Abstract
The coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread rapidly worldwide, seriously endangering human health. Although SARS‐CoV‐2 had a lower impact on paediatric population, children with COVID-19 have been reported as suffering from gastrointestinal (GI) symptoms at a higher rate than adults. The aim of this work was to evaluate faeces as a source of potential biomarkers of severity in the paediatric population, with an emphasis on intestinal microbiota and faecal immune mediators, trying to identify possible dysbiosis and immune intestinal dysfunction associated with the risk of hospitalization. This study involved 19 patients with COVID-19 under 24 months of age hospitalized during the pandemic at 6 different hospitals in Spain, and it included a comparable age-matched healthy control group (n = 18). Patients and controls were stratified according to their age in two groups: newborns or young infants (from 0 to 3 months old) and toddlers (infants from 6 to 24 months old). To characterize microbial intestinal communities, sequencing with Illumina technology of total 16S rDNA amplicons and internal transcribed spacer (ITS) amplicons of bifidobacteria were used. Faecal calprotectin (FC) and a range of human cytokines, chemokines, and growth factors were measured in faecal samples using ELISA and a multiplex system. Significant reduction in the abundance of sequences belonging to the phylum Actinobacteria was found in those infants with COVID-19, as well as in the Bifidobacteriaceae family. A different pattern of bifidobacteria was observed in patients, mainly represented by lower percentages of Bifidobacterium breve, as compared with controls. In the group of hospitalized young infants, FC was almost absent compared to age-matched healthy controls. A lower prevalence in faecal excretion of immune factors in these infected patients was also observed.
Conclusion: Hospitalized infants with COVID-19 were depleted in some gut bacteria, such as bifidobacteria, in particular Bifidobacterium breve, which is crucial for the proper establishment of a functional intestinal microbiota, and important for the development of a competent immune system. Our results point to a possible immature immune system at intestine level in young infants infected by SARS-CoV2 requiring hospitalization.
What is Known: • Although SARS‐CoV‐2 had a lower impact on paediatric population, children with COVID-19 have been reported as suffering from gastrointestinal symptoms at a higher rate than adults. • Changes in microbial composition have been described in COVID-19 adult patients, although studies in children are limited. | |
What is New: • The first evidence that hospitalized infants with COVID-19 during the pandemic had a depletion in bifidobacteria, particularly in Bifidobacterium breve, beneficial gut bacteria in infancy that are crucial for the proper establishment of a competent immune system. • In young infants (under 3 months of age) hospitalized with SARS-CoV2 infection, the aberrant bifidobacterial profile appears to overlap with a poor intestinal immune development as seen by calprotectin and the trend of immunological factors excreted in faeces. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread rapidly worldwide, seriously endangering human health. COVID-19 was declared a global pandemic in early 2020 by the World Health Organization (WHO), and to date, it has been diagnosed in more than 633 million people worldwide with 6,603,384 deaths (https://coronavirus.jhu.edu/data).
It has been observed that age is a differential factor in the prevalence and severity of SARS-CoV-2 [1]. The accumulated epidemiological and clinical data indicate that children, often more susceptible to respiratory virus infections [2], are less frequently and severely infected with SARS-CoV-2 than adults [1, 3, 4]. Most infected children were asymptomatic or had mild symptoms, being fever, cough, appetite loss, or gastrointestinal symptoms such as abdominal pain, nausea, diarrhoea, and vomiting the most frequent ones [5, 6]. In addition, some children with COVID-19 developed autoimmune and autoinflammatory complications, with features similar to Kawasaki disease, the so-called multisystem inflammatory syndrome in children (MIS-C) with significant digestive involvement [7, 8].
It has been estimated that the human body contains 1014 commensal microorganisms, tenfold the number of human cells, called microbiota, whose integrity is pivotal for human health. The majority of the microbiota reside in the gastrointestinal tract, taking part in the regulation of immune responses, inflammation status, and mucosal homeostasis, as well as in the defense against pathogens [9]. The establishment of gut microbiota takes place in the first stages of life, getting to relative stability by the age of approximately 2–3 years old, when its composition is more similar to the adult microbiota [10]. However, the microbial colonization can undergo fluctuations due to different factors such as type of delivery and breastfeeding, antibiotic administration, or hospital admission [11, 12]. In this context, while some studies have found changes in gut microbiota composition between patients infected with SARS-CoV-2 and healthy controls in adult populations [13, 14], the role of the gut microbiota in the susceptibility to COVID-19 infection in children remains unclear [8, 15, 16].
The aim of the present study was to examine gut microbiota composition and immune faecal factors in a paediatric population under 24 months of age hospitalized for COVID-19 disease, trying to identify potential relationships between intestinal dysbiosis and local inflammation associated with severe viral infection.
Material and methods
Participants
The study sample comprised 19 paediatric patients infected with SARS-CoV-2 under 24 months of age and 18 age-matched healthy controls. Patient recruitment was carried out in different public hospitals in Spain: “Hospital Universitario Central de Asturias” (Oviedo), “Complejo Hospitalario Universitario de Santiago de Compostela” (Santiago de Compostela), “Hospital Universitario y Politécnico La Fe” (Valencia), “Hospital Universitario Infantil Niño Jesús” (Madrid), “Hospital Universitario Virgen del Rocío” (Sevilla), and “Hospital Regional Universitario de Málaga” (Málaga). The recruitment period took place from October 2020 to July 2021. Inclusion criteria for patients’ selection were as follows: less than 24 months old, positive swab test for SARS-CoV-2 using reverse transcriptase quantitative polymerase chain reaction (qPCR) assay, and need for hospital admission. The control group was recruited by paediatricians in Spanish primary care centres at the time of scheduled well-child care visits, and inclusion criteria were as follows: (i) no previous antibiotic intake along the 3 months prior to sample collection, (ii) no clinical history of chronic and/or digestive tract related diseases, (iii) have not been diagnosed with COVID-19 by qPCR or antigen test, (iv) have not been in contact with people diagnosed or with suspicious of SARS-CoV-2 infection, and (v) do not present any symptoms of COVID-19 at the time of sampling.
Parents or legal guardians of all participants were informed of the objectives of the study, and they gave informed written consent. After that, stool samples were collected from each participant by its relative allowed to be at the hospital room. For that, families were provided with gloves, a sterile spoon and a stool sample tube. In the case of patients, the collection of samples was performed within the first 12 h after hospital admission. Clinical data was collected by clinicians and managed using Research Electronic Data Capture (REDCap) tools [17], hosted at “Sociedad Española de Gastroenterología, Hepatología y Nutrición Pediátrica” (SEGHNP) (redcap.seghnp.org) with assist from AEGREDCap Support Unit, shared with “Asociación Española de Gastroenterología” (AEG).
Ethical approval for this study was obtained from the Regional Ethics Committee for Clinical Research (Servicio de Salud del Principado de Asturias, no. 112/13) and from the Bioethics Committee of Consejo Superior de Investigaciones Científicas (CSIC) in compliance with the Declaration of Helsinki.
Stool sample preparation
After stool collection, faecal samples were immediately frozen at the recruitment institutions. Transport to the Institute of Dairy Products of Asturias (IPLA-CSIC) was performed on dry ice in shipping biological containers type CTM03 according to UN3373 standard. At IPLA-CSIC, biological samples were stored at − 80 °C until use. Stool samples were thawed on ice for 30 min in a biosafety cabinet. Viral inactivation was performed with MagMAX Viral/Pathogen Binding Solution (Thermo Fisher Scientific Inc., MA, USA) for 10 min at room temperature in a relation 1:2 (w/v) [18]. Subsequently, stool samples were used or divided in aliquots for further analytical determinations.
DNA extraction and quantification
Faecal dilutions (1:2) were homogenized in phosphate-buffered saline (PBS) to reach a dilution 1:10 for DNA extraction. This was based on the International Human Microbiome Standards (IHMS), protocol Q [19] using the QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany) with slight modifications. A mechanical lysis was performed in a Fisherbrand Bead Will 24 homogenizer (Thermo Fisher Scientific Inc., MA, USA) with 3 cycles of lysis for 45 s each and leaving the samples on ice for 5 min between each treatment. The DNA was eluted and resuspended in 50 µl of molecular-biology grade water (Sigma-Aldrich, Saint Louis, USA), and stored at − 20 °C until use. DNA concentration was quantified in a Qubit fluorometer with dsDNA assay kits (Thermo Fisher, Waltham, USA).
High-throughput sequencing of 16S rRNA gene amplicons and ITS region of rRNA genes amplicons of bifidobacteria
The 16S rDNA was amplified from the DNA of samples according to Milani et al. (2013) with primers pair “Probio_Uni/Probio_Rev” targeting the V3 region of the 16S rRNA gene, and 250 bp paired-end sequences were obtained using an Illumina MiSeq System (Illumina, San Diego, USA) at the spin-off of the University of Parma Genprobio srl (Italy) [20]. The sequences were processed using the Quantitative Insights Into Microbial Ecology (QIIME) software suite and were classified to the lowest possible taxonomic rank considered, using the SILVA database v. 132 as reference. In the same way, the internal transcribed spacer (ITS) region of rRNA genes of Bifidobacterium genus was amplified and subjected to high-throughput sequencing. For this, the primers “Probio_bif_uni” and “Probio_bif_rev” were used [21], together with an improved bifidobacterial ITS database and Genprobio personalized bioinformatic scripts. The raw sequences data were deposited in the Sequence Read Archive (SRA) of the NCBI (https://www.ncbi.nlm.nih.gov/sra) under BioProject ID: PRJNA911135 (sequence library IDs SAMN32161911 to SAMN32161927) and BioProject ID PRJNA914097 (sequence library IDs SAMN32320403 to SAMN32320420).
Intestinal inflammatory biomarkers
Faecal calprotectin determination
Calprotectin levels were quantified using the commercially available enzyme-linked immunosorbent assay (ELISA) kit CALPROLAB™ (Calpro, Lysaker, Norway) according to the manufacturer’s instructions.
Faecal immune factors
The concentration of 27 cytokines, chemokines, and growth factors was determined using a Bio-Plex 200 system (Bio-Rad, Hercules, USA) and the Bio-Plex Pro Human Cytokine 27-plex Assay kit (Bio-Rad). Before analysis, faeces diluted tenfold (w/v) in PBS were centrifuged for 15 min at 20,000 g at 4 °C, and the supernatants were collected, diluted, and treated following the manufacturer’s protocol. Standards and samples were determined in duplicate. Data acquisition was performed with the Bio-Plex Manager 6.0 software and the standard curves fitted to a 5-parameter logistic regression.
Statistical analyses
Statistical analyses were performed using IBM SPSS Statistics v.28.0.1 (IBM, Armonk, NY, USA). To examine the changes between patients and controls, we used the non-parametric U-Mann Whitney test and two-tailed probability values of p ≤ 0.05 were considered significant. In turn, medians, means, and interquartile ranges (Q1 and Q3) were represented in box and whisker graphics using the Origin Pro-2021 software (OriginLab, Northampton, MA, USA). Principal component analysis (PCA) was performed using R v.4.2.1. (“FactoMineR” and “Factorextra” packages).
Results
Participant characteristics
For the analysis of the results, the data was processed according to two groups of age: newborns or young infants including those between the age of 0–3 months, and toddlers, between 6 and 24 months of age. Information of age, gender, prematurity, mode of delivery, and type of feeding for both controls and patients is shown in Suppl. Table 1.
A total of 20 newborns were included, 11 patients with COVID-19, and 9 healthy controls. While patients in the newborns’ group had an average age of 1.9 ± 0.6 months, the average age of controls was 1.7 ± 0.8 months. On the other hand, the toddlers’ group was composed of 8 patients with COVID-19 and 9 healthy controls. The median age of patients in this group was 17 ± 7.7 months and 11 ± 4.8 months for controls. The hospital of origin, as well as comorbidities/previous chronic pathology, the main reason for admission, the days in hospital, and the treatment for each patient are shown in Table 1. No significant differences were found between patients and controls with respect to the variables type of delivery, type of feeding, and prematurity in the different age groups.
Fever was the main reason for admission in patients with COVID-19 in both groups. However, other clinical problems were observed (Fig. 1). Among newborns, 18.18% of patients presented digestive symptoms, with diarrhoea being the main clinical finding. On the other hand, 62.50% of toddlers’ cases showed digestive symptoms: abdominal pain (12.50%), nausea (25.00%), and diarrhoea (25.00%). Regarding other symptoms, respiratory problems were found in about 50.00% of patients (45.50% in newborns versus 50.00% in toddlers). It was observed that 25.00% of the patients in the toddler group presented skin alterations. (Fig. 1). In the newborns’ group 18.18% were treated with antibiotics; meanwhile, in the toddlers’ group, this percentage was 25.00% (Table 1). In this last group, 37.50% of patients received immunosuppressive therapy at hospital.
Faecal microbiota and compositional profile
Analysis of faecal microbial 16S sequences showed that Actinobacteria’ abundancy was lower in COVID-19 patients in both age groups (newborns and toddlers) as compared with controls (Fig. 2). In particular, in the newborn group, Actinobacteria phylum appeared reduced in COVID-19 infants (28.02 ± 32.76 patients versus 53.52 ± 28.69 controls), but no statistically significant differences were observed (p-value = 0.113). A significant reduction was observed in toddlers (p-value = 0.006), with lower Actinobacteria proportion in COVID-19 patients (15.10 ± 20.19) compared to healthy controls (49.53 ± 24.22) (Fig. 2B). On the other hand, while in the newborn patients the main microbial phyla were represented by Proteobacteria and Firmicutes followed by a low percentage of Bacteroidetes, in newborn controls, the microbial profile was distributed by Proteobacteria, Firmicutes, Bacteroidetes, and a reduced number of sequences belonging to Verrucomicrobia phyla (0.56% ± 1.19) (Fig. 2A). In contrast, looking at faecal microbial composition among toddlers (patients), the reduction of Actinobacteria provided a redistribution of the abundance of the rest of the phyla in comparison with controls, dominated by sequences of Firmicutes and Actinobacteria (Fig. 2B). Percentages of sequences of Proteobacteria, and Bacteroidetes, were higher in patients with COVID-19, although no significant differences were found.
In the newborns’ group, we observed that the most abundant families were represented by Bifidobacteriaceae and Enterobacteriaceae (Suppl. Table 2). Sequences belonging to the family Bifidobacteriaceae were more abundant in faecal samples from the control group (mean relative abundance 51.21%) compared with samples from patients with COVID-19 (mean relative abundance 25.84%). On the other hand, the relative abundance of Enterobacteriaceae family was higher in patients with COVID-19 than in controls (36.11% and 16.60%, respectively). In the toddlers’ group, a significant reduction in the proportions of sequences belonging to Bifidobacteriaceae family in patients (mean relative abundance 11.20%) compared to controls (mean relative abundance 44.34%) was observed (p-value = 0.004) (Suppl. Table 2). Relative abundance of Enterococcaceae family sequences was significantly higher in controls compared with patients (p-value = 0.027), without reaching percentages higher than 2% in any case.
At genus level, Bifidobacterium was the predominant genus in newborns, with a higher relative abundance in controls (51.21 ± 27.90) compared to patients (25.82 ± 32.51) (Suppl. Table 3). Moreover, a high percentage of assigned reads to the enterobacteria Escherichia-Shigella was observed in the samples of newborn patients (mean relative abundance 26.83%). In the toddlers’ group, we found a greater diversity of anaerobic genera belonging to different families, such as Ruminococcaceae, Lachnospiraceae, and Bacteroidaceae among others. The genus Bifidobacterium also showed a significant higher representation in the control group (p-value = 0.004).
Therefore, we investigated whether the reduction of Actinobacteria observed for COVID-19 patients was associated with changes in the bifidobacterial profile. We analysed the presence and diversity of bifidobacteria species through sequencing the ITS region. As depicted in Fig. 3, a higher diversity of bifidobacteria in patients with COVID-19 was detected in both groups of age as compared to healthy children. In newborns, we observed that the majority of sequences corresponded to Bifidobacterium longum and Bifidobacterium breve species (Fig. 3A). In newborn patients, a slight reduction in B. breve and an increased number of B. dentium sequences was observed as the main characteristics. Also of note was the presence of an ITS profile characterized by other less abundant species that were not found in the healthy group, such as B. longum spp. suis, B. animalis, and other bifidobacteria not classified yet (Fig. 3A).
Profile of internal transcribed spacer (ITS) sequences of bifidobacteria represented as relative abundance in patients and controls in newborns (9 patients and 9 controls) (A) and toddlers (8 patients and 9 controls) (B). Data showed percentages of species of Bifidobacterium above 5% at least in one group
On the other hand, among toddlers, we found statistical differences at genus level. Specifically, patients showed reduced levels of Bifidobacterium through 16S sequencing (p-value = 0.004). Similarly, when we analysed the ITS profile, data showed that patients with COVID-19 had a greater diversity of bifidobacteria species than controls (Fig. 3B). Also, at this age range, reduction in the proportion of B. breve was also observed as compared with controls, together with an increase of sequences belonging to undetermined species of Bifidobacterium (new taxa).
A complementary analysis was carried out considering GI symptoms in the full sample studied: 19 patients and 18 healthy controls. Although no significant differences were found, results showed a differential microbial profile of the patients with GI characterized by Proteobacteria and Firmicutes as the major phyla, an increase in Bacteroidetes and a decrease in Actinobacteria sequences when compared to healthy controls and patients without GI symptoms (data not shown).
Faecal immune biomarkers
Calprotectin levels
As illustrated in Fig. 4A, faecal calprotectin (FC) concentration was significantly different among patients and controls in the group of newborns (p-value = 0.045). Contrary to what we expected the results showed that the mean FC was lower in patients (38.87 µg/g of faeces) than in healthy controls (98.53 µg/g). It should be noticed that the determination of FC was only possible in 4 patients and 5 controls, which considerably reduced the size of the newborns’ group.
Calprotectin concentration (μg/g of faeces) in the faecal samples of the newborns (6 patients and 5 controls) (A) and toddlers (5 patients and 9 controls) (B). The lines inside the rectangle show the medians, and the whiskers show the maximum and minimum values. Comparisons were made with the U-Mann Whitney test to examine changes between patients and controls
On the other hand, the values of FC in toddlers did not show a statistical difference (p-value = 0.606). Patients had a FC concentration of 292.26 ± 441.28 µg/g (median ± standard deviation), whereas controls showed 118.84 ± 103.73 µg/g (Fig. 4B). Also here, we were unable to determine the calprotectin concentration in faeces for all participants of this age group due to the low quantity of faeces received (n = 5 for children with COVID-19) which could explain, in part, why we found no significant differences. Interestingly, the infant with COVID-19 who showed the highest FC level (1,077.12 µg/g of faeces) has been diagnosed with MIS-C following the WHO criteria [22]. In our analyses, this data was an extreme value (> 3 × interquartile ranges), so it was not considered for the statistical analysis.
Faecal immune factors
A total of 27 cytokines, chemokines, and grown factors, measured as immune markets of intestinal barrier maturation and inflammation, were determined. For statistical analysis, cytokines that were detected in at least half of the individuals in one of the groups were evaluated. Out of 27 immune factors, 12 of them were detected, whereas the rest were undetectable or below the limit of quantification based on the standard curves of the immunoassays. Table 2 shows these faecal immune factors according to the group of age, with the exception of platelet-derived growth factor-BB (PDGF-BB) which is showed in Fig. 5.
Plots showing faecal concentration of platelet-derived growth factor-BB (PDGF-bb) in COVID-19 patients and controls. A Newborns (0–3 months, 7 patients and 7 controls). B Toddlers (6–24 months, 7 patients and 7 controls). The lines inside the rectangle show the medians, and the whiskers show the maximum and minimum values. Comparisons were made with the U-Mann Whitney test to examine changes between patients and controls
For all quantified immune factors, but PDGF-BB, we did not find statistical differences. However, it should be noted that in both age groups, we observed a general trend towards a higher number of individuals excreting immune factors in controls compared to patients (Table 2), especially in the newborns. In this particular age group, a statistical difference in the levels of PDGF-BB was found (p-value = 0.026) between patients and controls, with newborns hospitalized for COVID-19 presenting higher faecal values (Fig. 5).
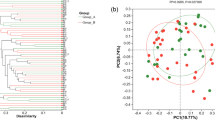
For multivariable analysis and comparison between patients and controls, PCA analysis with two components and all variables studied was performed. An association between Actinobacteria and most of the faecal immune factors was observed (Fig. 6) as they significantly correlated with the first same dimension, explaining 25.90% of the total variability.
Discussion
Several studies have been performed since the beginning of the pandemic, reflecting that SARS-CoV-2 infects not only the respiratory tract but also other epithelia such as the gastrointestinal mucosa where the angiotensin-converting enzyme 2 (ACE-2) receptors for the virus are found [23]. This direct viral infection may result in cytokine release and neutrophil activation at local intestinal level [24]. The intestinal epithelial cell damage caused by SARS-CoV-2 was thought to be associated with gastrointestinal symptoms, and particularly, children are reported to have more digestive symptomatology than adults [6]. Confirmed SARS-CoV-2 infection with gastrointestinal symptoms and changes in microbiota associated with COVID-19 severity have been reported in adults [25]. These types of studies in children are really scarce [8, 15, 16]. However, only in Spain, more than 1000 children were hospitalized due to COVID-19 [26]. In this work, we presented a multicentre study of infants with SARS-CoV-2 infection hospitalized in 6 Spanish hospitals. We observed that the presence of digestive symptoms was higher than 50%, at least in the case of infants from 6 to 24 months of age; however, we did not find statistical differences in the composition of the microbiota between patients with and without digestive symptoms.
Analysis of faecal microbial composition revealed a marked change with respect to control healthy infants in both groups (newborns and toddlers) that mainly consisted in a reduction in the percentage of sequences belonging to the Actinobacteria filum and Bifidobacteriaceae family. In agreement with our results, previous studies in children with COVID-19 reported a reduction in the abundance of Actinobacteria in the microbiota of patients compared to controls [8, 15]. Depletion of commensal bacteria (bifidobacteria) and increase of opportunistic pathogens have been also observed in adults [27]. In our work, among the genera Bifidobacterium, we also observed differences when comparing the ITS bifidobacterial profiles. A higher diversity of bifidobacterial, in terms of number of different species, was observed in patients with COVID-19 as compared with controls. In literature, it is reported that healthy children younger than 3 years of age possess low (alpha) diversity of the gut microbiota dominated by few bacterial species [28], representing Bifidobacterium the dominant bacterial genus [29]. In on work, relative abundance and number of species of bifidobacteria were different, with more presence of sequences assigned to Bifidobacterium breve in healthy control infants. Bifidobacterium breve, which is the main constituent of the intestinal microbiota of healthy newborns, is responsible for the development of intestinal biocenosis as well as for the activation of the immature immune system [30]. In fact, there are studies in animals’ models showing a direct activation of immature immunity by strains of this species [31]. As such, children with food allergy were observed to have decreased numbers of this species [32]. In brief, this bifidobacterial species that colonize the infant’s gut can induce epithelial barrier maturation and may protect against pathogenic bacteria [33]. In patients, we observed the presence of an ITS profile characterized by other bifidobacterial species that were not found in the healthy group, such as B. animalis or B. dentium which are known as cosmopolitan bifidobacterial species, not particularly associated to infancy [28].
FC is considered a useful diagnostic biomarker for intestinal mucosal inflammation, although their reference values are more clearly defined in adults than in children [34]. Calprotectin is an immunomodulatory, antimicrobial, and antiproliferative protein that is present in the cytoplasm of neutrophils, but also in the membranes of macrophages, in activated monocytes, and in mucosal epithelial cells, being of importance in defense mechanisms and physiological functions of the immune system [35]. However, no clear cut-offs have convincingly been established throughout infancy [36, 37]. Additionally, some studies have reported no clear consistence with digestive symptoms in COVID-19 [38, 39]. In this work, consistently with others previous observations [16], we did not find differences between values obtained in healthy and hospitalized toddlers ranging from 6 to 24 months of age. It is true that in this group of patients, a case of MIS-C with a FC over 1000 µg/g was included, which clearly reflects high inflammation and compromised gut barrier integrity which may make the surface of the enterocytes more susceptible to invasion by pathogens in the gut [40]. In fact, this patient presented an elevated colonization (45% relative abundance) by Gram-negative Proteobacteria as revealed by 16S sequence analysis (data not shown).
Unexpectedly, we found statistical differences between FC levels in newborns, ranging from 0 to 3 months of age, with higher values in healthy controls. This tendency was also described in babies under 1 year of age with cystic fibrosis in which controls had higher calprotectin values than patients [41]. This can point to an immature immune system at intestinal level with less neutrophils migration in hospitalized newborns with COVID-19. Velasco Rodríguez-Belvís and colleagues (2020) have already described that healthy babies between 1 and 5 months have elevated FC values probably due to the activation of the immune system after birth [34], with a high calling for neutrophils to the local mucosa and the activation of anti-defense mechanisms. Consistent with our observations, in adults, a dysfunctional immune response associated with gut microbiota dysbiosis has been proposed in severe COVID-19 [40, 42]. Trevelin and colleagues (2022) have already observed that the intestinal immune response is compromised in adults with severe COVID-19 [25]. These authors found a depletion of germinal centres in ileal Peyer´s patches and decreased potential B and T cell interaction and argue for a link with gut microbial dysbiosis.
Reinforcing this hypothesis, in this study, we found that faecal immune excretion (both anti and pro-inflammatory mediators) was more prevalent in healthy newborns than in those hospitalized with COVID-19. The only factor for which significant higher values were found in the faeces of patients was PDGF-BB. This factor, derived from platelets and related to vascular remodelling and angiogenesis, was shown in several studies to be strongly associated with severe COVID-19 [43, 44], although these results were observed in soluble plasma and in adults’ patients. This difference was not observed in the group of toddlers in whom the lower faecal cytokine excretion in patients was not so remarkable. It seems that in the small babies hospitalized with COVID-19, the intestinal immune system could not be completely mature and the mucosal lymphoid tissue might be still under development. Consistent with our theory, a higher frequency of faecal detection of immune factors in samples from healthy controls than in patients was shown in other work in infants (less than 18 months of age) with bronchiolitis [45]. In addition, local mucosal immune mediators in preterm infants were seen to be reduced or undetectable with similar faecal cytokine profiling technique [46]. However, in our cohort of study, there were not preterm infants (Suppl. Table 1).
On the one hand, SARS-CoV-2 may affect the intestinal microbiota promoting dysbiosis, as we have seen in this study in both neonates and toddlers. Nevertheless, the pre-existing microbiota may play an important role in determining individual susceptibility and resilience to COVID-19 as it has been suggested by other authors [40, 47]. Gut microbiota colonization in early infancy is linked with the immune system development and response [29]. Gut health at the time of SARS-CoV-2 infection may be critical and could be one of the reasons for the differences observed in the severity of symptoms among paediatric cases, from asymptomatic to requiring intensive care unit (ICU) admission. Underactive immune response, associated with microbial dysbiosis, such as that observed in infants in this work, may render the enteric mucosa more susceptible to invasion by pathogens, such as SARS-CoV-2, and therefore, it may affect disease progression, leading to potential complications.
This study was a preliminary work in infants younger than 2 years old, which explains some of its limitations, such as the reduced number of participants and the low quantity of sample recovered.This was derived to the difficulties experienced to access to this type of hospitalized patients during the pandemic due to the health alert situation and the critical studied population. All previous works with paediatric population with COVID-19 points how the sample size (with numbers varying from 9 to 13 patients) can be a limitation in finding relevant changes [15, 16]. Another potential bias in our work is the medical treatment received by the patients, and that despite samples were collected in the first deposition at hospital within the first 12 h after admission, we cannot dismiss some effect on the faecal parameters determined in the study. However, we consider that there is very few information regarding the affectation of the microbiota and the local immune reactivity in the GI tract in infants with SARS-CoV-2 infection, especially in those with moderate or severe manifestations. Unfortunately, in our work, it has not been possible to assess the different variants of the virus, which should be investigated in future studies to provide new hypotheses in the relationship between gut microbiota in infancy and COVID-19. More descriptive studies would contribute to fill this gap of knowledge and could give some light into the reasons leading to hospitalization.
Conclusions
This study is presenting the first evidence of an aberrant microbiota profile together with a poor intestinal immune maturation trend in hospitalized infants under 3 months of age with COVID-19. Although with a reduced sample size our results point to a scenario that could facilitate severe disease caused by SARS-CoV-2. Additionally, B. breve has been seen as main depleted beneficial commensal in these patients’ gut. Novelty results connecting microbiota with SARS-CoV-2 in infant population will be useful to generate new hypotheses to test dietary strategies with probiotics in viral diseases.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACE-2:
-
Angiotensin-converting enzyme 2
- AEG:
-
Asociación Española de Gastroenterología
- CSIC:
-
Consejo Superior de Investigaciones Científicas
- COVID-19:
-
Coronavirus disease 2019
- FC:
-
Faecal calprotectin
- GI:
-
Gastrointestinal
- ICU:
-
Intensive care unit
- ITS:
-
Internal transcribed spacer
- IHMS:
-
International Human Microbiome Standards
- IPLA-CSIC:
-
Institute of Dairy Products of Asturias
- MIS-C:
-
Multisystem inflammatory syndrome in children
- PBS:
-
Phosphate-buffered saline
- PDGF-BB:
-
Platelet-derived growth factor-BB
- PFAPA:
-
Periodic fever, aphthous stomatitis, pharyngitis, adenitis
- QIIME:
-
Quantitative Insights Into Microbial Ecology
- qPCR:
-
Quantitative polymerase chain reaction
- REDCap:
-
Research Electronic Data Capture
- SARS-Cov-2:
-
Severe acute respiratory syndrome coronavirus 2
- SEGHNP:
-
Sociedad Española de Gastroenterología, Hepatología y Nutrición Pediátrica
- SRA:
-
Sequence Read Archive
- WHO:
-
World Health Organization
References
Zimmermann P, Curtis N (2022) Why does the severity of COVID-19 differ with age?: Understanding the mechanisms underlying the age gradient in outcome following SARS-CoV-2 Infection. Pediatr Infect Dis J 41:e36–e45. https://doi.org/10.1097/INF.0000000000003413
Tregoning JS, Schwarze J (2010) Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev 23:74–98. https://doi.org/10.1128/CMR.00032-09
Ludvigsson JF (2020) Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr 109:1088–1095. https://doi.org/10.1111/APA.15270
Zimmermann P, Curtis N (2021) Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch Dis Child 106:429–439. https://doi.org/10.1136/archdischild-2020-320338
Gupta R, Beg S, Jain A, Bhatnagar S (2020) Paediatric COVID-19 and the gut. Indian J Med Microbiol 38:261–264. https://doi.org/10.4103/IJMM.IJMM_20_331
Gonzalez Jimenez D, Velasco Rodríguez-Belvís M, Ferrer Gonzalez P et al (2020) COVID-19 gastrointestinal manifestations are independent predictors of PICU admission in hospitalized pediatric patients. Pediatr Infect Dis J 39:e459–e462. https://doi.org/10.1097/INF.0000000000002935
Rhim JW, Kang JH, Lee KY (2022) Etiological and pathophysiological enigmas of severe coronavirus disease 2019, multisystem inflammatory syndrome in children, and Kawasaki disease. Clin Exp Pediatr 65:153–166. https://doi.org/10.3345/CEP.2021.01270
Romani L, Del Chierico F, Macari G et al (2022) The relationship between pediatric gut microbiota and SARS-CoV-2 infection. Front Cell Infect Microbiol 12:908492. https://doi.org/10.3389/FCIMB.2022.908492
Donaldson GP, Lee SM, Mazmanian SK (2016) Gut biogeography of the bacterial microbiota. Nat Rev Microbiol 14:20–32. https://doi.org/10.1038/NRMICRO3552
Yatsunenko T, Rey FE, Manary MJ et al (2012) Human gut microbiome viewed across age and geography. Nature 486:222–227. https://doi.org/10.1038/NATURE11053
Davenport ER, Sanders JG, Song SJ et al (2017) The human microbiome in evolution. BMC Biol 15:127. https://doi.org/10.1186/S12915-017-0454-7
Zimmermann P, Curtis N (2018) Factors influencing the intestinal microbiome during the first year of life. Pediatr Infect Dis J 37:e315–e335. https://doi.org/10.1097/INF.0000000000002103
Zuo T, Zhang F, Lui GCY et al (2020) Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology 159:944-955.e8. https://doi.org/10.1053/J.GASTRO.2020.05.048
Yamamoto S, Saito M, Tamura A et al (2021) The human microbiome and COVID-19: a systematic review. PLoS One 16:e0253293. https://doi.org/10.1371/JOURNAL.PONE.0253293
Xu R, Liu P, Zhang T et al (2021) Progressive deterioration of the upper respiratory tract and the gut microbiomes in children during the early infection stages of COVID-19. J Genet Genomics 48:803–814. https://doi.org/10.1016/j.jgg.2021.05.004
Nashed L, Mani J, Hazrati S et al (2022) Gut microbiota changes are detected in asymptomatic very young children with SARS-CoV-2 infection. Gut 71:2371–2373. https://doi.org/10.1136/GUTJNL-2021-326599
Harris PA, Taylor R, Thielke R et al (2009) Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381. https://doi.org/10.1016/J.JBI.2008.08.010
Ngo KA, Jones SA, Church TM et al (2017) Unreliable inactivation of viruses by commonly used lysis buffers. Applied Biosafety 22:56–59. https://doi.org/10.1177/1535676017703383
Costea PI, Zeller G, Sunagawa S et al (2017) Towards standards for human fecal sample processing in metagenomic studies. Nat Biotechnol 35:1069–1076. https://doi.org/10.1038/NBT.3960
Milani C, Hevia A, Foroni E et al (2013) Assessing the fecal microbiota: an optimized ion torrent 16S rRNA gene-based analysis protocol. PLoS One 8:e68739. https://doi.org/10.1371/JOURNAL.PONE.0068739
Milani C, Mancabelli L, Lugli GA et al (2015) Exploring vertical transmission of bifidobacteria from mother to child. Appl Environ Microbiol 81:7078–7087. https://doi.org/10.1128/AEM.02037-15
Multisystem inflammatory syndrome in children and adolescents with COVID-19: scientific brief, 15 May 2020. https://apps.who.int/iris/handle/10665/332095. Accessed 24 Mar 2023
Gu J, Han B, Wang J (2020) COVID-19: gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology 158:1518–1519. https://doi.org/10.1053/J.GASTRO.2020.02.054
Wong SH, Lui RNS, Sung JJY (2020) COVID-19 and the digestive system. J Gastroenterol Hepatol 35:744–748. https://doi.org/10.1111/JGH.15047
Trevelin SC, Pickering S, Todd K et al (2022) Disrupted Peyer’s patch microanatomy in COVID-19 including germinal centre atrophy independent of local virus. Front Immunol 13:838328. https://doi.org/10.3389/fimmu.2022.838328
Tagarro A, Cobos-Carrascosa E, Villaverde S et al (2022) Clinical spectrum of COVID-19 and risk factors associated with severity in Spanish children. Eur J Pediatr 181:1105–1115. https://doi.org/10.1007/S00431-021-04306-6
Gu S, Chen Y, Wu Z et al (2020) Alterations of the gut microbiota in patients with Coronavirus Disease 2019 or H1N1 Influenza. Clin Infect Dis 71:2669–2678. https://doi.org/10.1093/CID/CIAA709
Arrieta MC, Stiemsma LT, Amenyogbe N et al (2014) The intestinal microbiome in early life: health and disease. Front Immunol 5:427. https://doi.org/10.3389/fimmu.2014.00427
Milani C, Duranti S, Bottacini F et al (2017) The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev 81:e0036-e117. https://doi.org/10.1128/MMBR.00036-17
Inoue Y, Iwabuchi N, Xiao JZ et al (2009) Suppressive effects of bifidobacterium breve strain M-16V on T-helper type 2 immune responses in a murine model. Biol Pharm Bull 32:760–763. https://doi.org/10.1248/BPB.32.760
Ohtsuka Y, Ikegami T, Izumi H et al (2012) Effects of Bifidobacterium breve on inflammatory gene expression in neonatal and weaning rat intestine. Pediatr Res 71:46–53. https://doi.org/10.1038/PR.2011.11
Fieten KB, Totté JEE, Levin E et al (2018) Fecal microbiome and food allergy in pediatric atopic dermatitis: a cross-sectional pilot study. Int Arch Allergy Immunol 175:77–84. https://doi.org/10.1159/000484897
Cukrowska B, Bierła JB, Zakrzewska M et al (2020) The relationship between the infant gut microbiota and allergy. The role of Bifidobacterium breve and prebiotic oligosaccharides in the activation of anti-allergic mechanisms in early life. Nutrients 12:946. https://doi.org/10.3390/NU12040946
Rodríguez-Belvís MV, Viada Bris JF, Fernández CP et al (2020) Normal fecal calprotectin levels in healthy children are higher than in adults and decrease with age. Paediatr Child Health 25:286–292. https://doi.org/10.1093/PCH/PXZ070
Stríz I, Trebichavský I (2004) Calprotectin — a pleiotropic molecule in acute and chronic inflammation. Physiol Res 53:245–253
Li F, Ma J, Geng S et al (2015) Fecal calprotectin concentrations in healthy children aged 1–18 months. PLoS One 10:e0119574. https://doi.org/10.1371/JOURNAL.PONE.0119574
Roca M, Rodriguez Varela A, Carvajal E et al (2020) Fecal calprotectin in healthy children aged 4–16 years. Sci Rep 10:20565. https://doi.org/10.1038/S41598-020-77625-7
Gonzalez Jimenez D, Velasco Rodríguez-Belvís M, Domínguez Ortega G et al (2021) Faecal calprotectin in children with multisystem inflammatory syndrome: a pilot case-control study. Acta Paediatr 110:22466–32248. https://doi.org/10.1111/APA.15856
Shokri-Afra H, Alikhani A, Moradipoodeh B et al (2021) Elevated fecal and serum calprotectin in COVID-19 are not consistent with gastrointestinal symptoms. Sci Rep 11:22001. https://doi.org/10.1038/S41598-021-01231-4
Kim HS (2021) Do an altered gut microbiota and an associated leaky gut affect COVID-19 severity? mBio 12:1–9. https://doi.org/10.1128/MBIO.03022-20
Garg M, Leach ST, Coffey MJ et al (2017) Age-dependent variation of fecal calprotectin in cystic fibrosis and healthy children. J Cyst Fibros 16:631–636. https://doi.org/10.1016/J.JCF.2017.03.010
Yeoh YK, Zuo T, Lui GCY et al (2021) Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 70:698–706. https://doi.org/10.1136/gutjnl-2020-323020
Young BE, Ong SWX, Ng LFP et al (2021) Viral dynamics and immune correlates of coronavirus disease 2019 (COVID-19) Severity. Clin Infect Dis 73:e2932–e2942. https://doi.org/10.1093/CID/CIAA1280
Petrey AC, Qeadan F, Middleton EA et al (2021) Cytokine release syndrome in COVID-19: innate immune, vascular, and platelet pathogenic factors differ in severity of disease and sex. J Leukoc Biol 109:55–66. https://doi.org/10.1002/JLB.3COVA0820-410RRR
Alba C, Aparicio M, González-Martínez F et al (2021) Nasal and fecal microbiota and immunoprofiling of infants with and without RSV bronchiolitis. Front Microbiol 12:667832. https://doi.org/10.3389/fmicb.2021.667832
Lemme-Dumit JM, Song Y, Lwin HW et al (2022) Altered gut microbiome and fecal immune phenotype in early preterm infants with leaky Gut. Front Immunol 13:815046. https://doi.org/10.3389/FIMMU.2022.815046
Donati Zeppa S, Agostini D, Piccoli G et al (2020) Gut microbiota status in COVID-19: an unrecognized player? Front Cell Infect Microbiol 10:576551. https://doi.org/10.3389/FCIMB.2020.576551
Acknowledgements
The technical assistance of Lydia Sariego is greatly appreciated. We also want to thank Dr. Ignacio Carbajal and Dra. Agueda Merino from Primary Care Centers of Asturias Principality for the help in providing us control samples from the project “Microalergymilk” for this study. The Spanish Society of Pediatric Gastroenterology, Hepatology and Nutrition (SEGHNP) is acknowledged for its support in this proposal.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This word was funded in part by a CSIC (Consejo Superior de Investigaciones Científicas) internal project PIE reference 202070E237. The research was also funded by the European Commission-Next Generation EU (REGULATION EU 2020/2094), through CSIC’s Global Health Platform (PTI Salud Global). I. G-D. has a grant FJC2021-047052-I financed by MCIN/AEI/10.13039/501100011033 and by European Union “NextGenerationEU/PRTR.” Grant AYUDA-20201-50910 from the Government of Asturias Principality (FICYT, supported by FEDER) is also received.
Author information
Authors and Affiliations
Contributions
Conceptualization, S. D. and J. J. D.; methodology, I. G-D., M. S., and A.M.C.; software and data curation, V. M. N-L. and I. G-D.; resources, M. V. R-B., N. C., S. J., C. M., M. Q., M. H., and R. M-M.; writing—original draft preparation, I. G-D. and S. D.; writing—review and editing, P. F., V. N., B. E., R. L., and J. J. D.; funding acquisition, S.D. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
The studies involving human participants were reviewed and approved by Regional Ethics Committee for Clinical Research (Servicio de Salud del Principado de Asturias, no. 112/13). Written informed consent to participate in this study was provided by the family or legal guardian.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Peter de Winter
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gutiérrez-Díaz, I., Sanz-Martinez, M., Castro, A.M. et al. Microbial and immune faecal determinants in infants hospitalized with COVID-19 reflect bifidobacterial dysbiosis and immature intestinal immunity. Eur J Pediatr 182, 4633–4645 (2023). https://doi.org/10.1007/s00431-023-05140-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-023-05140-8