Abstract
Infective endocarditis (IE) is a rare disease in children and is associated with significant morbidity and mortality. In recent years, significant changes have occurred in pediatric care that could have influenced the microbiology and presentation of IE. The aim of this work was to study epidemiological, microbiological, and clinical features of IE treated at a Pediatric Cardiac Surgery Reference Center located in Madrid (Spain) in a 10-years’ period. A descriptive observational retrospective study was performed, including pediatric patients < 16 years old with definite or possible IE admitted to a reference center between January 2012 and December 2021. Thirty-two IE episodes were identified. Twenty-eight (87.5%) had congenital heart disease (CHD), 8 (25.0%) were preterm infants, 1 (3.1%) was immunocompromised and 6 (18.8%) had other chronic conditions; in 11 (34.4%) episodes more than one underlying condition was associated. In 20 (62.5%) episodes there was an indwelling central venous catheter (CVC); children with other comorbidities (preterm, immunocompromised, other chronic conditions) were more likely to have a CVC at diagnosis compared with patients with isolated CHD (p < 0.001). Thirty-six microbiological isolates were obtained in the 32 episodes; 4 (12.5%) episodes had 2 isolated microorganisms. Microbiological isolates were 20 (55.6%) Gram-positive bacteria (GPB), 10 (27.8%) non-HACEK Gram-negative bacteria (GNB), 1 (2.8%) HACEK-group bacterium, 4 (11.1%) fungi and 1 (2.8%) Coxiella burnetii. In 10 (31.3%) episodes, patients were colonized by multidrug-resistant bacteria (MDRB) and the etiology of IE in 3 (30.0%) of those episodes was the colonizing MDRB. MDRB colonization was associated with MDRB IE (p = 0.007). The most common complication was septic embolism: 11 (34.4%) episodes (9 pulmonary and 2 cerebral). In-hospital mortality was 6.3% (n = 2), all of them due to underlying conditions and not to IE or its complications. Clinical features and complications of IE episodes caused by non-HACEK GNB and those caused by GPB were compared, finding no statistically significant differences.
Conclusion: Risk factors for developing IE, the proportion of embolic complications, and mortality rate were consistent with previously published findings. Proportion of IE cases attributed to non-HACEK GNB was higher than previously reported, suggesting an evolving epidemiology of IE. One-third of children colonized with MDRB subsequently developed IE caused by the same MDRB strains, so empirical coverage of MDRB organisms must be considered when IE is suspected in MDRB colonized patients. No significant differences in clinical features and complications were observed when comparing IE episodes caused by non-HACEK GNB and those caused by GPB, however larger cohort studies are needed.
What is Known: • Infective endocarditis (IE) is a rare disease in children, associated with significant morbidity and mortality. • The main risk factor for developing IE in children is an underlying congenital heart disease. | |
What is New: • With current changing epidemiology in pediatric IE, a higher proportion of IE caused by non-HACEK Gram-negative bacteria should be expected. • A significant percentage of children colonized by multidrug-resistant bacteria can develop an IE due to those bacteria. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infective endocarditis (IE) is an infection of the cardiac endothelium that has low incidence in the pediatric population; however, it has an important morbidity and mortality (described mortality of 5–10% [1, 2]).
Congenital heart disease (CHD) is currently the main risk factor to develop IE in children (as much as 15–140 times higher compared to general population [3, 4]), due to the increase in survival in these patients that currently undergo surgical corrections that previously were not technically possible [5, 6] and the presence of prosthetic material after corrective surgery [7, 8]. However, with the increasing complexity in healthcare in pediatrics, a rise of IE in pediatric patients without CHD has been described [2], being other risk factors associated with pediatric IE prolonged indwelling central venous catheter (CVC) [9,10,11], immunocompromised patients (especially those receiving oncological treatment [12] or hematopoietic stem cell transplantation (HSCT) recipients [13]) or pediatric patients admitted to neonatal or pediatric intensive care units (NICU, PICU) that undergo invasive procedures [14, 15].
Staphylococcus aureus and viridans streptococci are still the leading causes of pediatric IE [2, 4, 5], nonetheless in recent years there has been an increase in pediatric IE caused by other less frequent microorganisms, such as coagulase-negative staphylococci (CoNS), Gram-negative bacteria (GNB), and fungi [11, 16]. Risk factors for CoNS IE are prosthetic material and prolonged indwelling CVC [5]. Neonatal period and prolonged indwelling CVC are risk factors for GNB IE [5]. NICU admission (especially in preterm infants), cancer patients, and prolonged indwelling CVC are well described risk factors for fungal IE [17, 18].
In pediatric IE, the complication with the highest morbidity is septic embolism, which can occur up to 40% of the cases (higher risk than adult population) [19]. The reported in-hospital mortality in pediatric series of IE ranges from 5 to 10%; with higher rates in patients with S. aureus IE, preterm infants (31%), patients with tetralogy of Fallot and pulmonary atresia (48%) and patients with prosthetic valve IE (8%) [1, 2, 10, 20].
It is important to have an updated knowledge of IE epidemiology, risk factors, clinical features, and complications in children in order to provide an adequate management. The aim of the present study is to describe these features of the IE episodes treated at a tertiary Spanish pediatric hospital in a 10-years’ period.
Materials and methods
Study design
An observational retrospective study was performed, describing the characteristics of pediatric patients (< 16 years old) with IE treated at a tertiary hospital in Madrid (Spain), national reference for complex CHD patients with advanced heart surgery, between January 2012 and December 2021.
Patients
Patients were eligible if they fulfilled all the following inclusion criteria: age < 16 years, treated at any unit at Hospital Universitario 12 de Octubre, with definite or possible IE (according to the ESC 2015 modified Duke criteria [21]).
Clinical data
Clinical data were obtained from the review of informatized clinical records and collected in a REDCap online database elaborated for this study. Patients’ date of birth, gender, date of diagnosis, fulfilled modified Duke criteria, and underlying conditions were noted. Also, previous surgical correction of CHD, presence of indwelling CVC, and multidrug-resistant bacteria (MDRB) colonization were recorded. Recorded data of the IE episodes included clinical presentation, echocardiographic findings, and microbiologic data. Also, medical, and surgical treatment, complications, length of hospitalization, and outcomes were noted.
Statistical analysis
Categorical variables were reported as frequencies and percentages, and continuous variables as median and interquartile range (IQR). To evaluate the possible association between microorganism and clinical presentation and complications, Fisher’s exact test and Wilcoxon-Mann-Whitney test were used. The significance level was α = 0.05. Statistical analysis was performed using IBM SPSS Statistics 25.
Results
Demographic features
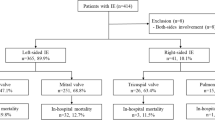
During the study period, 32 episodes of IE were identified (demographic and clinical features shown in Table 1); considering the Duke modified criteria, 28 (87.5%) were definite IE and 4 (12.5%) possible IE.
The median age at diagnosis was 9.1 (4.2–129.8) months; 17 (53.1%) episodes were diagnosed in infants younger than 1 year of age, 3 (9.4%) in patients between 1–5 years, 4 (12.5%) in patients between 6–10 years and 8 (25.0%) in patients between 11–15 years.
Regarding underlying conditions, 28 (87.5%) patients had CHD (Table 2), 8 (25.0%) were preterm infants (median gestational age at birth: 27.5 weeks, IQR: 25.0–32.8 weeks), 1 (3.1%) was immunocompromised (metastatic medulloblastoma) and 6 (18.8%) had other chronic conditions (3 had a polymalformative syndrome, 1 short bowel syndrome, 1 esophageal atresia, and 1 Down syndrome); in 11 (34.4%) episodes, more than one underlying condition was associated (6 episodes: CHD plus preterm birth, 5 episodes: CHD plus other chronic conditions).
Regarding the 28 episodes of IE diagnosed in patients with CHD, a corrective cardiac surgery or therapeutic cardiac catheterization was previously performed in 20 (71.4%). The median time of IE diagnosis after the cardiac procedure was 47.5 (18.3–348.0) days. At diagnosis, in 20 (62.5%) episodes the patients had an indwelling CVC, and the median time of IE diagnosis after CVC placement was 8.5 (4.0–14.3) days. Eight out of 8 (100%) preterm infants, 1 out of 1 (100%) immunocompromised patient and 5 out of 6 (83.3%) patients with other chronic conditions had an indwelling CVC at diagnosis (i.e., 14 out of 15, 93.3%), compared to 6 out of 17 (35.3%) patients with isolated CHD that had and indwelling CVC at diagnosis (p < 0.001).
Clinical manifestations
Presenting signs were fever in 27 (84.4%) episodes, shock in 16 (50.0%), heart failure in 1 (3.1%), vascular phenomena in 14 (43.8%) (11 (34.4%) septic embolisms, 2 (6.3%) intracranial hemorrhage, 1 (3.1%) Janeway’s lesions), immunological phenomena in 1 (3.1%) (glomerulonephritis and rheumatoid factor elevation in the same episode). The presenting signs in preterm infants compared to other patients were different: fever was only present in 4 out of 8 (50.0%) compared to 23 out of 24 (95.8%) of the non-preterm patients (p < 0.001) and shock was present in 7 out of 8 (87.5%) compared to 9 out of 24 (37.5%) of the non-preterm patients (p < 0.001).
Echocardiogram
An echocardiogram was performed in all episodes, transthoracic echocardiography was used in 29 (90.6%) episodes and both transthoracic and transesophageal echocardiography was used in 3 (9.4%). There were echocardiographic findings in 27 (84.4%), which are shown in Table 3.
Microbiology
Thirty-six microbiological isolates were obtained in the 32 episodes (Table 4).
Blood cultures were positive in 30 (93.8%) episodes; in 4 (12.5%) episodes, more than one microorganism was isolated (2 microorganisms isolated per episode). In those 4 episodes, the isolated microorganisms were: Staphylococcus aureus and Enterococcus faecalis, Enterococcus faecalis and Staphylococcus epidermidis, Enterococcus faecalis and extended spectrum beta-lactamase (ESBL) Escherichia coli, and Stenotrophomonas maltophilia and carbapenemase-producing Klebsiella pneumoniae. In those 2 episodes without positive blood cultures, Trichosporon inkin was isolated at surgical specimen in one and serology for Coxiella burnetii was positive (confirmed by PCR in surgical specimen and blood) in another case.
All S. aureus isolates were methicillin-sensitive and all E. faecalis isolates were ampicillin-sensitive. Among those 10 non-HACEK GNB, 5 (13.9%) were MDRB: 1 MDR Pseudomonas aeruginosa, 1 ESBL Escherichia coli, 1 carbapenemase-producing Klebsiella pneumoniae, and 2 AmpC beta-lactamase producing GNB (Enterobacter cloacae and Serratia marcescens).
In 10 (31.3%) episodes, patients were previously colonized by MDRB (clinical features of those patients and comparison with non-colonized patients in Table 5). Of those 10 patients, the colonizing bacteria were isolated in 3 (30.0%) of the patients’ blood cultures: 1 MDR Pseudomonas aeruginosa, 1 ESBL Escherichia coli, and 1 AmpC beta-lactamase producing Enterobacter cloacae. MDRB colonization was associated with MDRB IE (p = 0.007), with the presence of indwelling CVC (p = 0.030), and with longer time of PICU/NICU stay (p = 0.005).
Regarding the 4 episodes of fungal IE, one Candida albicans IE occurred in a premature newborn (25 weeks of gestational age at birth) with a patent ductus arteriosus and a 20-days’ CVC, the other in an infant with esophageal atresia and an 8-days’ CVC, and both Trichosporon inkin IE occurred in an adolescent with pulmonary atresia without CVC but with a prosthetic pulmonary conduit (relapse). All of them where right IE, located at native tricuspid valve, interventricular septum (right ventricle), and prosthetic pulmonary conduit respectively. Both patients with C. albicans IE where previously admitted to NICU/PICU. Both the patient with Trichosporon inkin IE and the infant with esophageal atresia and C. albicans IE had pulmonary embolisms and both required surgical treatment.
Treatment
The median of total antibiotic treatment duration was 47.0 (IQR 34.8–55.5) days, with a median duration of empirical antibiotic treatment of 4.5 (IQR 3.0–12.0) days. The median delay in initiating antibiotic treatment after the symptoms’ onset was 2.0 (IQR 0.0–5.0) days. The most used empirical antibiotic regimen was meropenem plus a glycopeptide (vancomycin or teicoplanin) with or without an aminoglycoside (gentamicin or amikacin) in 6 (18.8%) episodes, followed by a glycopeptide (vancomycin or teicoplanin) plus an aminoglycoside (gentamicin or amikacin) in 5 (15.6%).
Surgical management was required in 11 (34.4%) episodes, with a median time from diagnosis to surgical intervention of 5.0 (IQR 2.0–22.0) days. The indication of surgery was the difficult to eradicate isolated microorganism in 6 (18.8%) episodes (3 (9.4%) S. aureus, 2 (6.3%) P. aeruginosa, 1 (3.1%) C. albicans; with persistent bacteriemia in 5 (15.6%)), embolism prevention in 2 (6.3%), severe valve regurgitation in 2 (6.3%), and severe pulmonary conduit obstruction in 1 (3.1%).
Complications
PICU/NICU admission related to IE or its complications was required in 26 (81.3%) episodes, with a median stay of 26.0 (6.5–87.3) days. Complications secondary to IE were present in 25 (78.1%) episodes, 17 (53.1%) of the episodes with more than one complication. Registered complications were septic embolisms in 11 (34.4%) (8 (25.0%) pulmonary, 1 (3.1%) pulmonary and soft tissue, 1 (3.1%) cerebral, 1 (3.1%) cerebral and hepatic), shock in 8 (25.0%), heart failure in 7 (21.9%), valve regurgitation in 7 (21.9%), acute renal failure in 4 (12.5%), neurological complications in 3 (9.4%), local extension of infection in 3 (9.4%), and heart rhythm disturbances in 1 (3.1%). In-hospital mortality occurred in 2 (6.3%) episodes which was not attributed to IE or its complications, but to complications related to the patients’ underlying condition (pulmonary atresia and polymalformative syndrome (CHARGE syndrome) respectively).
Outcomes
There were 6 (18.8%) recurrences: 4 (12.5%) in-hospital relapses and 2 (6.3%) outpatient recurrences (1 relapse, 1 reinfection). At discharge, there were sequelae in 10 (31.3%) episodes, 3 (9.4%) of the episodes with more than one sequela: valvular regurgitation in 7 (21.9%) (2 (6.3%) of them associating heart failure), neurological sequelae in 3 (9.4%), heart rhythm disturbances in 1 (3.1%), valvular double lesion in 1 (3.1%), and chronic renal failure in 1 (3.1%). At follow-up (median time 3.4 years, IQR 0.7–7.2 years), 6 (18.8%) of those patients had persistent sequelae (1 (3.1%) with more than one sequela): valvular regurgitation in 4 (12.5%), heart failure in 2 (6.3%), neurological sequelae in 1 (3.1%), heart rhythm disturbances in 1 (3.1%), and chronic renal failure in 1 (3.1%).
Comparison of clinical features and complications depending on causative microorganism of IE
Clinical features and complications of IE episodes caused by non-HACEK GNB were compared to those episodes caused by GPB, and no statistically significant differences were found between them (Table 6).
Although children with non-HACEK GNB IE exhibited a higher proportion of PICU admission (100% vs. 70.6%), shock (77.8% vs. 47.1%), heart failure (33.3% vs. 11.8%), neurological complications (22.2% vs. 5.9%), more than one underlying condition (55.6% vs. 29.4%), and presence of indwelling CVC (88.9% vs. 58.8%), these distinctions did not reach statistical significance. On the other hand, children with GPB IE showed a trend towards higher rate of residual valve regurgitation (29.4% vs 0.0%).
Discussion
Although IE is a rare disease in childhood, its incidence is increasing in the last years due to increase in health-care complexity [2]. For this same reason, epidemiology, risk factors and causative microorganisms are changing [11], so it is of paramount importance to have an updated knowledge of them in our setting to manage this disease adequately. We designed our study to describe the characteristics of the pediatric IE episodes treated in a cardiology reference center in a 10 years’ period.
As shown in previous reports [5], the main underlying condition was CHD (87.5%), suggesting that CHD is the main risk factor to develop IE in the pediatric population. The second risk factor for developing IE (25.0%), was being preterm (especially those extremely preterm, with a median of 27.5 weeks of gestational age at birth in our study); which has been also previously published as risk factor due to the prolonged indwelling CVC [9] and the fact that they undergo invasive procedures [14, 15]. In our study, only 3.1% episodes occurred in immunocompromised children, a population that may have been underrepresented due to the characteristics of our center, where HSCT (a well described risk factor for developing IE in children [13]) is not performed. Additionally, indwelling CVC was present in 93.3% of the recorded IE episodes in patients that were preterm, immunocompromised or had other chronic conditions compared to 35.3% episodes where CHD was the only underlying condition. This finding is in line with other studies where indwelling CVC is the main risk factor to develop an IE in these 3 groups of patients [9,10,11]. In our series, there were two incidence peaks regarding age at presentation: 53.1% presented in patients younger than 1 year and 25.0% in patients older than 10 years, similar to what is reported in previous literature where episodes peak in the first year of life (where surgical correction of the more severe CHD is performed) and adolescence [2].
Regarding clinical manifestations, we found that the preterm infant group had significantly different clinical presentation compared to the other patients: only 50.0% of preterm infants in our study had fever (vs. 95.8% in non-preterm children) and 87.5% presented with shock (vs. 37.5% in non-preterm children). These findings suggest that maybe other diagnostic criteria of IE should be applied in preterm patients, as previously has been proposed by other group [22].
Regarding microbiological isolates, the most frequent isolates were CoNS (19.4%) and S. aureus (16.7%) in contrast with what is reported in the literature [2, 4, 5, 20] and other recent series [23, 24] where the most frequently isolated microorganisms are S. aureus and viridans streptococci (which in our series were only isolated in 8.3% of the episodes). Remarkably, our study showed an important proportion of non-HACEK GNB (27.8%) which is significantly above what has been previously reported (0–9.1%) [23, 25, 26]. The proportion of fungal IE in our series (11.1%) was similar to other Spanish series [25, 27], being C. albicans the most frequent isolate [5].
Of note, we registered the MDRB colonization. Among 10 MDRB colonized children with IE, 3 (30.0%) had the same bacteria in blood cultures. This significantly high proportion of MDRB IE has not been previously reported in pediatric IE literature. Due to this finding, it might be advisable to consider empirical antibiotic coverage of these MDRB when suspecting IE in colonized patients. MDRB colonization was also associated with the presence of CVC and with longer NICU/PICU stay. Children with severe CHD spend long periods admitted to NICU/PICU and those with chronic conditions need a prolonged indwelling CVC, both situations with high risk for MDRB colonization and therefore possibility for MDRB IE.
Regarding IE complications, the main complication was septic embolism, present in 34.4% (n = 11) of the episodes (28.1% pulmonary embolisms and 6.3% cerebral embolisms). Proportion of embolism was similar to that reported in other pediatric study (40%) [18], although in our study the proportion of cerebral embolisms was lower (6.3% vs 20%) and that of pulmonary embolisms was higher (28.1% vs 8%), probably due to a lower proportion of left IE in our cohort (18.8% vs 55.9%). Previous studies showed that left sided IE have a higher risk for cerebral embolism compared to right IE (32% vs 2.8%) [28].
In-hospital mortality in our study was 6.3% (n = 2), like that reported in other studies [1, 2]. None of the deaths was due to IE or its complications. One of the patients had pulmonary atresia (a described risk factor for higher mortality -48%- [2]) and the other patient had a polymalformative syndrome. In our study, 18.8% (n = 6) of the patients had persistent sequelae at follow-up. Mortality and sequelae rates are consistent with previous literature [1, 8, 11, 23,24,25]. Despite IE is a rare disease, long-term sequelae in children with IE are still common, which may be relevant in populations with higher risk of IE, like patients with CHD or preterm infants.
We did not find statistically significant differences in clinical features or in complication rates between non-HACEK GNB and GPB IE, probably due to a limited sample size, but we could observe some interesting trends in this study (Table 6). There was a higher proportion of non-HACEK GNB IE in children with more than one underlying condition (55.6%) and children with indwelling CVC (88.9%), probably due to frequent contact with the health-care system. Non-HACEK GNB IE showed a higher proportion of PICU admission (100%), shock (77.8%), heart failure (33.3%) and neurologic complications (22.2%), which may be due to their higher virulence. On the other hand, GPB IE showed a higher proportion of residual valve regurgitation (29.4%), which may be caused by tendency of biofilm formation in GPB with higher potential for valve disruption.
Limitations of our study are those inherent to retrospective studies (missing data, prone to biases). Limited sample size is a major limitation of the study as IE is a rare condition in children, even in a reference center with advanced heart surgery like in this study.
In conclusion, risk factors for developing IE, the proportion of embolic complications, and mortality rate were consistent with previously published findings. Notably, the proportion of IE cases attributed to non-HACEK GNB was higher than previously reported, suggesting an evolving epidemiology of IE. It is noteworthy that one-third of children colonized with MDRB subsequently developed IE caused by the same MDRB strains; this emphasizes the potential importance of considering empiric antibiotic coverage against colonizing MDRB in those patients. While no statistically significant differences in clinical features and complications were observed when comparing IE episodes caused by non-HACEK GNB and those caused by GPB, larger cohort studies are needed for further investigation.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- CHD:
-
Congenital heart disease
- CoNS:
-
Coagulase-negative staphylococci
- CVC:
-
Central venous catheter
- ESBL:
-
Extended spectrum beta-lactamase
- GNB:
-
Gram-negative bacteria
- GPB:
-
Gram-positive bacteria
- HSCT:
-
Hematopoietic stem cell transplantation
- IE:
-
Infective endocarditis
- IQR:
-
Interquartile range
- MDRB:
-
Multidrug-resistant bacteria
- NICU:
-
Neonatal intensive care unit
- PICU:
-
Pediatric intensive care unit
References
Dixon G, Christov G (2017) Infective endocarditis in children: an update. Curr Opin Infect Dis 30(3):257–267. https://doi.org/10.1097/QCO.0000000000000370
Day MD, Gauvreau K, Shulman S, Newburger JW (2009) Characteristics of children hospitalized with infective endocarditis. Circulation 119(6):865–870. https://doi.org/10.1161/CIRCULATIONAHA.108.798751
Rushani D, Kaufman JS, Ionescu-Ittu R, Mackie AS, Pilote L, Therrien J, Marelli AJ (2013) Infective endocarditis in children with congenital heart disease: cumulative incidence and predictors. Circulation 128(13):1412–1419. https://doi.org/10.1161/CIRCULATIONAHA.113.001827
Jortveit J, Klcovansky J, Eskedal L, Birkeland S, Døhlen G, Holmstrøm H (2018) Endocarditis in children and adolescents with congenital heart defects: a Norwegian nationwide register-based cohort study. Arch Dis Child 103(7):670–674. https://doi.org/10.1136/archdischild-2017-313917
Baltimore RS, Gewitz M, Baddour LM, Beerman LB, Jackson MA, Lockhart PB, Pahl E, Schutze GE, Shulman ST, Willoughby R Jr, American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young and the Council on Cardiovascular and Stroke Nursing (2015) Infective endocarditis in childhood: 2015 update. A scientific statement from the American Heart Association. Circulation 132(15):1487–1515. https://doi.org/10.1161/CIR.0000000000000298
Oster ME, Lee KA, Honein MA, Riehle-Colarusso T, Shin M, Correa A (2013) Temporal trends in survival among infants with critical congenital heart defects. Pediatrics 131(5):e1502–e1508. https://doi.org/10.1542/peds.2012-3435
Khoo B, Buratto E, Fricke TA, Gelbart B, Brizard CP, Brink J, d’Udekem Y, Konstantinov IE (2019) Outcomes of surgery for infective endocarditis in children: a 30-year experience. J Thorac Cardiovasc Surg 158(5):1399–1409. https://doi.org/10.1016/j.jtcvs.2019.06.024
Cox DA, Tani LY (2020) Pediatric infective endocarditis: a clinical update. Pediatr Clin N Am 67:875–888. https://doi.org/10.1016/j.pcl.2020.06.011
Lin YT, Hsieh KS, Chen YS, Huang IF, Cheng MF (2013) Infective endocarditis in children without underlying heart disease. J Microbiol Immunol Infect 46(2):121–128. https://doi.org/10.1016/j.jmii.2012.05.001
Gupta S, Sakhuja A, McGrath E, Asmar B (2017) Trends, microbiology, and outcomes of infective endocarditis in children during 2000–2010 in the United States. Congenit Heart Dis 12(2):196–201. https://doi.org/10.1111/chd.12425
Rosenthal LB, Feja KN, Levasseur SM, Alba LR, Gersony W, Saiman L (2010) The changing epidemiology of pediatric endocarditis at a children’s hospital over seven decades. Pediatr Cardiol 31(6):813–820. https://doi.org/10.1007/s00246-010-9709-6
Rech A, Loss JF, Machado A, Brunetto AL (2004) Infective endocarditis (IE) in children receiving treatment for cancer. Pediatr Blood Cancer 43(2):159–163. https://doi.org/10.1002/pbc.20093
Kuruvilla J, Forrest DL, Lavoie JC, Nantel SH, Shepherd JD, Song KW, Sutherland HJ, Toze CL, Hogge DE, Nevill TJ (2004) Characteristics and outcome of patients developing endocarditis following hematopoietic stem cell transplantation. Bone Marrow Transplant 34(11):969–973. https://doi.org/10.1038/sj.bmt.1704655
Elder RW, Baltimore RS (2015) The changing epidemiology of pediatric endocarditis. Infect Dis Clin N Am 29(3):513–524. https://doi.org/10.1016/j.idc.2015.05.004
Eleyan L, Khan AA, Musollari G, Chandiramani AS, Shaikh S, Salha A, Tarmahomed A, Harky A (2021) Infective endocarditis in paediatric population. Eur J Pediatr 180(10):3089–3100. https://doi.org/10.1007/s00431-021-04062-7
Gilleece A, Fenelon L (2000) Nosocomial infective endocarditis. J Hosp Infect 46(2):83–88. https://doi.org/10.1053/jhin.2000.0802
Millar BC, Jugo J, Moore JE (2005) Fungal endocarditis in neonates and children. Pediatr Cardiol 26(5):517–536. https://doi.org/10.1007/s00246-004-0831-1
Ganesan V, Ponnusamy SS, Sundaramurthy R (2017) Fungal endocarditis in paediatrics: a review of 192 cases (1971–2016). Cardiol Young 27(8):1481–1487. https://doi.org/10.1017/S1047951117000506
Thom K, Hanslik A, Russell JL, Williams S, Sivaprakasam P, Allen U, Male C, Brandão LR (2018) Incidence of infective endocarditis and its thromboembolic complications in a pediatric population over 30 years. Int J Cardiol 252:74–79. https://doi.org/10.1016/j.ijcard.2017.10.085
Cahill TJ, Jewell PD, Denne L, Franklin RC, Frigiola A, Orchard E, Prendergast BD (2019) Contemporary epidemiology of infective endocarditis in patients with congenital heart disease: a UK prospective study. Am Heart J 215:70–77. https://doi.org/10.1016/j.ahj.2019.05.014
Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, Dulgheru R, El Khoury G, Erbaa PA, Iung B et al (2015) 2015 ESC guidelines for the management of infective endocarditis: the Task force for the management of infective endocarditis of the European Society of Cardiology (ESC). Eur Heart J 36(44):3075–3128. https://doi.org/10.1093/eurheartj/ehv319
Daher AH, Berkowitz FE (1995) Infective endocarditis in neonates. Clin Pediatr (Phila) 34(4):198–206. https://doi.org/10.1177/000992289503400405
Kelchtermans J, Grossar L, Eyskens B, Cools B, Roggen M, Boshoff D, Louw J, Frerich S, Veloso TR, Claes J et al (2019) Clinical characteristics of infective endocarditis in children. Pediatr Infect Dis J 38(5):453–458. https://doi.org/10.1097/INF.0000000000002212
Mahony M, Lean D, Pham L, Horvath R, Suna J, Ward C, Veerappan S, Versluis K, Nourse C (2021) Infective endocarditis in children in Queensland, Australia: epidemiology, clinical features and outcome. Pediatr Infect Dis J 40(7):617–622. https://doi.org/10.1097/INF.0000000000003110
Goycochea-Valdivia WA, Aboza-García M, Moreno-Pérez de Tudela R, Carazo-Gallego B, Roldan-Tormo E, Ruiz-Sáez B, Vázquez-Pérez Á, Peromingo-Matute E, Croche-Santander B, Obando-Pacheco P, Obando-Santaella I, REPA (Red de Endocarditis Pediátrica) (2022) Endocarditis infecciosa en pacientes pediátricos de Andalucía (España), 2008–2020. Enferm Infecc Microbiol Clin. https://doi.org/10.1016/j.eimc.2022.05.014
Johnson JA, Boyce TG, Cetta F, Steckelberg JM, Johnson JN (2012) Infective endocarditis in the pediatric patient: a 60-year single-institution review. Mayo Clin Proc 87(7):629–635. https://doi.org/10.1016/j.mayocp.2012.02.023
Carreras-Blesa C, Martínez del Villar M, Melendo Pérez S, Guijarro Casas E, Betrián Blasco P (2016) Características clínicas y epidemiológicas de la endocarditis infecciosa en pediatría: 26 años de experiencia. Acta Pediatr Esp 74(3–4):93–99
Cao GF, Bi Q (2019) Pediatric infective endocarditis and stroke: a 13-year single-center review. Pediatr Neurol 90:56–60. https://doi.org/10.1016/j.pediatrneurol.2018.07.001
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. DBG was supported by a Research grant (INT23/00039) from the Spanish Ministry of Science and Innovation Instituto de Salud Carlos III and co-funded by the European Regional Development Fund. The authors declare that no other funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
IMC and DBG contributed to the study conception and design. Material preparation, data collection and analysis were performed by IMC. Statistical analysis was also performed by RPT. The first draft of the manuscript was written by IMC and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Hospital Universitario 12 de Octubre (Date: 23/10/2017, No: TP17/0370) in view of the retrospective nature of the study and all the procedures being performed were part of the routine care.
Consent to participate and publish
Due to the retrospective observational nature of the study and the anonymization of the data included, no informed consent to participate and publish was needed according to the Ethics Committee of Hospital Universitario 12 de Octubre (Date: 23/10/2017, No: TP17/0370).
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Tobias Tenenbaum
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marín-Cruz, I., Pedrero-Tomé, R., Toral, B. et al. Infective endocarditis in pediatric patients: a decade of insights from a leading Spanish heart surgery reference center. Eur J Pediatr 183, 3905–3913 (2024). https://doi.org/10.1007/s00431-024-05606-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-024-05606-3