Abstract
Recently, the importance of post-COVID-19 in children has been recognized in surveys and retrospective chart analysis. However, objective data in the form of cardiopulmonary exercise test as performed in adults suffering from this condition are still lacking. This study aimed to investigate the cardiopulmonary effects of post-COVID-19 on children and adolescents. In this cross-sectional study (the FASCINATE study), children fulfilling the criteria of post-COVID-19 and an age- and sex-matched control group underwent cardiopulmonary exercise testing on a treadmill and completed a questionnaire with regard to physical activity before, during and after the infection with SARS-CoV-2. We were able to recruit 20 children suffering from post-COVID-19 (mean age 12.8 ± 2.4 years, 60% females) and 28 control children (mean age 11.7 ± 3.5 years, 50% females). All participants completed a maximal treadmill test with a significantly lower \(\dot{{\text{V}}}{{\text{O}}}_{2}{\text{peak}}\) in the post-COVID-19 group (37.4 ± 8.8 ml/kg/min vs. 43.0 ± 6.7 ml/kg/min. p = 0.019). This significance did not persist when comparing the achieved percentage of predicted \(\dot{{\text{V}}}{{\text{O}}}_{2}{\text{peak}}\). There were no significant differences for oxygen pulse, heart rate, minute ventilation or breathing frequency.
Conclusion: This is the first study to investigate post-COVID-19 in children using the cardiopulmonary exercise test. Although there was a significantly reduced \(\dot{{\text{V}}}{{\text{O}}}_{2}{\text{peak}}\) in the post-COVID-19 group, this was not true for the percent of predicted values. No pathological findings with respect to cardiac or pulmonary functions could be discerned. Deconditioning was the most plausible cause for the experienced symptoms.
Trial registration: clinicaltrials.gov, NCT054445531, Low-field Magnetic Resonance Imaging in Pediatric Post Covid-19—Full Text View—ClinicalTrials.gov.
What is Known: • The persistence of symptoms after an infection with SARS-CoV 2, so-called post-COVID-19 exists also in children. • So far little research has been conducted to analyze this entity in the pediatric population. | |
What is New: • This is the first study proving a significantly lower cardiopulmonary function in pediatric patients suffering from post-COVID-19 symptoms. • The cardiac and pulmonary function appear similar between children suffering from post-COVID-19 and those who don’t, but the peripheral muscles seem affected. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Besides having caused millions of cases and thousands of deaths worldwide during acute infection [1], there is growing concern about the long-term effects of SARS-CoV 2 infection [2, 3]. Even though children are less affected by severe COVID-19 than adults [4, 5], more and more data is emerging about the persistence of symptoms, so-called post-COVID-19, also in children [6, 7]. This condition is still insufficiently defined but includes signs and symptoms that persist, develop or fluctuate after SARS-CoV-2 infection for at least 2 months and cannot be explained by an alternative diagnosis [8].
Most of the research being conducted about the symptoms of post-COVID-19 focuses on the adult population with limited information about pediatric patients [5, 6]. The most common symptoms being reported in children are fatigue [6, 9,10,11], exertional dyspnea [11,12,13] and exercise intolerance [10, 11, 13, 14]. So far, all studies investigating post-COVID-19 in children have relied on surveys [9,10,11, 15], chart reviews [13], pulmonary function tests [13, 14] and rarely an occasional 6 min-walk-test (6MWT) [13].
In the adult population many studies have investigated the effects of post-COVID-19 on patients using cardiopulmonary exercise testing (CPET) [16]. CPET remains the standard for measuring exercise capacity on top of aiding in the differential diagnosis of the exercise limitations [17, 18]. Several studies established a reduction in peak oxygen uptake (\(\dot{{\text{V}}}{{\text{O}}}_{2}{\text{peak}}\)), as well as in the predicted \(\dot{{\text{V}}}{{\text{O}}}_{2}{\text{peak}}\) and the \(\dot{{\text{V}}}{{\text{O}}}_{2}\) at the first ventilatory threshold [16, 19, 20] in post-COVID-19 patients. However, the causes for this reduction are unclear.
While pulmonary dysfunction has been observed on advanced magnetic resonance imaging [21, 22], one possible explanation is the effect of deconditioning combined with alteration in muscular oxygen utilization [16, 23]. Apart from dysfunctional breathing, chronotropic incompetence and abnormal heart recovery have been observed [16, 24]. Interestingly, cardiac output, indirectly measured as the oxygen pulse (O2pulse), is not affected in patients suffering from post-COVID-19 [16].
To this date, no study has investigated the effects of post-COVID-19 in children using CPET. As a consequence of being aware of the long-term sequelae of an infection with SARS-CoV-2, concerns have arisen regarding the return to sports after the disease. Although it is clear that a lack of sports and physical exercise has led to severe consequences for children during the pandemic [25, 26], some authors suggest a prolonged rest period with gradual return to sports after extensive medical testing including cardiac screening [27, 28].
Material and methods
The study was approved by the Ethics Committee of the University of Erlangen-Nuremberg, FRG (206_21B). All study participants as well as their legal guardians gave written informed consent according to the standards set by the Declaration of Helsinki. The study is part of a bigger study and was registered under clinicaltrials.gov as part of the FASCINATE study (NCT054445531) https://clinicaltrials.gov/ct2/show/NCT05445531?term=FASCINATE&draw=2&rank=2.
Participants
Participants consisted of children between the age of 5 and 17 years with symptoms of post-COVID-19 as defined by the “Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften” AWMF S1 (step 1) guideline [29]. The comparison group consisted of children and teenagers with proof of SARS-CoV-2 infection but post-COVID-19 criteria [29] not fulfilled. All participants were recruited via media (newspaper, homepage).
The inclusion criteria for the patients in the post-COVID-19 group were:
-
Positive SARS-CoV-2 infection confirmed by polymerase chain reaction (PCR)
-
Post-COVID-19 criteria according to the German guideline AWMF S1 [29] fulfilled
The inclusion criteria for the comparison group were:
-
Proof of SARS-CoV-2 infection
-
Post-COVID-19 criteria not met
The exclusion criteria for both groups were:
-
Clinical presentation of acute infection either by SARS-CoV-2 or other
-
Necessary quarantine
-
Pregnancy, lactation
-
Known pleural or pericardial effusion
-
Critical condition (need for respiratory support, ventilation, oxygen administration, shock, symptomatic heart failure)
-
Marked thoracic deformities
-
Previous lung surgery
-
Injuries that do not allow for physical stress testing
-
Suspicion of pulmonary disease
-
Inhaled therapy (e.g., steroids or beta-mimetics)
-
Immunosuppression
-
Any condition that may lead to a respiratory limitation (e.g., pain)
-
Obesity (> 97% of age percentile)
Height and weight were measured using a stadiometer and electronic scale (Seca 704 S, Hamburg, Germany).
Extracurricular physical activity was assessed differentiating between the type and amount (in hours per week) of sports performed out of school in the year prior to infection with SARS-CoV-2, as well as at the moment of testing, and the length of a rest period from physical activity due to the infection.
Measurement of gas exchange
A small, low-dead-space respiratory valve (88 ml) with a size-matched mouthpiece and headgear was used (Metalyzer 3B, Cortex, Leipzig, Germany). During each test, the gas exchange was measured continuously using a breath-by-breath method and averaged over 15 s intervals. We used the following physiological criteria for completion of a valid \(\dot{{\text{V}}}{{\text{O}}}_{2}{\text{peak}}\), two of which needed to be met for validation: (1) peak heart rate (peak HR) within 5% of the age-predicted maximum, (2) respiratory exchange ratio (RER) ≥ 1.0 and (3) volitional fatigue [30, 31]. We chose a threshold of 1.00 RER for the completion of a valid \(\dot{{\text{V}}}{{\text{O}}}_{2}{\text{peak}}\) as it is difficult to achieve higher RER values when performing CPET on a treadmill with children [32]. The peak oxygen uptake was put in relation to normal values from Kalden et al. [33] for children below the age of 8 and from Bongers et al. [34] for children between 9 and 16 years of age. For adolescents above the age of 16 years, normal values for adults [35] were used.
The V-slope method proposed by Beaver et al. [36] was used by the same experienced researcher to determine the ventilatory thresholds VT1 and VT2. Plotting \(\mathrm{oxygen uptake} (\dot{{\text{V}}}{{\text{O}}}_{2}\)) (ml/min) against the logarithm of minute ventilation (\(\dot{{V}_{{\text{E}}}}\)) (ml/min) and calculating the slope of this linear relation through single regression analysis [30] determined the oxygen uptake efficiency slope (OUES).
The ventilatory response during exercise was assessed using a linear regression function by plotting minute ventilation (\(\dot{{V}_{{\text{E}}}}\)) against carbon dioxide procution (\({\dot{V}}_{{\text{CO}}2}\)) without the data above the ventilatory compensation point, and the slope (\({\dot{V}}_{{\text{E}}}/{\dot{V}}_{{\text{CO}}2}\)) was obtained from the regression line [37].
The breathing reserve represents the percentage of the achieved maximal voluntary ventilation (MVV), which was calculated from the FEV1 × 35.
A half-time recovery of \(\dot{{\text{V}}}{{\text{O}}}_{2}\) (\({T}_{1/2}\dot{{\text{V}}}{{\text{O}}}_{2}\)) was assessed during off-transient after peak-graded CPET and was defined as the time needed for \(\dot{{\text{V}}}{{\text{O}}}_{2}{\text{peak}}\) to decrease by half [38].
Heart rate recovery was monitored during the first minute of the recovery phase (HRR).
Cardiopulmonary exercise test
All subjects were equipped with a 12-lead ECG (Custo®, Ottobrunn, Germany) for monitoring heart rate and ECG changes.
An incremental step test on a treadmill (COSMED T 170, COSMED, Italy) was performed for cardiopulmonary exercise testing. We used an age-appropriate treadmill testing protocol derived from a previous study [30]. In this protocol the starting speed is set at 3 km/h, with the following steps set at 6 km/h, 8 km/h, and then an increase of 1 km/h every 2 min. We used an increment of 1% for simulation of a natural environment. All participants were encouraged verbally to run until exhaustion and all tests were performed by the same researchers.
Statistical analysis
Statistical analysis was performed using Microsoft Excel 2000® for data collection and SPSS 12.0® (SPSS Inc., Chicago, IL) for statistical evaluation. All measured values are reported as means and standard deviations. The Kolmogorow-Smirnov test was used to check for normal distribution. Homogeneity of variance was investigated using Levene’s F-test. For normally distributed variables, differences between the former preterm children and their healthy control group were assessed with unpaired t-tests; otherwise, the Wilcoxon or the Whitney-Mann U-tests were used. Statistical significance was set at p < 0.05.
Since the study was part of a bigger study that had been powered to find differences in the magnetic resonance imaging performed in the two groups, we performed Cohen’s D as well as the correlation coefficient r for the primary outcome, namely the peak oxygen uptake \(\dot{{\text{V}}}{{\text{O}}}_{2}{\text{peak}}\), in order to measure the effect size.
Results
Subjects
Overall, 20 participants suffering from post-COVID-19, and 28 participants for the comparison group were recruited. There were no significant differences between the patients suffering from post-COVID-19 and the controls with respect to age, height, weight or sex (Table 1).
The type and amount of physical activity were also comparable between the two groups except for the rest period from physical activity after SARS-CoV-2 infection which amounted to over 4 weeks in the group suffering from post-COVID-19 compared to 0.3 weeks in the control group (Table 2). Overall, the children had performed significantly less physical activity during the pandemic (2.1 ± 2.7 h) than before (3.5 ± 2.8 h).
The children suffering from post-COVID-19 stated a lower exercise tolerance.
Cardiopulmonary exercise test
The data from the cardiopulmonary exercise test is summarized in Table 3.
There was a significant difference with regard to exercise time with the post-COVID-19 group achieving less than the control group (855.6 ± 152.9 s. vs. 969.9 ± 152.9 s.). The achieved peak exertion was comparable between the two groups with similar RER, heart rate reserve (HRR) and breathing reserve (BR). No arrhythmias were discerned either at rest, or during exercise or recovery.
The peak oxygen uptake was significantly lower in the group suffering from post-COVID-19 symptoms (Fig. 1). In a post-hoc analysis, it became apparent that this finding was limited to the female participants (mean values of 33 ± 7.4 ml/kg/min for the girls affected by post-COVID-19 vs. 40.1 ± 5.3 ml/kg/min for the non-affected girls with a p-value of 0.005), whereas the boys showed comparable values for \(\dot{{\text{V}}}{{\text{O}}}_{2}{\text{peak}}\) (mean values of 43.4 ± 7.2 ml/kg/min for the boys affected by post-COVID-19 vs. 45.7 ± 6.7 ml/kg/min for the non-affected boys, with a p-value of 0.451). Furthermore, the predicted \(\dot{{\text{V}}}{{\text{O}}}_{2}{\text{peak}}\) did not differ significantly between the two groups, not even when only investigating the girls.
The oxygen uptake at VT1, as well as the percentage of achieved \(\dot{{\text{V}}}{{\text{O}}}_{2}{\text{peak}}\) at VT1, was comparable between the two groups and was not affected by sex.
Pulmonary function
When analyzing the pulmonary measurements from the CPET, there were no significant differences with respect to minute ventilation either at peak exercise \(\dot{{\text{V}}}{\text{Epeak}}\) or at the first ventilatory threshold (\(\dot{{\text{V}}}{\text{EVT}}1\)). Nor were there differences with regard to the breathing reserve (BR).
The slope of \({\dot{V}}_{{\text{E}}}/{\dot{V}}_{{{\text{CO}}}_{2}}\) below the first ventilatory threshold VT1 showed no significant differences between the two groups.
Cardiac function
All parameters related to cardiac function (Peak O2pulse, Peak HR, HRR, \({\dot{V}}_{{\text{E}}}/{\dot{V}}_{{{\text{CO}}}_{2}}\)-slope, chronotropic index) were comparable between the two groups (Table 3).
Peripheral function
Peripheral function represents the third physiologic compartment in the gas transport mechanisms for coupling cellular to pulmonary respiration according to Wasserman [39]. The third compartment represents the mitochondrial function of the muscle [39]. One further parameter for analyzing peripheral function is the \({T}_{1/2}\dot{{\text{V}}}{{\text{O}}}_{2}\), the time needed for \(\dot{{\text{V}}}{{\text{O}}}_{2}{\text{peak}}\) to decrease by half (Table 3). There were no significant differences between the two groups in this study.
Correlations
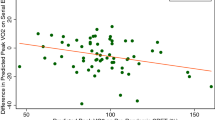
When calculating possible correlations only one proved significant: the negative correlation between the duration of rest from physical activity after an infection with SARS-CoV-2 and the \(\dot{{\text{V}}}{{\text{O}}}_{2}{\text{peak}}\) with a p-value of 0.044 (Fig. 2).
Discussion
Physical activity, exercise time and peak oxygen uptake
Interestingly, there were no significant differences between the two groups with regard to the amount of physical activity before the infection with SARS-CoV-2, nor in their school transport habits, or in their subjective exercise tolerance. The notion that premorbid \(\dot{{\text{V}}}{{\text{O}}}_{2}{\text{peak}}\) may have been low in the post-COVID-19 group, one possible explanation for the previously lower values observed in the adult population, therefore seems unlikely [40]. However, after the infection, the participants in the post-COVID-19 group needed a longer rest period from physical activity than the control group (over 4 weeks vs. less than 1 week) and felt subjectively less exercise-tolerant. This is in accordance with previous survey studies investigating the effects of post-COVID-19 in children [6, 9,10,11, 13, 15].
Almost all participants achieved maximal exercise (defined as an RER > 1.00). There were also no differences with regard to heart rate recovery (HRR) or breathing reserve (BR). In previous studies in adults, the fact that HRR was lower in patients suffering from post-COVID-19 was taken as a sign of earlier termination of exercise due to deconditioning and fatigue [41]. Raman et al. [19] who also observed shorter walk distances in a 6-min-walk-test observed that many patients stopped CPET early because of generalized muscle ache rather than breathlessness. Interestingly, the majority of participants who suffered from post-COVID-19 in the current study also reported muscle fatigue as the main cause for stopping the exercise.
The absolute value of peak oxygen uptake was lower in the post-COVID19 group. This is in accordance with numerous studies conducted in the adult population [23, 24, 40] and reflects observations from survey and chart review studies conducted in the pediatric population [6, 9, 13,14,15]. However, when comparing the values for the percentage of predicted \(\dot{{\text{V}}}{{\text{O}}}_{2}{\text{peak}}\) achieved, there was no significant difference. This could be a consequence of a relatively small sample size. Normal values for children represent an approximation and are gathered according to age. This leads to a smoothing of the results, which could therefore have annihilated the differences observed for \(\dot{{\text{V}}}{{\text{O}}}_{2}{\text{peak}}\). Nor was there any significant difference in the oxygen uptake efficiency slope (OUES). These findings suggest that children affected by post-COVID-19 do not show a measurable impairment of their cardiopulmonary function, a fact that is underlined by values of 90% and more of their predicted values in both groups.
When differentiating the data according to sex, it became apparent that the lower \(\dot{{\text{V}}}{{\text{O}}}_{2}{\text{peak}}\) as well as the lower percentage of predicted \(\dot{{\text{V}}}{{\text{O}}}_{2}{\text{peak}}\) was limited to the girls. The fact that females seem to experience more pronounced symptoms for post-COVID-19 has been described before, using surveys and 6MWT [42, 43], although the pathomechanism behind this finding is yet unclear.
Pulmonary function
The fact that there were no significant differences between the pulmonary variables (\({\dot{{\text{V}}}}_{{\text{E}}}{\text{peak}}\), \({\dot{V}}_{{\text{E}}}\) at VT1, breathing reserve BR, breathing frequency BF) recorded during CPET between the two groups underlines the fact that pulmonary function does not seem to be the cause for exertional dyspnea or reduced exercise tolerance in children.
One possible explanation for dyspnea after COVID-19 is dysfunctional breathing [44]. A marker for ventilatory efficiency is the ventilatory equivalent (\({\dot{V}}_{{\text{E}}}/{\dot{V}}_{{{\text{CO}}}_{2}}\)-slope) [40]. Altered pulmonary diffusion capacity, ventilation/perfusion mismatch and hyperventilation-syndrome are possible causes for dysfunctional breathing and have been documented in COVID-19 survivors [45]. Interestingly the ventilatory equivalent between the two groups in our study did not differ significantly. Thus, ventilatory inefficiency seems an unlikely candidate for the subjective reduced exercise tolerance reported by children suffering from post-COVID-19.
Cardiac function
Parameters for unmasking cardiovascular limitation using CPET are the O2pulse, the peak HR, or an abnormal increase of the \(\dot{V}{{\text{O}}}_{2}/P\)-slope. Despite the concerns around cardiac involvement during the SARS-CoV-2 pandemic, most studies showed normal values for the O2pulse in patients suffering from post-COVID-19 [23] or recovering from severe illness [46]. This was also true in this study.
At least a mild chronotropic incompetence has been observed in most studies conducting CPET in adults after infection with SARS-CoV-2 [46]. Lower peak HR was discussed either being due to chronotropic incompetence or a pharmaceutical betablockade or as a consequence of ceasing exercise early [41]. The children in this study did not show any significant differences with regard to chronotropic incompetence, and the HRR was well above the pathological 12 beats/minute [47]. This difference between the adult population and the children studied here may be due to several factors. First of all, the children showed a high willingness to reach peak exertion, reflected in the fact that almost all participants reached RER values above 1, irrespective of their symptoms, whereas the adults had probably ceased exercise before reaching peak exertional capacity, which may have been related to dyspnea unrelated to post-COVID-19 [41]. On the other hand, the use of beta-blocker was wide-spread in the investigated adult cohorts, also offering an explanation for the chronotropic incompetence. In contrast, none of the children involved in this study was on beta-blockers.
A further parameter which is typically reduced in patients with cardiovascular disease is the \(\dot{{\text{V}}}{{\text{O}}}_{2}/P\)-slope reflecting limitations in the supply and/or metabolism of oxygen. None of the children investigated in this study exhibited pathological values, defined as values below 10 ml/min/W [48]. This stands in contrast to studies investigating this parameter in the adult population, where the fact that it was slightly reduced was taken as a sign for a potential contribution of cardiovascular factors to the observed low \(\dot{{\text{V}}}{{\text{O}}}_{2}{\text{peak}}\) [49].
Peripheral function
The third compartment influencing \(\dot{{\text{V}}}{{\text{O}}}_{2}{\text{peak}}\) is the periphery (musculature and mitochondria). Peripheral limitations can be assessed through an abnormal response in RER, abnormal \(\dot{{\text{V}}}{{\text{CO}}}_{2}\)-kinetics throughout exercise, a shallower \(\dot{V}{O}_{2}/P\)-slope or a reduced VT1 in relation to \(\dot{{\text{V}}}{{\text{O}}}_{2}{\text{peak}}\). However, all of these parameters are unspecific and are also used to assess the cardiac compartment (see above) [40]. It is thus difficult to attribute abnormal findings in these parameters to deconditioning [40]. Some authors have therefore declared the observed reduction in \(\dot{{\text{V}}}{{\text{O}}}_{2}{\text{peak}}\) to be a consequence of deconditioning in the absence of ventilatory and cardiac exercise limitations.
None of these parameters showed any significant difference between the group of children suffering from post-COVID-19 and those who did not. After completing the exercise, all children suffering from post-COVID-19 stated that they had to stop the exercise due to muscular fatigue, which was not the case in the comparison group. This observation suggests muscular deconditioning as the possible mechanism for the observed reduction in \(\dot{{\text{V}}}{{\text{O}}}_{2}{\text{peak}}\).
Another parameter reintroduced by Longobardi et al. [24], the \({T}_{1/2}\dot{{\text{V}}}{{\text{O}}}_{2}\), is defined as the time needed for \(\dot{{\text{V}}}{{\text{O}}}_{2}{\text{peak}}\) to decrease by half [24]. This value proved to be significantly longer in adults suffering from post-COVID-19, suggesting a slower replenishment of energy stores in peripheral muscles underlying this defective off-transient \(\dot{{\text{V}}}{{\text{O}}}_{2}\) kinetic response [24]. Even though there were no significant differences between the two groups in this study, the children suffering from post-COVID-19 had values above a cut-off value of 90 s, which is generally assumed to be the upper limit of normal [24]. This supports the idea of a peripheral mechanism as the cause for the reduced exercise tolerance.
Interestingly, the only other significant difference between the two groups was the duration of rest after an infection with SARS-COV-2. The children suffering from post-COVID-19 stated a mean absence from physical activity of 4 weeks compared to 0.3 weeks in the comparison group. Furthermore, the only significant correlation of all the study parameters was between the duration of the rest period and \(\dot{{\text{V}}}{{\text{O}}}_{2}{\text{peak}}\). A study investigating the effects of early deconditioning of human skeletal muscle found several deconditioning processes to be initiated within the first 5 days of hypoactivity [50]. A rest period of a mean of 4 weeks as observed in our cohort of post-COVID-19 children could explain the observed decrease of \(\dot{{\text{V}}}{{\text{O}}}_{2}{\text{peak}}\) and abnormal \({T}_{1/2}\dot{{\text{V}}}{{\text{O}}}_{2}\). Since neither the pulmonary nor the cardiac compartment seems to be affected by infection with SARS-CoV-2, it seems reasonable to expect the cause in the peripheral compartment, i.e. the muscles. The combination of a significant difference between the two groups regarding the duration of rest with the negative correlation between rest period and \(\dot{{\text{V}}}{{\text{O}}}_{2}{\text{peak}}\) suggests a connection between inactivity and the occurrence of post-COVID-19 symptoms. However, as this is a retrospective study, it is impossible to say whether the inactivity was the cause of the prolonged symptoms after SARS-CoV-2 infection or whether a more serious course of the infection leads to prolonged inactivity and prolonged symptoms at the same time. The fact remains that long rest periods before returning to sports as recommended with myocarditis [51] are probably not warranted as the heart seems unaffected. Instead we should encourage children after a Coronavirus infection to return to sports within the limits of an infectious disease[52] in order to improve the peripheral compartment and consequently the \(\dot{{\text{V}}}{{\text{O}}}_{2}{\text{peak}}\). This recommendation obviously exclusively applies to children with post-COVID-19 syndrome and does not apply to children with paediatric inflammatory multisystem syndrome temporally associated with COVID-19 (PIMSts)/multisystem inflammatory syndrome in children (MIS-C) who need to be followed up as currently recommended [53].
Conclusion
As observed previously in post-COVID-19 studies in the adult population, the absolute \(\dot{{\text{V}}}{{\text{O}}}_{2}{\text{peak}}\) (but not the percentage of predicted \(\dot{V}{O}_{2}peak\)) was reduced in children suffering from post-COVID-19 symptoms. However, neither pulmonary, nor cardiac parameters proved to be significantly different from a group with no symptoms. The only other difference between the two groups was the rest period taken from physical activity after the infection with SARS-CoV-2. This suggests peripheral deconditioning as a possible cause for the reduction in cardiopulmonary function.
Limitations
The study has several limitations. For one, the sample size was comparably small with 20 children in the post-COVID-19 group. Thankfully, the number of children suffering from post-COVID-19 is rather low, which made the recruitment challenging. However, many studies in the adult population are also limited to a comparable number of patients.
This study is a retrospective study. There is no data on the further course of post-COVID-19 in children.
Data availability
As a consequence of the small cohort, the data can only be made available for direct inquiries directed at the authors. Otherwise patient confidentiality cannot be ensured.
Abbreviations
- ANS:
-
Autonomic nervous system
- AWMF S1:
-
Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften step 1
- BPD:
-
Bronchopulmonary dysplasia
- BF:
-
Breathing frequency
- BR:
-
Breathing reserve
- CPET:
-
Cardiopulmonary exercise test
- HR:
-
Heart rate
- HRR:
-
Heart rate recovery
- OUES:
-
Oxygen uptake efficiency slope
- Peak HR:
-
Peak heart rate
- peakRER:
-
RER at maximal exertion
- RER:
-
Respiratory exchange ratio
- VCO2 :
-
Carbon dioxide elimination
- VE:
-
Minute ventilation
- VEpeak:
-
Peak minute ventilation
- V E/V CO2 :
-
Breathing efficiency
- VO2 :
-
Oxygen uptake
- VO2 peak:
-
Peak oxygen uptake
- vpeak:
-
Peak speed
- VT1:
-
Ventilatory threshold 1
- VT2:
-
Ventilatory threshold 2
References
WHO (2023) COVID-19 weekly epidemiological update. Weekly. World Health Organization, World Health Organization
Montani D, Savale L, Noel N, Meyrignac O, Colle R, Gasnier M, Corruble E, Beurnier A, Jutant EM, Pham T, Lecoq AL, Papon JF, Figueiredo S, Harrois A, Humbert M, Monnet X (2022) Post-acute COVID-19 syndrome. Eur Respir Rev 31
Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, Kang L et al (2021) 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 397:220–232
Chua PEY, Shah SU, Gui H, Koh J, Somani J, Pang J (2021) Epidemiological and clinical characteristics of non-severe and severe pediatric and adult COVID-19 patients across different geographical regions in the early phase of pandemic: a systematic review and meta-analysis of observational studies. J Investig Med 69:1287–1296
Behnood SA, Shafran R, Bennett SD, Zhang AXD, O’Mahoney LL, Stephenson TJ, Ladhani SN, De Stavola BL, Viner RM, Swann OV (2022) Persistent symptoms following SARS-CoV-2 infection amongst children and young people: a meta-analysis of controlled and uncontrolled studies. J Infect 84:158–170
Lopez-Leon S, Wegman-Ostrosky T, Ayuzo Del Valle NC, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, Villapol S (2022) Long-COVID in children and adolescents: a systematic review and meta-analyses. Sci Rep 12:9950
Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, Cook JR et al (2021) Post-acute COVID-19 syndrome. Nat Med 27:601–615
Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV (2022) A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis 22:e102–e107
Buonsenso D, Pujol FE, Munblit D, Pata D, McFarland S, Simpson FK (2022) Clinical characteristics, activity levels and mental health problems in children with long coronavirus disease: a survey of 510 children. Future Microbiol 17:577–588
Brackel CLH, Lap CR, Buddingh EP, van Houten MA, van der Sande L, Langereis EJ, Bannier M, Pijnenburg MWH, Hashimoto S, Terheggen-Lagro SWJ (2021) Pediatric long-COVID: an overlooked phenomenon? Pediatr Pulmonol 56:2495–2502
Ashkenazi-Hoffnung L, Shmueli E, Ehrlich S, Ziv A, Bar-On O, Birk E, Lowenthal A, Prais D (2021) Long COVID in children: observations from a designated pediatric clinic. Pediatr Infect Dis J 40:e509–e511
Doležalová K, Tuková J, Pohunek P (2022) The respiratory consequences of COVID-19 lasted for a median of 4 months in a cohort of children aged 2–18 years of age. Acta Paediatr 111:1201–1206
Leftin Dobkin SC, Collaco JM, McGrath-Morrow SA (2021) Protracted respiratory findings in children post-SARS-CoV-2 infection. Pediatr Pulmonol 56:3682–3687
Bogusławski S, Strzelak A, Gajko K, Peradzyńska J, Popielska J, Marczyńska M, Kulus M, Krenke K (2023) The outcomes of COVID-19 pneumonia in children-clinical, radiographic, and pulmonary function assessment. Pediatr Pulmonol 58:1042–1050
Osmanov IM, Spiridonova E, Bobkova P, Gamirova A, Shikhaleva A, Andreeva M, Blyuss O et al (2022) Risk factors for post-COVID-19 condition in previously hospitalised children using the ISARIC global follow-up protocol: a prospective cohort study. Eur Respir J 59
Durstenfeld MS, Sun K, Tahir P, Peluso MJ, Deeks SG, Aras MA, Grandis DJ, Long CS, Beatty A, Hsue PY (2022) Use of cardiopulmonary exercise testing to evaluate long COVID-19 symptoms in adults: a systematic review and meta-analysis. JAMA Netw Open 5:e2236057
American Thoracic S, College A, of Chest P, (2003) ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 167:211–277
Balady GJ, Williams MA, Ades PA, Bittner V, Comoss P, Foody JM, Franklin B, Sanderson B, Southard D (2007) Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: a scientific statement from the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; the Councils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation 115:2675–2682
Raman B, Cassar MP, Tunnicliffe EM, Filippini N, Griffanti L, Alfaro-Almagro F, Okell T et al (2021) Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine 31:100683
Barbagelata L, Masson W, Iglesias D, Lillo E, Migone JF, Orazi ML, Maritano Furcada J (2022) Cardiopulmonary exercise testing in patients with post-COVID-19 syndrome. Med Clin (Barc) 159:6–11
Matheson AM, McIntosh MJ, Kooner HK, Lee J, Desaigoudar V, Bier E, Driehuys B, Svenningsen S, Santyr GE, Kirby M, Albert MS, Shepelytskyi Y, Grynko V, Ouriadov A, Abdelrazek M, Dhaliwal I, Nicholson JM, Parraga G (2022) Persistent (129)Xe MRI pulmonary and CT vascular abnormalities in symptomatic individuals with post-acute COVID-19 syndrome. Radiology:220492
Heiss R, Tan L, Schmidt S, Regensburger AP, Ewert F, Mammadova D, Buehler A, Vogel-Claussen J, Voskrebenzev A, Rauh M, Rompel O, Nagel AM, Levy S, Bickelhaupt S, May MS, Uder M, Metzler M, Trollmann R, Woelfle J, Wagner AL, Knieling F (2022) Pulmonary dysfunction after pediatric COVID-19. Radiology:221250
Skjørten I, Ankerstjerne OAW, Trebinjac D, Brønstad E, Rasch-Halvorsen Ø, Einvik G, Lerum TV, Stavem K, Edvardsen A, Ingul CB (2021) Cardiopulmonary exercise capacity and limitations 3 months after COVID-19 hospitalisation. Eur Respir J 58
Longobardi I, Prado D, Goessler KF, Meletti MM, de Oliveira Júnior GN, de Andrade DCO, Gualano B, Roschel H (2022) Oxygen uptake kinetics and chronotropic responses to exercise are impaired in survivors of severe COVID-19. Am J Physiol Heart Circ Physiol 323:H569-h576
Ammar A, Brach M, Trabelsi K, Chtourou H, Boukhris O, Masmoudi L, Bouaziz B et al (2020) Effects of COVID-19 home confinement on eating behaviour and physical activity: results of the ECLB-COVID19 International Online Survey. Nutrients 12
Stäbler T, Weigelt A, Rottermann K, Wällisch W, Hübner M, Dittrich S, Schöffl I (2022) COPHYD (Corona Owed Physical Deficiency): The impact of lockdown on cardiopulmonary function in paediatric cardiology patients. Klin Padiatr
Calcaterra G, Fanos V, Cataldi L, Cugusi L, Crisafulli A, Bassareo PP (2022) Need for resuming sports and physical activity for children and adolescents following COVID-19 infection. Sport Sci Health 18:1179–1185
Thompson LA, Kelly MN (2021) Return to play after COVID-19 infection in children. JAMA Pediatr 175:875
Koczulla AR, Ankermann T, Behrends U, Berlit P, Berner R, Böing S, Brinkmann F et al (2022) S1-Leitlinie long-/post-COVID. Pneumologie 76:855–907
Schoffl I, Ehrlich B, Stanger S, Rottermann K, Dittrich S, Schoffl V (2020) Exercise field testing in children: a new approach for age-appropriate evaluation of cardiopulmonary function. Pediatr Cardiol
Rottermann K, Weigelt A, Stäbler T, Ehrlich B, Dittrich S, Schöffl I (2022) New kids on the CPET: age-appropriate outdoor cardiopulmonary exercise testing in preschoolers. Eur J Appl Physiol
Rottermann K, Weigelt A, Stabler T, Ehrlich B, Dittrich S, Schoffl I (2022) New kids on the CPET: age-appropriate outdoor cardiopulmonary exercise testing in preschoolers. Eur J Appl Physiol 122:791–800
Kalden P, Schoeffl I, Rottermann K, Loeffelbein F, Michaelis A, Markel F, Brosig S, Gebauer RA, Daehnert I, Paech C (2021) Open field stress testing: finally an optimal method in young children? Reference values for mobile cardiopulmonary exercise testing in healthy children aged 4–8 years. Cardiol Young 32:1598–1602
Bongers B, van brussel M, Hulzebos HJ, Takken T, (2014) Pediatric norms for cardiopulmonary exercise testing in relation to sex and age. Uitgeverij BOXpress, Hertogenbosch, Netherlands
Koch B, Schäper C, Ittermann T, Spielhagen T, Dörr M, Völzke H, Opitz CF, Ewert R, Gläser S (2009) Reference values for cardiopulmonary exercise testing in healthy volunteers: the SHIP study. Eur Respir J 33:389–397
Beaver WL, Wasserman K, Whipp BJ (1986) A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 60:2020–2027
Dorelli G, Braggio M, Gabbiani D, Busti F, Caminati M, Senna G, Girelli D, Laveneziana P, Ferrari M, Sartori G, Dalle Carbonare L, Crisafulli E, On Behalf Of The Respicovid Study I (2021) Importance of cardiopulmonary exercise testing amongst subjects recovering from COVID-19. Diagnostics (Basel) 11
Grassi B (2005) Delayed metabolic activation of oxidative phosphorylation in skeletal muscle at exercise onset. Med Sci Sports Exerc 37:1567–1573
Wasserman K (1988) The Dickinson W. Richards lecture. New concepts in assessing cardiovascular function. Circulation 78:1060–1071
Schwendinger F, Knaier R, Radtke T, Schmidt-Trucksäss A (2022) Low cardiorespiratory fitness post-COVID-19: a narrative review. Sports medicine (Auckland, NZ):1–24
Kimmig LM, Rako ZA, Ziegler S, Richter MJ, G SA, Roller F, Grimminger F, Vadász I, Seeger W, Herold S, Tello K, Matt U, (2022) Long-term comprehensive cardiopulmonary phenotyping of COVID-19. Respir Res 23:263
Asadi-Pooya AA, Akbari A, Emami A, Lotfi M, Rostamihosseinkhani M, Nemati H, Barzegar Z, Kabiri M, Zeraatpisheh Z, Farjoud-Kouhanjani M, Jafari A, Sasannia F, Ashrafi S, Nazeri M, Nasiri S, Shahisavandi M (2021) Risk factors associated with long COVID syndrome: a retrospective study. Iran J Med Sci 46:428–436
Spicuzza L, Campisi R, Alia S, Prestifilippo S, Giuffrida ML, Angileri L, Ciancio N, Vancheri C (2023) Female sex affects respiratory function and exercise ability in patients recovered from COVID-19 pneumonia. J Womens Health (Larchmt) 32:18–23
Boulding R, Stacey R, Niven R, Fowler SJ (2016) Dysfunctional breathing: a review of the literature and proposal for classification. Eur Respir Rev 25:287–294
Aparisi Á, Ladrón R, Ybarra-Falcón C, Tobar J, San Román JA (2022) Exercise intolerance in post-acute sequelae of COVID-19 and the value of cardiopulmonary exercise testing- a mini-review. Front Med (Lausanne) 9:924819
Joris M, Minguet P, Colson C, Joris J, Fadeur M, Minguet G, Guiot J, Misset B, Rousseau AF (2021) Cardiopulmonary exercise testing in critically ill Coronavirus disease 2019 survivors: evidence of a sustained exercise intolerance and hypermetabolism. Crit Care Explor 3:e0491
Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS (1999) Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 341:1351–1357
Piepoli MF, Corrà U, Agostoni PG, Belardinelli R, Cohen-Solal A, Hambrecht R, Vanhees L (2006) Statement on cardiopulmonary exercise testing in chronic heart failure due to left ventricular dysfunction. Recommendations for performance and interpretation. Eur J Cardiovasc Prev Rehabil 13:10–12
Mancini DM, Brunjes DL, Lala A, Trivieri MG, Contreras JP, Natelson BH (2021) Use of Cardiopulmonary stress testing for patients with unexplained dyspnea post-coronavirus disease. JACC Heart Fail 9:927–937
Fovet T, Guilhot C, Stevens L, Montel V, Delobel P, Roumanille R, Semporé MY, Freyssenet D, Py G, Brioche T, Chopard A (2021) Early deconditioning of human skeletal muscles and the effects of a thigh cuff countermeasure. Int J Mol Sci 22
Pelliccia A, Solberg EE, Papadakis M, Adami PE, Biffi A, Caselli S, La Gerche A, Niebauer J, Pressler A, Schmied CM, Serratosa L, Halle M, Van Buuren F, Borjesson M, Carre F, Panhuyzen-Goedkoop NM, Heidbuchel H, Olivotto I, Corrado D, Sinagra G, Sharma S (2019) Recommendations for participation in competitive and leisure time sport in athletes with cardiomyopathies, myocarditis, and pericarditis: position statement of the Sport Cardiology Section of the European Association of Preventive Cardiology (EAPC). Eur Heart J 40:19–33
Scharhag J, Meyer T (2014) Return to play after acute infectious disease in football players. J Sports Sci 32:1237–1242
Schlapbach LJ, Andre MC, Grazioli S, Schöbi N, Ritz N, Aebi C, Agyeman P et al (2021) Best practice recommendations for the diagnosis and management of children with pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS; multisystem inflammatory syndrome in children, MIS-C) in Switzerland. Front Pediatr 9:667507
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the “Bayrisches Staatsministerium für Wissenschaft und Kunst”.
Author information
Authors and Affiliations
Contributions
Author’s name | Conception and design of the study | Data acquisition | Data analysis and interpretation | Drafting of manuscript | Critical revision of manuscript | Accountability for all aspects of work, ensuring integrity and accuracy |
|---|---|---|---|---|---|---|
IS | √ | √ | √ | √ | √ | √ |
RR | √ | √ | √ | √ | √ | |
TJP | √ | √ | √ | √ | √ | |
APR | √ | √ | √ | √ | ||
CK | √ | √ | √ | |||
WW | √ | √ | √ | |||
RT | √ | √ | √ | |||
JW | √ | √ | √ | |||
SD | √ | √ | √ | |||
RH | √ | √ | √ | |||
FK | √ | √ | √ | √ | √ | |
AW | √ | √ | √ | √ | √ |
Corresponding authors
Ethics declarations
Ethics approval
The study was approved by the Ethics Committee of the University of Erlangen-Nuremberg, FRG (206_21B).
Consent to participate
All study participants as well as their legal guardians gave written informed consent according to the standards set by the Declaration of Helsinki. The study was registered under clinicaltrials.gov as part of the FASCINATE study (NCT054445531) https://clinicaltrials.gov/ct2/show/NCT05445531?term=FASCINATE&draw=2&rank=2.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Tobias Tenenbaum
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schoeffl, I., Raming, R., Tratzky, JP. et al. Cardiopulmonary function in paediatric post-COVID-19: a controlled clinical trial. Eur J Pediatr 183, 1645–1655 (2024). https://doi.org/10.1007/s00431-024-05421-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-024-05421-w