed fibrotic endoscopic features among responders.
What is Known: • Proton pump inhibitors are widely used to induce and maintain remission in EoE in real practice, despite other first-line alternative therapies possibly providing higher effectiveness. | |
What is New: • Proton pump inhibitors represent up to two-thirds of first-line monotherapies used to induce EoE remission in pediatric and adolescent patients with EoE. The choice of STC as first-line treatment for EoE was significantly associated with fibrotic features at baseline endoscopy and recruitment in Italian centers; age less than 12 years was associated with dietary therapy. • PPI effectiveness was found to be determined by use of high doses, attendance at pediatric facilities, presenting inflammatory instead of fibrotic or mixed phenotypes, and younger age. Among responders, PPI therapy reversed both inflammatory and fibrotic features of EoE after short-term treatment. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eosinophilic esophagitis (EoE) is a chronic, immune‐mediated inflammatory disease that is characterized by esophageal dysfunction and transmural infiltration by eosinophils restricted to the esophagus [1, 2], and generally triggered by exposure to dietary antigens [3]. EoE presents a chronic natural course in the vast majority of cases, and appears to be progressive, with long-standing eosinophilic inflammation leading to esophageal remodeling with stricture formation and functional damage [4,5,6]. Dysphagia and food impaction are the most characteristic symptoms in adults and adolescents, while symptoms reminding gastroesophageal reflux disease and feeding disturbances predominates in children [1, 7, 8].
The global prevalence of EoE currently exceed 60 cases per 100,000 inhabitants [9]; with a steady rise in EoE incidence and prevalence rates having been observed over time in patients of all ages [10]. Peak ages for EoE diagnosis are pediatric [11] and the third to fourth decades in life in adulthood [12]. Despite been a single disease across the age range, some symptoms are considered characteristic of pediatric EoE [13]; however, there is little research comparing the effectiveness of different treatment options for different age groups [7].
Anti-inflammatory first-line therapies for EoE consist of swallowed topical corticosteroids (STC), dietary modifications aimed at avoiding exposure to trigger foods, and proton pump inhibitors (PPIs) [14, 15]; an interleukine-4 receptor monoclonal antibody has recently been added to anti-inflammatory therapies [16]. Esophageal dilation should be considered a solution to fibrotic sequelae of EoE [17]. Despite its limited effectiveness of only 50% in inducing histological remission [18], off-label PPI therapy is currently the preferred initial therapy for EoE in patients of all ages and in most clinical settings [19,20,21,22,23,24]. This is likely due to drug availability, convenience, cost, and safety profile. However, the reasons determining treatment choice in pediatric EoE have not yet been analyzed, and determinants for PPI effectiveness in younger patients have been only limitedly assessed [25, 26].
In this study, we compare the short-term effectiveness of PPI, SCT, and dietary therapies in reversing clinical, histological, and, whenever possible, endoscopic features of EoE in pediatric patients, and aim to identify determinants for treatment choice and aspects underlying PPI therapy effectiveness in a large, collaborative, multicenter European registry.
Methods
Study design and data collection
The “European Registry of Clinical, Environmental and Genetic Determinants in Eosinophilic Esophagitis” (EoE CONNECT) is an international, multicenter, non-interventional registry initially promoted by United European Gastroenterology and currently supported by EUREOS, the European Consortium for Eosinophilic Diseases of the Gastrointestinal Tract (www.eureos.online).
A cross-sectional analysis of EoE CONNECT was performed [27]; patients aged younger than 18 years old and diagnosed with EoE based on evidence-based [1] and AGREE consensus criteria [2], and who received PPIs, STC, or any dietary interventions as first-line monotherapy to induce EoE remission were identified. Prospective clinical and demographic data from EoE patients had been imputed onto the registry by practitioners during face-to-face clinical appointments.
Variables collected for this study were sex, age, time of diagnosis, country of recruitment, type of hospital facility (pediatric or adult), EoE phenotype, histology findings, endoscopic features, and treatment response. Definitions, detailed study protocols and operational procedures of EoE CONNECT have been published elsewhere [27]. EoE CONNECT runs in accordance with the Declaration of Helsinki and has been approved by La Princesa University Hospital Research Ethics Committee (as central Committee) and Ethics Committees at all participating sites; assent and written informed consent to participate in the EoE CONNECT project was obtained, respectively, from all patients and their legal guardians.
Main definitions
Histological remission was defined as an eosinophil peak count of < 5 eosinophils/high power field (eos/hpf) at all esophageal levels after therapy; histological response was considered as a peak count between 5 and 14 eos/hpf. Symptomatic improvement in adolescents was assessed by changes in the Dysphagia Symptom Score (DSS) [27], reported by patients or by clinicians’ perception. For younger children, any subjective improvement in symptoms reported by either children or parents was considered as a clinical response. In addition, clinicians semi-quantitatively expressed changes in symptoms from treatment initiation as a clinical response or no response.
Clinico-histological response was defined as the simultaneous combination of symptomatic improvement and histological response (peak eosinophil count below 15 eos/hpf) in the same patient after therapy; clinico-histological remission was defined as any symptomatic improvement together with < 5 eos/hpf after therapy.
Endoscopic features in the esophagus were graded by the presence and severity of edema, rings, exudates, furrows, and stricture(s) in accordance with the EREFS grading scoring system [28]. Rings and strictures were classified as fibrotic features, while edema, furrows, and exudates were defined as inflammatory ones [29].
Standard doses of PPI included omeprazole 20 mg, pantoprazole 40 mg, esomeprazole 20 mg, lansoprazole 30 mg, and rabeprazole 20 mg daily. Double doses or higher were considered high-dose PPI, and a low dose was defined when PPIs were given at standard doses or below [30,31,32,33]. For children under 12 years, the dosage in mg/kg was estimated by dividing the daily PPI dose by expected weight for age and sex in the pediatric population [34, 35].
Statistical analysis
Means and standard deviations (or medians and interquartile ranges for non-normal variables) were reported for continuous variables, and proportions for categorical data. Frequency tables were generated for first-line treatment choice and effectiveness (clinico-histological response). Contingency tables to assess demographic and clinical factors influencing treatment choice and effectiveness were produced and analyzed by chi-square or Fisher exact (univariate) tests.
A binary logistic multivariate regression analysis was performed to assess demographical and clinical factors influencing treatment response rates of PPI treatment identified in univariate analyses. Additionally, a multinomial logistic regression was performed to assess demographic and clinical factors influencing first-line treatment choice identified in univariate analyses. Odds ratio (OR) and their 95% confidence interval (95%CI) were reported for those variables reaching statistical significance in each model.
Changes from baseline in EREFS score and sub-scores induced by PPI therapy were compared by the McNemar test (qualitative variables) and paired t-test (quantitative variables).
All analyses were carried out using PASW v18.0 statistical analysis software (SPSS Inc, Chicago, ILL, USA). A p-value < 0.05 was considered significant.
Results
Demographic and clinical characteristics of pediatric patients with EoE included in the study
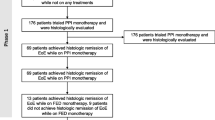
On the search date, August 25th, 2023, 416 patients registered in EoE CONNECT and recruited across 29 study sites had received their first treatment for EoE when under 18 years old. PPIs were the main first-line treatment option (67.5%), while combination treatments were rare (5.3%) (Suppl Table 1). The main demographic and clinical characteristics of the 393 EoE patients treated with the three most common options (PPIs, STCs, and dietary interventions) in monotherapy were summarized in Table 1. They were mainly male (80%), adolescents between 12 and 18 years old (64%), with an inflammatory phenotype (90%) lacking endoscopic features of fibrosis (68%), and recruited in adult facilities (78%) at Spanish sites (84%).
Choice and effectiveness of first-line therapy
Among PPIs, omeprazole was the preferred drug (37%), used at 20 mg twice daily (43% of omeprazole treatments) (Suppl Table 2). As for STC, fluticasone propionate was more frequently prescribed than budesonide, with metered-dose formulation (inhalation or spray devices applied in the mouth and then swallowed), being the most common choice (34%) (Suppl Table 3). Finally, the vast majority of first-line dietary interventions were empiric elimination diets (77%) (Suppl Table 4).
Next, we evaluated and compared clinical and histological response rates to PPIs, STCs, and diets used in monotherapy (Table 2). No differences were found for clinical response overall, being 72%, 86%, and 73%, respectively (p = 0.178). Regarding histological endpoints, STC were the most effective choice, with 65% patients overall achieving < 15 eos/hpf, higher than the 43% and 42% obtained, respectively, for PPIs and diets (p = 0.017). Finally, and concordant with these results, clinico-histological response (clinical improvement together with < 15 eos/hpf) was better for STCs (66%) than for PPIs and diets (44% and 42%, respectively) (p = 0.018); with most patients (60%, 35%, and 28% of those respectively treated with SCT, PPIs, and diets) achieving clinico-histological remission (symptomatic improvement together with < 5 eos/hpf in esophageal biopsies).
Determinant factors involved in the selection of first-line therapy for pediatric EoE
Six variables were analyzed by univariate analysis to evaluate clinical and demographic factors determining first-line treatment choice: sex, age, EoE phenotype, features of fibrosis at baseline endoscopy, country, and recruiting facility (Table 2). STC treatments were preferably used in patients with a mixed/stricturing phenotype (p = 0.023), and when fibrotic features were found in baseline endoscopy (p = 0.001). Conversely, PPI prescriptions were more common in pediatric facilities compared to adult ones (p = 0.024). In addition, a trend towards more common use of dietary interventions in children under 12 years old, compared to adolescents, was also observed (p = 0.056).
A multinomial logistic regression model, adjusted by sex and type of facility (pediatric or adult), confirmed the relevance of these findings (Table 3). Being younger than 12 years old was associated with a preferred use of dietary therapy over drugs (PPI or STC) (p = 0.034). The presence of fibrotic features at baseline endoscopy and being recruited in Italy, rather than Spain, were independently associated with the use of STC over PPI or diet (p < 0.001 and p = 0.003, respectively).
PPI treatment to induce clinico-histological remission and determinants for effectiveness
Given that PPI therapy was the preferred first-line option, and the highest number of patients belonged to this group, subsequent sub-analyses were performed to determine which variables could influence PPI effectiveness, measured as clinico-histological response. For this purpose, we identified 29 additional patients who received PPIs as second-line treatment, after failure to respond to STCs or dietary options. Of these, 23 were fully evaluated for clinical and histological responses, and they presented a lower response rate than when PPIs were used in first-line (30% vs. 44%), despite the difference not being significant (p = 0.211). Therefore, 262 patients with their clinico-histological response assessed were included in these analyses.
First, univariate statistical analyses were performed on variables affecting the PPI treatment itself (Table 4). The only variable associated with higher clinico-histological response was PPI dose, either as being high (double doses or higher) in the overall pediatric population (p = 0.030), or being higher than 1 mg/kg in those children under 12 years old (p = 0.041). Additional variables considered (type of PPI drug, intakes per day, days until evaluation, and line of treatment) did not significantly influence effectiveness. Next, similar analyses were undertaken on clinical and demographical variables (Table 5). Here, the type of recruiting facility was the only factor associated with the response to PPIs, as pediatric sites showed better response rates than adult ones (59% vs. 37%, p = 0.002).
When these variables were included in a multivariate analysis, recruitment in a pediatric facility and the use of high PPI doses significantly associated with improved clinico-histological response to PPI therapy (p = 0.002 and p = 0.033, respectively) (Table 6). When analyses were adjusted by line of treatment and recruitment country, two more variables — age under 12 years old and having an inflammatory phenotype — showed a trend towards improved response (p = 0.051 and p = 0.085, respectively) (Table 6).
Effectiveness of induction PPI treatment to reverse fibrotic endoscopic features short term
From those patients treated with PPIs to induce EoE remission as first- or second-line therapy, 146 had data registered on EREFS score for both baseline and post PPI endoscopies, including 43% PPI-responders and 57% non-responders (Table 7). Reduction in total EREFS score from baseline was higher among responders (2.63 ± 1.56 vs. 0.40 ± 0.66; p < 0.001) compared to non-responders (2.94 ± 1.29 vs. 2.52 ± 1.53; p = 0.050).
When changes in EREFS inflammatory subscore components were analyzed, all the features (edema, furrows, and exudates) experienced a significant reduction from baseline (p < 0.001), while no significant changes were observed in non-responders. More importantly, EREFS fibrotic subscore for rings also decreased among responders (0.27 ± 0.63 vs. 0.05 ± 0.22, p = 0.005). Strictures were present at baseline in only three pediatric PPI-responder patients, and disappeared in all of them after PPI therapy. However, no changes for rings and strictures were noted among non-responders, and even an increase in the latter was found (Table 7).
Discussion
This is the first analysis of the EoE CONNECT database focused on assessing treatment effectiveness exclusively in the pediatric population. To our knowledge, this is one of the largest real-world practice-based studies defining effectiveness of treatment in EoE in real life, and the first one to provide evidence on determinants for treatment choice, and on the effectiveness of PPIs in pediatric populations. According to our data, PPI monotherapy was the preferred first-line treatment choice in pediatric EoE patients, with an overall effectiveness similar to dietary options, but lower than STC. Treatment choice depended on patients’ age (with diets being more commonly used in younger children), endoscopic phenotypes at baseline (PPI being preferred in patients with inflammatory-predominant features at initial endoscopy), and country of patient’s origin (with PPIs being predominantly prescribed as a first-line choice in Spain). Effectiveness of PPI treatment was linked to the use of high doses and disease management by pediatric care facilities.
Our study reproduces results found in the literature previously [25], which shows that despite their limited effectiveness, PPIs are the main treatment option for EoE in both adults [19,20,21, 24] and children [20, 22, 23, 25]. Although there are more effective alternatives, clinicians seem to avoid pharmacological options based on STC in the initial therapeutic approach to EoE, and this trend is also reproduced among pediatricians. The effectiveness of PPIs in terms of clinical-histological remission in children, in general, parallels the figures reported in adults, although our results deserve further comment: as in previous reports, higher doses (both overall and adjusted by weight for age) achieved better results. However, we could not demonstrate that the number of doses into which the daily dose is divided was relevant. A recent study on 305 adults with EoE treated with PPI showed that doses of 20 mg of omeprazole per day, divided into two doses, were significantly superior to a single daily dose, with higher doses not achieving better results [36]. As previously shown in adults [19], the different PPI drugs used at equivalent doses provided comparable effectiveness. However, unlike what has been documented in adults, we were not able to demonstrate that prolonging treatment length beyond 8 weeks and up to 12 weeks associated with greater effectiveness [19], largely because few patients used short treatment times (the median time of PPI treatment until response assessment was 96 days).
Our work is the first, to our knowledge, that attempts to define reasons for the choice of first-line PPI therapy in younger EoE patients. Previous analyzes of the EoE CONNECT registry, on a predominantly adult population, had already shown significant differences in the choice of first-line therapies according to patients’ age, referral hospital, and EoE phenotype [37]. The present study shows that these factors also determined treatment choice in populations under 18 years old. The limited treatment strategies for EoE made it predictable that determinants would concur across different age groups in sites contributing to the same registry.
The other relevant result of this analysis is that we evaluated, for the first time, the ability of PPIs to reverse esophageal fibrosis features in children with EoE. Few patients showed rings or strictures at baseline endoscopy (only 32.5% of our series), thus coinciding with other reports in children [26], or were classified by their physicians as having a stricturing or mixed phenotype (10%), which was likely due to the short diagnostic delay in our cohort (only 2 years on average). However, the prevalence of both rings and strictures significantly reduced among PPI responders, while they showed no change among non-responders. These data confirm that PPIs also constitute an effective treatment for reversing the fibrosis associated with EoE, in the short term, in children. This had previously been demonstrated in a large series of adults [38], in whom endoscopic reversal of fibrosis was associated with an increase in esophageal caliber, measured by planimetry with endoFLIP [39] in a subgroup of cases.
The strengths of our study include the use of a large, multicenter registry of patients with EoE prospectively recruited at several European hospitals. All patients started EoE therapy at an age younger than 18-year-old, and data were prospectively collected for the majority. Treatment effectiveness was objectively evaluated in the vast majority of patients with homogeneous histological, clinical, and endoscopic criteria. However, some limitations should be also acknowledged, as our data came from somewhat expert facilities in the management of EoE and most patients were recruited at Spanish sites, and therefore not necessarily representative of practice patterns in other places. A reduced number of patients received STC or dietary interventions as first-line treatment for EoE, which may affect real effectiveness of these therapies. Furthermore, very few patients used combination therapies, and this prevented establishing their potential advantages over monotherapy. Anyway, no guidelines recommend such practice, and this is reflected in the expertise of recruiting sites in EoE CONNECT. The DSS score is not validated for measuring symptoms of EoE in children and adolescents, despite its demonstrated ability to capture changes induced by therapy [19, 37, 40, 41], even in randomized placebo-controlled trials [42]. Adherence to EoE treatment was not systematically assessed, and no efforts were made to determine medication compliance beyond the usual advice in each physician’s practice [27]; Therefore, the expected effectiveness of each intervention in a conventional clinical use setting is provided. Although we endeavored to thoroughly record and ensure data quality, a proportion of patients had missing data and bias in the information included cannot be ruled out. Finally, multivariate analysis was performed for the clinico-histological response endpoint, symptomatic improvement together with < 15 eos/hpf, as more strict criteria are not required for practice-based observational studies [43].
In conclusion, our results provide comparative effectiveness of first-line anti-inflammatory therapies in children and adolescents with EoE, and establish that the choice of initial therapy depends on the endoscopic features of the disease, patient’s age, and the country of origin of the patients. The effectiveness of PPI treatment was directly related to the use of high doses and management in pediatric facilities; among responders, this treatment option was able to significantly reverse the fibrotic findings associated with the disease. These data should be considered to inform future clinical practice guidelines and recommendations for the treatment of EoE in the pediatric age.
Data Availability
No datasets were generated or analysed during the current study.
Abbreviations
- CI:
-
Confidence interval
- DSS:
-
Dysphagia Symptom Score
- EoE:
-
Eosinophilic esophagitis
- IQR:
-
Interquartile range
- OR:
-
Odds ratio
- PPI:
-
Proton pump inhibitors
- STC:
-
Swallowed topical corticosteroids
References
Lucendo AJ, Molina-Infante J, Arias A et al (2017) Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United Eur Gastroenterol J 5:335–358. https://doi.org/10.1177/2050640616689525
Dellon ES, Liacouras CA, Molina-Infante J et al (2018) Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference. Gastroenterology 155:1022–1033. https://doi.org/10.1053/j.gastro.2018.07.009
Feo-Ortega S, Lucendo AJ (2022) Evidence-based treatments for eosinophilic esophagitis: insights for the clinician. Ther Adv Gastroenterol 15:17562848211068664. https://doi.org/10.1177/17562848211068665
Schoepfer AM, Safroneeva E, Bussmann C et al (2013) Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology 145(1230–1236):e1-2. https://doi.org/10.1053/j.gastro.2013.08.015
Lipka S, Kumar A, Richter JE (2016) Impact of diagnostic delay and other risk factors on eosinophilic esophagitis phenotype and esophageal diameter. J Clin Gastroenterol 50:134–140. https://doi.org/10.1097/MCG.0000000000000297
Dellon ES, Kim HP, Sperry SLW et al (2014) A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc 79:577-585.e4. https://doi.org/10.1016/j.gie.2013.10.027
Laserna-Mendieta EJ, Navarro P, Casabona-Francés S et al (2023) Differences between childhood- and adulthood-onset eosinophilic esophagitis: an analysis from the EoE connect registry. Dig Liver Dis 55:350–359. https://doi.org/10.1016/j.dld.2022.09.020
Hoofien A, Dias JA, Malamisura M et al (2019) Pediatric eosinophilic esophagitis: results of the European Retrospective Pediatric Eosinophilic Esophagitis Registry (RetroPEER). J Pediatr Gastroenterol Nutr 68:552–558. https://doi.org/10.1097/MPG.0000000000002215
Hahn JW, Lee K, Shin JI et al (2023) Global incidence and prevalence of eosinophilic esophagitis, 1976–2022: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 21:3270–3284. https://doi.org/10.1016/j.cgh.2023.06.005
Arias Á, Lucendo AJ (2020) Epidemiology and risk factors for eosinophilic esophagitis: lessons for clinicians. Expert Rev Gastroenterol Hepatol 14:1069–1082. https://doi.org/10.1080/17474124.2020.1806054
Robson J, O’Gorman M, McClain A et al (2018) Incidence and prevalence of pediatric eosinophilic esophagitis in Utah based on a 5-year population-based study. Clin Gastroenterol Hepatol 17:107–114. https://doi.org/10.1016/j.cgh.2018.06.028
Arias Á, Lucendo AJ (2019) Incidence and prevalence of eosinophilic oesophagitis increase continiously in adults and children in Central Spain: a 12-year population-based study. Dig Liver Dis 51:55–62. https://doi.org/10.1016/j.dld.2018.07.016
Visaggi P, Savarino E, Sciume G et al (2021) Eosinophilic esophagitis: clinical, endoscopic, histologic and therapeutic differences and similarities between children and adults. Therap Adv Gastroenterol 14:1756284820980860
Hirano I, Chan ES, Rank MA et al (2020) AGA Institute and the Joint Task Force on Allergy-Immunology Practice Parameters Clinical Guidelines for the Management of Eosinophilic Esophagitis. Gastroenterology 158:1776–1786. https://doi.org/10.1053/j.gastro.2020.02.038
Visaggi P, Barberio B, Del Corso G et al (2023) Comparison of drugs for active eosinophilic oesophagitis: systematic review and network meta-analysis. Gut 72:2019–2030. https://doi.org/10.1136/gutjnl-2023-329873
Tamarit-Sebastian S, Ferrer-Soler FM, Lucendo AJ (2022) Current options and investigational drugs for the treatment of eosinophilic esophagitis. Expert Opin Investig Drugs 1–18. https://doi.org/10.1080/13543784.2022.2033207
Aceves SS, Alexander JA, Baron TH et al (2022) Endoscopic approach to eosinophilic esophagitis: American Society for Gastrointestinal Endoscopy Consensus Conference. Gastrointest Endosc 96:576-592.e1. https://doi.org/10.1016/j.gie.2022.05.013
Munoz-Persy M, Lucendo AJ (2018) Treatment of eosinophilic esophagitis in the pediatric patient: an evidence-based approach. Eur J Pediatr 177:649–663. https://doi.org/10.1007/s00431-018-3129-7
Laserna-Mendieta EJ, Casabona S, Guagnozzi D et al (2020) Efficacy of proton pump inhibitor therapy for eosinophilic oesophagitis in 630 patients: results from the EoE connect registry. Aliment Pharmacol Ther 52:798–807. https://doi.org/10.1111/apt.15957
Tourlamain G, Garcia-Puig R, Gutiérrez-Junquera C et al (2020) Differences in management of eosinophilic esophagitis in Europe: an assessment of current practice. J Pediatr Gastroenterol Nutr 71:83–90. https://doi.org/10.1097/MPG.0000000000002672
Chang JW, Saini SD, Mellinger JL et al (2019) Management of eosinophilic esophagitis is often discordant with guidelines and not patient-centered: results of a survey of gastroenterologists. Dis Esophagus Off J Int Soc Dis Esophagus 1(32):doy133. https://doi.org/10.1093/dote/doy133
Hannan N, Steel A, McMillan SS, Tiralongo E (2020) Health service use and treatment choices for pediatric eosinophilic esophagitis: findings from a cross-sectional survey of Australian carers. Front Pediatr 8:147. https://doi.org/10.3389/fped.2020.00147
Zifman E, Banai H, Shamir R et al (2018) Practice differences in the diagnosis and management of eosinophilic esophagitis among adult and pediatric gastroenterologists in Israel. J Pediatr Gastroenterol Nutr 67:34–39. https://doi.org/10.1097/MPG.0000000000001909
Miehlke S, von Arnim U, Schlag C et al (2019) Clinical management of eosinophilic esophagitis — a nationwide survey among gastroenterologists in Germany. Z Gastroenterol 57:745–752. https://doi.org/10.1055/a-0885-1963
Gutiérrez-Junquera C, Fernández-Fernández S, Domínguez-Ortega G et al (2023) Proton pump inhibitor therapy in pediatric eosinophilic esophagitis: predictive factors and long-term step-down efficacy. J Pediatr Gastroenterol Nutr 76(2):191–198. https://doi.org/10.1097/MPG.0000000000003660
Oliva S, Dias JA, Rea F et al (2022) Characterization of eosinophilic esophagitis from the European Pediatric Eosinophilic Esophagitis Registry (pEEr) of ESPGHAN. J Pediatr Gastroenterol Nutr 75:325–333. https://doi.org/10.1097/MPG.0000000000003530
Lucendo A, Santander C, Savarino E et al (2022) EoE CONNECT, the European registry of clinical, environmental and genetic determinants in eosinophilic esophagitis: Rationale, design and study protocol of a large-scale epidemiological study in Europe. Ther Adv Gastroenterol
Hirano I, Moy N, Heckman MG et al (2013) Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: Validation of a novel classification and grading system. Gut 62:489–495. https://doi.org/10.1136/gutjnl-2011-301817
Lucendo AJ, Arias A, Molina-Infante J, Arias-Gonzalez L (2017) The role of endoscopy in eosinophilic esophagitis: from diagnosis to therapy. Expert Rev Gastroenterol Hepatol 11:1135–1149. https://doi.org/10.1080/17474124.2017.1367664
Armstrong D, Marshall JK, Chiba N (2005) Canadian Consensus Conference on the management of gastroesophageal reflux disease in adults — update 2004. Can J Gastroenterol Hepatol 19:15–35. https://doi.org/10.1155/2005/836030
Graham DY, Tansel A (2018) Interchangeable use of proton pump inhibitors based on relative potency. Clin Gastroenterol Hepatol 16:800–808.e7. https://doi.org/10.1016/j.cgh.2017.09.033
Klok RM, Postma MJ, van Hout BA, Brouwers JRBJ (2003) Meta-analysis: comparing the efficacy of proton pump inhibitors in short-term use. Aliment Pharmacol Ther 17:1237–1245. https://doi.org/10.1046/j.1365-2036.2003.01562.x
Kirchheiner J, Glatt S, Fuhr U et al (2009) Relative potency of proton-pump inhibitors-comparison of effects on intragastric pH. Eur J Clin Pharmacol 65:19–31. https://doi.org/10.1007/s00228-008-0576-5
Carrascosa A, Yeste D, Moreno-Galdó A et al (2018) Índice de masa corporal e índice de masa triponderal de 1.453 niños no obesos ni malnutridos de la generación del milenio. Estudio longitudinal de Barcelona An Pediatría 89:137–143. https://doi.org/10.1016/j.anpedi.2017.12.016
Carrascosa Lezcano A, Fernández-Cancio M, Yeste D et al. Milennials’ Grouth. Estudio longitudinal de crecimiento. Barcelona 1995–2017
Muftah M, Goldin AH, Barshop K et al (2024) Twice daily PPI induces higher remission rate in eosinophilic esophagitis than once daily regimen regardless of total daily dose. Am J Gastroenterol 119:991–995. https://doi.org/10.14309/ajg.0000000000002712
Laserna-Mendieta EJ, Casabona S, Savarino E et al (2020) Efficacy of therapy for eosinophilic esophagitis in real-world practice. Clin Gastroenterol Hepatol 18:2903-2911.e4. https://doi.org/10.1016/j.cgh.2020.01.024
Navarro P, Laserna-Mendieta EJ, Guagnozzi D et al (2021) Proton pump inhibitor therapy reverses endoscopic features of fibrosis in eosinophilic esophagitis. Dig Liver Dis 53:1479–1485. https://doi.org/10.1016/j.dld.2021.05.025
Casabona-Francés S, Sanz-García A, Ortega GJ et al (2024) A new method to evaluate lower esophageal distension capacity in eosinophilic esophagitis by using functional lumen imaging probe (EndoFLIP™). Diagn Basel Switz 14:218. https://doi.org/10.3390/diagnostics14020218
Gonsalves N, Yang G-Y, Doerfler B et al (2012) Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology 142:1451–9.e1; quiz e14–15. https://doi.org/10.1053/j.gastro.2012.03.001
Laserna-Mendieta EJ, Navarro P, Casabona-Francés S et al (2024) Swallowed topical corticosteroids for eosinophilic esophagitis: Utilization and real-world efficacy from the EoE CONNECT registry. United Eur Gastroenterol J. https://doi.org/10.1002/ueg2.12533
Miehlke S, Hruz P, Vieth M et al (2016) A randomised, double-blind trial comparing budesonide formulations and dosages for short-term treatment of eosinophilic oesophagitis. Gut 65:390–399. https://doi.org/10.1136/gutjnl-2014-308815
Ma C, Schoepfer AM, Dellon ES et al (2021) Development of a core outcome set for therapeutic studies in eosinophilic esophagitis (COREOS). J Allergy Clin Immunol S0091674921010599. https://doi.org/10.1016/j.jaci.2021.07.001
Acknowledgements
EoE CONNECT was established with funds from the United European Gastroenterology (National Societies Link Award Program), and is supported by the European Consortium for Eosinophilic Diseases of the GI Tract (EUREOS).
Author information
Authors and Affiliations
Contributions
Guarantor of article: Alfredo J Lucendo; specific author contributions: Pilar Navarro, Emilio J Laserna‐Mendieta and Alfredo J Lucendo: study design and writers; Pilar Navarro and Emilio J Laserna‐Mendieta: database monitoring and quality assessment; Pilar Navarro, Emilio J Laserna‐Mendieta and Ángel Arias: data extraction and analysis. All authors collected and registered the data, and approved the final version of the article, including the authorship list.
Corresponding author
Ethics declarations
Competing interests
The authors declare that there are no conflicts of interest.
Additional information
Communicated by Peter de Winter
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Navarro, P., Feo‐Ortega, S., Casabona‐Francés, S. et al. Determinant factors for first-line treatment choice and effectiveness in pediatric eosinophilic esophagitis: an analysis of the EUREOS EoE CONNECT registry. Eur J Pediatr (2024). https://doi.org/10.1007/s00431-024-05618-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00431-024-05618-z