Abstract
We would like to provide an updated comprehensive perspective and identify the components linked to chronic spontaneous urticaria (CSU) without specific triggers in autoimmune atrophic gastritis (AAG). AAG is an organ-specific autoimmune disease that affects the corpus-fundus gastric mucosa. Although we lack a unified explanation of the underlying pathways, when considering all paediatric patients reported in the literature, alterations result in gastric neuroendocrine enterochromaffin-like (ECL) cell proliferation and paracrine release of histamine. Several mechanisms have been proposed for the pathogenesis of CSU, with much evidence pointing towards AAG and ECL cell responses, which may be implicated as potential factors contributing to CSU. The excessive production/release of histamine into the bloodstream could cause or trigger exacerbations of CSU in AAG, independent of Helicobacter pylori; thus, the release of histamine from ECL cells may be the primary modulator.
Conclusion: Considering the understanding of these interactions, recognising the respective roles of AAG in the pathogenesis of CSU may strongly impact the diagnostic workup and management of unexplained/refractory CSU and may inform future research and interventions in the paediatric population.
What is Known: • Autoimmune atrophic gastritis is a chronic immune-mediated inflammatory disease characterised by the destruction of the oxyntic mucosa in the gastric body and fundus, mucosal atrophy, and metaplastic changes. • Autoimmune atrophic gastritis in paediatric patients is important because of the poor outcome and risk of malignancy and possibly underestimated entities primarily reported in single-case reports. | |
What is New: • Upper gastrointestinal inflammatory disorders, independent of H. pylori, have been implicated as potential inducing factors in the development of chronic spontaneous urticaria. • If a paediatric patient presents with symptoms such as anaemia, reduced vitamin B12 levels, recurrent urticaria with no other detectable aetiology, positive anti-parietal cell antibodies, and elevated gastrin levels, autoimmune atrophic gastritis should be considered a possible cause of chronic urticaria. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urticaria is widely considered a heterogeneous group of diseases that share a distinct skin reaction pattern: the development of urticarial skin lesions. Chronic spontaneous urticaria (CSU) is characterised by episodic itchy wheals that occur on most days of the week for more than six weeks without an evident triggering stimulus within 24 h [1]. The pathogenesis of this condition is complex. Despite careful clinical and laboratory investigations, a clear cause of CSU cannot be identified in approximately 75% of patients, and treatment is ineffective.
CSU can be caused by autoreactivity/autoimmunity in at least one-third of patients [2] and is more often associated with autoimmune diseases (e.g. thyreopathies, celiac disease, rheumatoid arthritis, systemic lupus erythematosus, Sjögren's syndrome, and type 1 diabetes mellitus) [3]. Several studies have raised concerns regarding the possible connection between chronic urticaria and infection, where an autoreactive immune response may be activated in predisposed patients. Infections of the gastrointestinal tract, such as Helicobacter pylori (H. pylori) infections, viral hepatitis, and parasitosis, may be associated with CSU [4]. The association of chronic urticaria with haematological malignancies has rarely been described. Furthermore, chronic inducible urticaria is less common, and its manifestation depends on the presence of specific triggers, such as physical stimuli, stress, food antigens, and drugs; however, both forms can occur simultaneously.
Autoimmune atrophic gastritis (AAG) is a well-established gastric pathological condition in adults; however, it is rarely described in paediatric patients. AAG is a chronic immune-mediated inflammatory disease characterised by the destruction of the oxyntic mucosa in the gastric body and fundus, mucosal atrophy, metaplastic changes, and the presence of two types of circulating autoantibodies: anti-parietal cell (APCA) and anti-intrinsic factor (AIFA) antibodies [5]. The parietal cells are destroyed by an immune-mediated process leading to hypo- or achlorhydria, which stimulates gastrin-producing G cells in the anthroduodenal region, leading to abnormally elevated gastrin levels, which stimulate gastric proliferation of enterochromaffin-like (ECL) cells. Long-term (and in the absence of parietal cells, ineffective) stimulation of ECL cells leads to hyperplasia, possibly even their transformation into neuroendocrine tumours (NETs) [6]. In advanced stages, when ECL cells become hyperplastic, histamine leaks into the bloodstream. Excess histamine in patients with AAG could trigger the emergence of chronic urticaria, contributing to its further progress.
The connection between gastrointestinal diseases and CSU has long been intensively investigated; however, our knowledge regarding the potential AAG pathogenetic mechanisms and the exact players that trigger CSU remains elusive; therefore, proactive case-finding strategies are needed. In this review, we focus on new pathophysiological aspects of AAG, including hyperplastic ECL cells producing histamine, as potential triggers for CSU. Therefore, close attention should be paid to the occurrence of AAG in children with a history of CSU.
Definition and classification of AAG in the context of CSU
Upper gastrointestinal (GI) inflammatory disorders, independent of H. pylori, have been implicated as potential inducing factors in the development of CSU. AAG in paediatric patients is important because of the poor outcome and risk of malignancy and possibly underestimated entities primarily reported in single-case reports. AAG is defined as the loss of appropriate gastric glands with or without metaplasia in the setting of chronic inflammation directed against the structures of gastric epithelial cells, which, in some cases, may be triggered by H. pylori. Regardless of the aetiology, the diagnosis of atrophic gastritis should be confirmed by histopathology. Although it has been described for several years, its actual pathophysiological mechanisms, natural history, and possible neoplastic complications are unclear [7]. AAG is characterised by the disruption of gastric epithelial cell development, targeting gastric parietal cells, leading to oxyntic-restricted mucosal atrophy, loss of intrinsic factors, and reduced acid output [5]. AAG is strongly associated with anaemia, atrophy, intestinal metaplasia, and ECL hyperplasia of the gastric fundus and body. These are hallmarks of this condition during childhood [8, 9]. However, to date, the long-term outcomes of AAG, including improvements in gastric atrophy and ECL hyperplasia in children, consistent with the known progression of adult AAG, remain debatable.
AAG is classified into two types according to the underlying etiopathogenic mechanisms: a predominant type in patients with the infection of H. pylori or due to other mechanisms of autoimmunity development, mainly limited to the corpus and fundus-dominant advanced atrophy.
The interaction between genetic and environmental factors has been implicated and may often be associated with other autoimmune diseases. In juvenile populations, autoimmune thyroid disease may be associated [10].
H. pylori infection may trigger an immune reaction against the antigens of the oxyntic mucosa itself, either based on cross-reactivity with H. pylori antigens or as a result of the stimulation of the immune system by neoantigens arising during tissue damage by inflammation. Several important factors help differentiate AAG from H. pylori-induced gastritis, including ECL hyperplasia, parietal cell pseudohypertrophy, and oxyntic gland involvement [11].
Identifying prolonged upper gastric inflammatory disorders that may lead to CSU and trigger exacerbations independent of H. pylori infection is important [12]. Hence, this might imply that in patients positive for H. pylori, urticaria is not linked to the bacterium itself or immunological reactions against the bacterium, but rather to the underlying inflammation [12]. Furthermore, in accordance with these findings, it was recently demonstrated that H. pylori-negative peptic ulcer disease and gastritis are comorbidities associated with an increased risk of CSU [12, 13]. Thus, as far as CSU alterations are concerned, these exciting findings suggest the importance of gastroenterological workup in paediatric patients with CSU independent of H. pylori. However, our understanding of the mechanisms, context, and roles of inflammation, ECL hyperplasia responses, and pathology in CSU is constantly evolving.
Discussed pathophysiological mechanism of chronic urticaria in AAG
To elucidate the complexity of the pathophysiology of AAG and its impact on the possible development of CSU, briefly discussing the histology and cellular composition of gastric glands, as well as their connections to various physiological processes and conditions would be useful.
The simple columnar mucus-secreting epithelium covers the entire luminal surface and comprises a continuous layer of surface mucous cells that release gastric mucus from the apical surface to form a thick protective layer over the gastric lining. Although all the gastric glands are tubular, they vary in form and cellular composition in different parts of the stomach. They can be divided into three groups as follows: the cardiac glands (glandulae cardiacae), principal glands in the body and fundus (glandulae gastricae propriae), and pyloric glands (glandulae pyloricae) [14]. Mucus-secreting cells are predominant in the cardiac glands. The other cell types were either absent or very few.
The pyloric glands are located in the pyloric antrum. These cells are primarily mucus-secreting cells. Parietal and chief cells are rare. By contrast, neuroendocrine cells are numerous, especially G cells, which secrete gastrin.
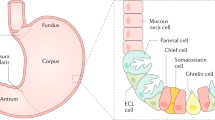
At least five distinct cell types are present in the walls of the principal glands: chief, parietal, mucous neck, stem, and neuroendocrine. The chief (zymogenic) cells are predominant in the lower regions of the gastric glands. They are strongly basophilic (owing to their secretory function) and contain numerous granules with the inactive enzyme pepsinogen, a precursor of pepsin. Parietal (oxyntic) cells are present in the mucous neck and throughout the deeper parts of the glands. They are a source of hydrogen ions (which create hydrochloric acid in the lumen) and an intrinsic glycoprotein necessary for the absorption of vitamin B12. Mucous neck cells are mainly localised in the necks of the gastric glands. These cells include many progenitors and immature surface mucous cells. Their primary function is to secrete mucus. Stem cells are predominantly located in the isthmus (the border between the gastric pits and gastric glands). These cells undergo mitosis, migration, and differentiation into various types of gastric cells. Among the chief cells, the neuroendocrine (enteroendocrine) cells are situated mainly in the deeper parts of the glands. These cells synthesise several biogenic amines and polypeptides that are important for controlling motility and glandular secretion. In the stomach, these include G cells that secrete gastrin, D cells that produce somatostatin, and ECL cells (Fig. 1) [15].
Structure of the gastric glands. Adapted from “Cells of Gastric Glands (Antrum vs Corpus)”, by BioRender.com (2023). Retrieved from https://app.biorender.com/biorender-templates
In addition to these basic cell types, other rarer and less-explored populations of cells are present in the gastric glands, such as tuft cells. Tuft cells are characterised by the presence of a luminally directed tuft that displays a distinct membrane-covered array of microtubules. The presence of the apical tuft apparatus suggests that tuft cells function in the detection and transmission of environmental signals. One key to understanding the role of tuft cells is that they share many characteristics with the chemosensory cells in taste buds. This suggests that the tuft cells can function as chemoreceptive cells that sense many chemical signals around them. The Dclk1-expressing tuft cell population in the gastric fundus increases in response to oxygenic atrophy, and elevated gastrin levels are necessary for dynamic changes [16].
The population of parietal cells that produce gastric acid and intrinsic factors is the focus of interest. Hydrochloric acid generates an acidic environment (pH < 2) in the gastric lumen. A strongly acidic environment facilitates food digestion, promotes the absorption of minerals, including iron, calcium, and phosphate, and kills food-derived bacteria. High and low levels of gastric acid secretion are potentially injurious to the integrity of the gastric mucosa. Therefore, the gastric mucosa must maintain a balance between gastric acid secretion and mucosal protection. The intrinsic and extrinsic neurohumoral regulation of the stomach balances the effects of the stimulatory (agonist) and inhibitory (antagonist) pathways to maintain a safe range of acid secretion. G cells are gastrin-producing enteroendocrine cells in the antrum that regulate parietal cell function via a feedback pathway from the distal part of the stomach. Gq-coupled receptors (chemical sensors, e.g. CaSR, GPCR6A, and LPAR5) for peptone and amino acids (digested proteins) are present in G cells and are likely to be involved in amino acid-induced gastrin secretion. Gastrin stimulates parietal cells to acid secretion [17,18,19].
Parietal cells secrete gastric acid via direct and indirect pathways. These cells are directly stimulated by gastrin through activation of the CCK2 receptor [20, 21]. CCK2 receptor is also important for parietal cell differentiation and maturation. Germline CCK2 receptor-deficient mice demonstrate decreased numbers of parietal and ECL cells owing to gastric mucosal atrophy, resulting in increased gastric pH and plasma gastrin levels. However, the primary pathway is the indirect effect of gastrin through the paracrine release of histamine from ECL cells, which directly stimulates parietal cells by activating H2 receptors for acid secretion [6, 22].
Histamine is a biogenic amine generated by histidine decarboxylase in ECL cells present in the corpus of the gastric mucosa. ECL cells are localised next to parietal cells and are closed-type enteroendocrine cells because they have no direct contact with the gastric lumen. Histamine molecules are released directly through these pathways: circulating gastrin stimulates ECL cells via CCK2R, and neuronal pituitary adenylate cyclase-activating peptide stimulates ECL cells via PAC1R. Somatostatin suppresses histamine release via SST2R on ECL cells [23].
Gs-coupled histamine H2 receptors are present in parietal cells and directly increase intracellular cAMP levels, stimulating acid secretion. Intraduodenal and intragastric HCl and nutrients stimulate antral D cells to secrete somatostatin, which inhibits gastric acid secretion. Somatostatin receptors (SST1–SST5R) are coupled with inhibitory G proteins and are distributed in the gastric mucosa (e.g. parietal cells, G cells, and ECL cells) (Fig. 2) [24].
Physiology mechanisms. Pathway parietal cell – G-cell – enterochromaffin-like cell and the regulation. Created with BioRender.com
The pathophysiology of CSU in AAG is complex and involves several complex changes in acid secretion, ECL hyperplasia cells, and an excess of histamine that leaks into the bloodstream, which could be a triggering factor in chronic urticaria (Fig. 3). The central point in the pathogenesis of oxyntic mucosal damage is the formation of an autoreactive clone of CD4 + T lymphocytes that stimulates a cellular and humoral immune response against various structures of parietal cells or intrinsic factors. Diffuse destruction of the oxyntic glands results in achlorhydria, pepsinogen deficiency, and a lack of intrinsic factors that participate in the absorption of vitamin B12 [25, 26]. Achlorhydria stimulates G cells in the anthroduodenal region to significantly and long-term increase gastrin secretion. Increased gastrin levels stimulate histamine-producing enhanced ECL cells. Long-term (and in the absence of parietal cells, ineffective) stimulation of ECL cells leads to hyperplasia, possibly even tumour transformation [27]. ECL cell hyperplasia has been reported in children in the AAG group [28]. ECL cell hyperplasia in the stomach may be the cause of CSU because histamine can leak into the bloodstream and cause chronic urticaria. Histamine is a vasoactive mediator and the central factor in the pathophysiology of urticaria. The effects of histamine on cells occur via four receptors: H1, H2, H3, and H4. Histamine receptors are G protein-coupled receptors. The H1 and H2 receptors are at the forefront of interest. The effects of H1 receptors are mediated by increased phospholipase C activity, increased cytoplasmic calcium, and a subsequent increase in protein kinase C activity [29]. H1 receptors are expressed throughout the body, including blood vessels, smooth muscle cells of the airway, and neurons. H1 receptors are activated and lead to allergic/anaphylactic reactions such as vasodilation, pruritus, hypotension, tachycardia, flushing, and bronchoconstriction. They regulate behaviour, memory, learning, locomotion, sleep–wake cycles, thermal regulation, and food intake. H1 receptor antagonists are used to treat the adverse effects of histamine. The effects of H2 receptors are mediated by increased intracellular cAMP levels and the subsequent activation of protein kinase A [30, 31]. H2 receptors are expressed in the parietal cells of the gastric mucosa, smooth muscle cells, and the heart. H2 receptors are activated and primarily lead to increased gastric acid secretion; however, if ECL cells are hyperplastic and parietal cells are damaged in autoimmune gastritis, histamine can leak into the bloodstream.
Pathophysiological mechanism of autoimmune gastritis and urticaria. The pathophysiology of chronic spontaneous urticaria in autoimmune atrophic gastritis is complex and involves changes in acid secretion, enterochromaffin-like hyperplasia, ensuing excess of histamine leaks into the bloodstream which could trigger chronic urticaria. Created with BioRender.com
Pathological and clinical characterization
Although the actual prevalence of chronic atrophic gastritis is unknown, and this entity may be largely underdiagnosed, patients in whom a diagnosis is established commonly present with advanced disease stages [32]. The diagnosis of AAG is often delayed owing to a lack of specific clinical manifestations and signs and remains reliant on pathological diagnosis. The predominant and characteristic symptoms of AAG include GI symptoms, deficiencies in iron and less commonly vitamin B12 [5, 31]. Complications in the clinical course of AAG include ECL hyperplasia, which may precede the formation of a NET from ECL cells due to long-term stimulation by high gastrin levels. Furthermore, adenocarcinoma of the stomach arises from a sequence of changes in intestinal metaplasia–dysplasia–adenocarcinoma. Furthermore, additional risk factors in the pathogenesis of urticaria, including AAG and ECL hyperplasia, need to be identified. A typical endoscopic finding in AAG is diffuse atrophy of the mucous membrane in the body and fundus of the stomach, which is macroscopically visible as the thinning of the mucous membrane with the disappearance of its folds (Fig. 4). Histologically, it presents as inactive atrophic chronic gastritis with pyloric and intestinal metaplasia (Fig. 5). Hyperplasia of neuroendocrine ECL cells indicates hypergastrinaemia in achlorhydria (Figs. 6 and 7). In patients with a histology compatible with autoimmune gastritis, gastroenterologists should consider checking for APCA antibodies and AIFA antibodies to assist with diagnosis. Gastroenterologists should also evaluate anaemia due to vitamin B12 and iron deficiencies.
Conclusion
Paediatricians and pathologists should consider the possibility of AAG in the paediatric population. This article describes the possible mechanisms underlying urticaria in patients with AAG. These findings suggest that the development of chronic urticaria could be caused by ECL hyperplasia with the subsequent excessive production of histamine. If a paediatric patient presents with symptoms such as anaemia, reduced vitamin B12 levels, recurrent urticaria with no other detectable aetiology, positive APCA, and elevated gastrin levels, AAG should be considered a possible cause of these skin manifestations. Physicians should suspect AAG in patients with a history of autoimmune disorders and/or urticaria.
References
Zuberbier T, Abdul Latiff AH, Abuzakouk M et al (2022) The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy 77(3):734–766. https://doi.org/10.1111/all.15090
Boguniewicz M (2008) The autoimmune nature of chronic urticaria. Allergy Asthma Proc 29(5):433–438. https://doi.org/10.2500/aap.2008.29.3148
Kolkhir P, Church MK, Weller K, Metz M, Schmetzer O, Maurer M (2017) Autoimmune chronic spontaneous urticaria: What we know and what we do not know. J Allergy Clin Immunol 139(6):1772-1781.e1. https://doi.org/10.1016/j.jaci.2016.08.050
Wedi B, Raap U, Kapp A (2004) Chronic urticaria and infections. Curr Opin Allergy Clin Immunol 4(5):387–396. https://doi.org/10.1097/00130832-200410000-00010
Lenti MV, Rugge M, Lahner E et al (2020) Autoimmune gastritis. Nat Rev Dis Primers 6(1):56. https://doi.org/10.1038/s41572-020-0187-8
Waldum HL, Hauso Ø, Fossmark R (2014) The regulation of gastric acid secretion - clinical perspectives. Acta Physiol 210(2):239–256. https://doi.org/10.1111/apha.12208
Massironi S, Zilli A, Elvevi A, Invernizzi P (2019) The changing face of chronic autoimmune atrophic gastritis: an updated comprehensive perspective. Autoimmun Rev 18(3):215–222. https://doi.org/10.1016/j.autrev.2018.08.011
Vanoli A, La Rosa S, Luinetti O et al (2013) Histologic changes in type A chronic atrophic gastritis indicating increased risk of neuroendocrine tumor development: the predictive role of dysplastic and severely hyperplastic enterochromaffin-like cell lesions. Hum Pathol 44(9):1827–1837. https://doi.org/10.1016/j.humpath.2013.02.005
Miguel N, Costa E, Santalha M Jr, Lima R, Vizcaino JR, Pereira F, Barbot J (2014) Refractory iron-deficiency anemia and autoimmune atrophic gastritis in pediatric age group: analysis of 8 clinical cases. J Pediatr Hematol Oncol 36(2):134–139. https://doi.org/10.1097/MPH.0000000000000069
Calcaterra V, Montalbano C, Miceli E et al (2020) Anti-gastric parietal cell antibodies for autoimmune gastritis screening in juvenile autoimmune thyroid disease. J Endocrinol Invest 43(1):81–86. https://doi.org/10.1007/s40618-019-01081-y
Amedei A, Bergman MP, Appelmelk BJ et al (2003) Molecular mimicry between Helicobacter pylori antigens and H+, K+ -adenosine triphosphatase in human gastric autoimmunity. J Exp Med 198(8):1147–1156. https://doi.org/10.1084/jem.20030530
Chu CY, Zuberbier T (2020) Urticaria and the gut. Curr Opin Allergy Clin Immunol 20(4):381–385. https://doi.org/10.1097/ACI.0000000000000653
Zhang C, Wei Y (2021) Association between helicobacter pylori-negative peptic ulcer disease and chronic urticaria: a retrospective observational study. Clin Cosmet Investig Dermatol 14:1637–1643. https://doi.org/10.2147/CCID.S336617
Mescher LA (2021) Junqueira’s basic histology text and atlas. McGraw Hill
Ross MH, Pawlina W (2016) Histology: a text and atlas with correlated cell and molecular biology. Lippincott Williams & Wilkins
Choi E, Petersen CP, Lapierre LA et al (2015) Dynamic expansion of gastric mucosal doublecortin-like kinase 1-expressing cells in response to parietal cell loss is regulated by gastrin. Am J Pathol 185(8):2219–2231. https://doi.org/10.1016/j.ajpath.2015.04.009
Rettenberger AT, Schulze W, Breer H, Haid D (2015) Analysis of the protein related receptor GPR92 in G-cells. Front Physiol 6:261. https://doi.org/10.3389/fphys.2015.00261
Haid DC, Jordan-Biegger C, Widmayer P, Breer H (2012) Receptors responsive to protein breakdown products in g-cells and d-cells of mouse, swine and human. Front Physiol 3:65. https://doi.org/10.3389/fphys.2012.00065
Iwanaga T (2023) Anatomical basis of gastrin- and CCK-secreting cells and their functions. A review. Biomed Res 44(3):81–95. https://doi.org/10.2220/biomedres.44.81
Wank SA, Pisegna JR, de Weerth A (1992) Brain and gastrointestinal cholecystokinin receptor family: structure and functional expression. Proc Natl Acad Sci U S A 89(18):8691–8695. https://doi.org/10.1073/pnas.89.18.8691
Kopin AS, Lee YM, McBride EW et al (1992) Expression cloning and characterization of the canine parietal cell gastrin receptor. Proc Natl Acad Sci U S A 89(8):3605–3609. https://doi.org/10.1073/pnas.89.8.3605
Gardner JD, Jackson MJ, Batzri S, Jensen RT (1978) Potential mechanisms of interaction among secretagogues. Gastroenterology 74(2Pt2):348–354. https://doi.org/10.1097/00004836-198312001-00006
Zeng N, Kang T, Lyu RM et al (1998) The pituitary adenylate cyclase activating polypeptide type 1 receptor (PAC1-R) is expressed on gastric ECL cells: evidence by immunocytochemistry and RT-PCR. Ann N Y Acad Sci 865:147–156. https://doi.org/10.1111/j.1749-6632.1998.tb11173.x
Allen JP, Canty AJ, Schulz S, Humphrey PP, Emson PC, Young HM (2002) Identification of cells expressing somatostatin receptor 2 in the gastrointestinal tract of Sstr2 knockout/lacZ knockin mice. J Comp Neurol 454(3):329–340. https://doi.org/10.1002/cne.10466
Toh BH, Sentry JW, Alderuccio F (2000) The causative H+/K+ ATPase antigen in the pathogenesis of autoimmune gastritis. Immunol Today 21(7):348–354. https://doi.org/10.1016/s0167-5699(00)01653-4
Hershko C, Patz J, Ronson A (2007) The anemia of achylia gastrica revisited. Blood Cells Mol Dis 39(2):178–183. https://doi.org/10.1016/j.bcmd.2007.03.006
Bordi C, D’Adda T, Azzoni C, Pilato FP, Caruana P (1995) Hypergastrinemia and gastric enterochromaffin-like cells. Am J Surg Pathol 19(Suppl 1):S8–S19
Demir AM, Berberoğlu Ateş B, Hızal G et al (2020) Autoimmune atrophic gastritis: the role of Helicobacter pylori infection in children. Helicobacter 25(5):e12716. https://doi.org/10.1111/hel.12716
Smit MJ, Hoffmann M, Timmerman H, Leurs R (1999) Molecular properties and signalling pathways of the histamine H1 receptor. Clin Exp Allergy 29(Suppl 3):19–28. https://doi.org/10.1046/j.1365-2222.1999.00007.x-i1
Dy M, Schneider E (2004) Histamine-cytokine connection in immunity and hematopoiesis. Cytokine Growth Factor Rew 15(5):393–410. https://doi.org/10.1016/j.cytogfr.2004.06.003
Panula P (2021) Histamine receptors, agonists, and antagonists in health and disease. Handb Clin Neurol 180:377–387. https://doi.org/10.1016/B978-0-12-820107-7.00023-9
Rodriguez-Castro KI, Franceschi M, Noto A et al (2018) Clinical manifestations of chronic atrophic gastritis. Acta Biomed 89(8-S):88–92. https://doi.org/10.23750/abm.v89i8-S.7921
Funding
The SVV–2023 No 260 651 grant and Cooperatio supported the work.
Author information
Authors and Affiliations
Contributions
J.B.: Conceptualization; Writing—original draft; Visualization; Writing—review & editing; Resources. J.S.:; Writing—original draft; Supervision; Conceptualization. L.V:: Conceptualization; Writing—original draft; Visualization. V.G.: Supervision. Š.K.: Supervision. O.D.: Supervision; Visualization. J.Sch.: Conceptualization; Supervision; Writing—review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Peter de Winter
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bufka, J., Sýkora, J., Vaňková, L. et al. Impact of autoimmune gastritis on chronic urticaria in paediatric patients – pathophysiological point of views. Eur J Pediatr 183, 515–522 (2024). https://doi.org/10.1007/s00431-023-05324-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-023-05324-2