Abstract
This study aimed to evaluate ceftriaxone pharmacokinetics that affects the achievement of targets in the treatment of critically ill children (meningitis, pneumonia, urinary tract infection, peritonitis, and infective endocarditis( who were admitted to Zagazig University Pediatric hospital in Egypt to monitor for the drug adverse effects.
Blood samples were obtained from 24 hospitalized pediatric patients (ages ranging from 2.5 months to 12 years) after administering the calculated dose of ceftriaxone via intravenous bolus route. Then, ceftriaxone plasma concentrations were measured using a validated HPLC method with ultraviolet detection. The pharmacokinetic analysis was conducted using Phoenix Winnonlin Program® software.
Data for total and free ceftriaxone best fitted on a one-compartment model with the first-order elimination process. Clearance of ceftriaxone is reduced for patients with reduced kidney function and increased with those with augmented renal clearance. The volume of distribution and the free fraction are increased in these patients, especially those with hypoalbuminemia with a shorter half-life time were detected. A slight increase in total bilirubin and liver enzymes has been observed after treatment with ceftriaxone in these patients.
Conclusion: In most critically ill pediatric patients, the current ceftriaxone treatment regimen (50 to 100 mg/kg) offers adequate pathogenic coverage. The clearance of free ceftriaxone in all patients correlates well with their renal function (eGFR), with r2 = 0.7252. During therapy with ceftriaxone at all doses ranging from 50 to 100 mg/kg, a rise in total bilirubin was observed in these patients. Moreover, liver enzymes (ALT and AST) increased moderately (p 0.0001). So, it is recommended to monitor total bilirubin and liver enzymes during the treatment with ceftriaxone, especially for a long duration (more than 5 days) or use another agent in patients with high baseline values.
What is Known: • The dosing regimen of ceftriaxone (50 to 100 mg/kg) provided optimum therapeutic outcomes. • Some studies show data for total and free Ceftriaxone best fitted on a one-compartment model while other studies show data for total and free Ceftriaxone best fitted on a two-compartment model. | |
What is New: • Up to my knowledge this is the first study ,considering individual pharmacokinetic analysis, conducted on hospitalized Egyptian pediatric population most of them with reduced kidney function with ages ranging from 2.5 months to 12 years. Data for total and free Ceftriaxone best fitted on a one-compartment model with linear clearance of the free ceftriaxone. • In all patients, total bilirubin and liver function tests were mildly increased, making them at risk for cholestasis or ceftriaxone-induced cholestatic hepatitis. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cephalosporins are a group of antibiotics that belong to β-lactams that were first isolated from the fungus Acremonium Chrysogenum. They exert their antibacterial activity via inhibition of the cell wall synthesis of the bacterial cell [1]. They bind to the penicillin-binding proteins (PBPs), essential in synthesizing bacterial cell wall peptidoglycans. As a result, cell lysis occurs in the hypo-osmotic or iso-osmotic environment surrounding the bacterial cell [2]. Cephalosporins are classified into five generations and are effective against aerobic and anaerobic microorganisms [3]. Ceftriaxone is a third-generation cephalosporin administered parenterally via intravenous or intramuscular injection. Ceftriaxone is commonly used in pediatrics and has indications for bloodstream infections caused by the following micro-organisms: Streptococcus pneumoniae, Klebsiella pneumoniae, Staphylococcus aureus, Hemophilus influenzae or Escherichia coli, septic arthritis, osteomyelitis, intra-abdominal infection, bacterial meningitis, lower respiratory tract infections (pneumonia), urinary tract infection (including cystitis and pyelonephritis) and acute otitis media [4]. Most adverse pharmacological effects associated with cephalosporin antibiotics in the pediatric population were brought on by inappropriate drug use [5]. Antibiotics can be categorized into time-dependent and concentration-dependent antibiotics based on their kill characteristics [6].
Ceftriaxone has time-dependent bacterial killing properties meaning that the maximum bacterial killing effect is achieved when the free plasma concentration of the drug remains above the minimum inhibitory concentration (fT > MIC) for more than or equal to 60 to 70% of the dosing interval [7]. For most common infections, ceftriaxone’s MIC varies from 0.06 to 2 mg/L [5]. Also, according to the European Committee on antimicrobial susceptibility testing (EUCAST), the optimum MIC of ceftriaxone against most susceptible organisms ranges from 0.5 to 2 mg/L.
Ceftriaxone has an FDA block box warning for use in neonates (less or equal to 28 days), especially prematures, because of the risk of development of bilirubin encephalopathy. Also, mixing ceftriaxone with calcium containing IV solution (for example parenteral nutrition solutions) is contraindicated because of the risk of precipitation of ceftriaxone-calcium complex [8].
By shedding light on ceftriaxone pharmacokinetics, it has a high affinity for binding with serum albumin (85–95%). It has an elimination half-life (t1/2) of 9 h up to 30 days in neonates and 4 to 6.6 h in infants and children, which is a relatively long range. Ceftriaxone’s free fraction, not the bound fraction to plasma protein, is the only portion of the entire medication responsible for the antibacterial activity and the only fraction susceptible to elimination. This means that any condition (such as hepatic or renal disorders causing hypoalbuminemia or hyperbilirubinemia) leading to changes in the free fraction of the drug will modify the antibiotic exposure and have an impact on clinical outcomes. Ceftriaxone has mixed renal elimination (33–67% as an unaltered drug in urine) and biliary elimination (as an inactive drug in feces) [4].
The pharmacokinetic parameters of ceftriaxone’s free fraction are linear and dose-dependent, meaning that when the drug’s dose is increased, the drug’s steady-state concentration will increase accordingly [9].
Ceftriaxone dosage for children ranges from 50 to 100 mg per kg per day divided every 12 to 24 h for an average duration of five days (maybe less or more according to type and severity of infection) [4].
A previous pharmacokinetic study on pediatric patients with community-acquired pneumonia shows that the data best fit on a one-compartment model with first-order elimination kinetics, and the optimum dose that maintains the MIC for ceftriaxone at 2 mg/L was 100 mg/kg every 24 h [5].
Hoy WE …et al. documented in a systematic review the significant differences in antibiotic pharmacokinetics that may occur between different ethnic groups, so rational for this study was to be conducted on Egyptian pediatric patients to reveal data from such population [10]. Ethnicity has a great impact on the renal function. A large-scale study involving about 71,638 individuals from four ancestries for whom genome-wide association studies are carried out for estimated eGFR. Which identified different genes that affect the performance and sensitivity of renal cells in these different groups [11]. Furthermore, another published study by Fabian, June, et al. considering ethnicity’s effect on renal function supports this concept [12]. Thus, pharmacokinetics of ceftriaxone is affected.
This pharmacokinetic study was conducted to evaluate and shed light on the pharmacokinetics of ceftriaxone and a clinical evaluation of Egyptian pediatric patients in our setting. A validated HPLC method (with some modifications) with ultraviolet detection has been used to determine ceftriaxone plasma concentrations [9].
Patients and methods
Patients and clinical samples [9]
This cross-section observational study was conducted from May 2020 to November 2020. It involved 24 patients from different departments at the Zagazig University Pediatrics Hospital, Egypt, who fulfilled the following inclusion criteria: Egyptian with ages ranging from 1 month to < 18 years old and clinical indication for administration of ceftriaxone antibiotic. Patients who are non-Egyptian, with age < 1 month (neonates) or > = 18 years old (adults), patients allergic to ceftriaxone, or patients with end-stage chronic kidney disease are excluded from this study. The ethics committee represented by the Institutional Review Board (IRB) at the Faculty of Medicine, Zagazig University, gave us approval for collecting blood samples from pediatric patients opportunistically during the duration of treatment (Approval Number; ZU-IRB#6070/26/4/2020). The sample was taken as a comprehensive sample due to the rare attendance of cases (4 cases or less/month), so the committee determined the sample to be 24 cases. It was previously calculated using the online version of EPI info. with the power of study of 80%.
The characteristics and laboratory data of the patients involved in this study are obtained from patients’ files at baseline and after completion of treatment with ceftriaxone (the decision of treatment with ceftriaxone was previously taken from the responsible staff for the selected patients). In addition, GFR was estimated for each patient according to the bedside Schwartz equation: [(0.413 × height (cm)) / Scr (mg/dL)] as recommended by the National Kidney Disease Education Program. Furthermore, serum creatinine values were determined using Jaffe Colorimetric method at Zagazig University Hospitals Central Laboratories. These collected data are presented in the "Results" section.
Then, ceftriaxone was administered in doses ranging from 50Footnote 1 to 100 mg/kg via intravenous bolus injection to each patient. Then, blood samples were obtained from each patient during specific time previously determined by department administrator (the time preceding the next dose, trough), as one blood sample from different dosing intervals. Three blood samples were obtained from each patient, then stored immediately after collection at 2–8 °C in an icebox. After that, plasma was separated using centrifugation at 6000 rpm for 15 min and then analyzed using a suitable High-Performance Liquid Chromatographic method.
The analysis of each sample was carried out immediately after obtaining each blood sample. Both total and free ceftriaxone were determined. A study flow chart is illustrated by Fig. 1.
Analysis of ceftriaxone
Blood samples were collected from patients (when available, 2 ml) in heparin tubes and then centrifuged at 6000 rpm for 15 min to obtain plasma. 250 μl of plasma is mixed with 250 μl of cold acetonitrile in an Eppendorf tube, then centrifuged at 8000 rpm for 6 min at 4 °C. The supernatant is then filtered and injected into the HPLC system for analysis [13].
Analysis was carried out as cited reference with some modifications using Thermo Fisher Scientific®HPLC system, which is composed of binary pumps, autosampler with a loop size of 10 μl, photodiode array detector (PDA) with detection at 260 nm, and Chromquest 5.0 software (Thermo Electron Corp., Bellefonte, PA, USA). Ceftriaxone analytical standard was kindly supplied from EIPICO, Egypt. Methanol, acetonitrile, and ammonium acetate (HPLC grades) were purchased from Fisher Scientific, USA. Double distilled water was applied all over the experiments and prepared in-house. A stock solution of ceftriaxone was freshly prepared by dissolving 20 mg of pure drug in 100 mL double distilled water to get a stock solution with a concentration of 200 µg mL−1 used for method validation.
Samples were analyzed by HPLC using Agenla Technologies C18 column (250 mm × 4.6 mm, 5 μm particle size) maintained at 25 °C temperature. Elution pumps ran an isocratic flow using a mobile phase consisting of methanol and ammonium acetate 20 mM buffer solution (freshly prepared by dissolving 0.77 g in 500 ml distilled water) in the ratio of (79:21%v/v) at 1 ml/min flow rate. The autosampler utilizes water as a rinse solution, and the injection volume was 10 μl. The detection of samples was carried out at λmax 260 nm with a run time of 8 min [14].
Pharmacokinetic analysis
Total and free ceftriaxone pharmacokinetics were analyzed separately with good fitting on a one-compartment model using Phoenix Winnonlin Program®(8.3.5.340, Core version 06-Feb. 2020). Then, the pharmacokinetic parameters were obtained and used to simulate the plasma concentration–time profile for each patient’s total and free ceftriaxone [9].
Statistical analysis
GraphPad Prism software was used for statistical analysis of the changed laboratory values.
Results
HPLC method validation results
Construction of calibration curves in plasma
Aliquots of standard solutions, ranging from 2.5 to 100 μg per ml, were prepared in a series of 10 ml volumetric flasks, mixed with an equal volume of plasma from a healthy volunteer, then stirred. After sample pre-treatment, 10 μl of the supernatant was injected into the HPLC System. Detection was performed at wavelength 260 nm. The calibration graphs were constructed by plotting the peak areas obtained versus the corresponding injected concentrations (Fig. S1A).
HPLC chromatograms for method validation and examples for those from patients’ samples are presented in Figs. S1B–D, S7, and S8.
The applied Method was validated according to the ICH and FDA guidelines in respect to certain parameters such as linearity, precision, accuracy and system suitability [15, 16].
Data validation
Linearity was established across the specified range by plotting the area under the curve against its corresponding plasma concentration (Fig. S1, A). Linearity drug standards were injected in triplicates, and average responses were calculated. Table S2 shows linearity data, correlation coefficient, slope and intercept, and limits of detection (LOD) and quantification (LOQ) were calculated and listed. The result of LOD (3.3σ/S) was calculated as a function of the standard deviation of intercept (σ) and the slope (S) of the calibration curve, and LOQ was calculated as (10σ/S).
The method’s accuracy was confirmed using three standard concentrations injected in triplicates. The closeness of the calculated percentage recovery results to the actual values proved the method’s validity.
Precision was performed by three different determinations of three ceftriaxone concentrations on the same day (intra-day) and three other days (inter-day). Precision results listed in Table S2 show that the proposed method has good repeatability and reproducibility.
System suitability test parameters of the chromatographic method were checked and presented in table to ensure that the system was working correctly during the bioanalysis including retention time (Rt), column efficiency (number of theoretical plates (N), height equivalent to theoretical plates (HETP), tailing factor, resolution between ceftriaxone and plasma peaks (Rs) Table S2.
The influence of slight variations in one of the critical chromatographic parameters was evaluated while keeping all the others constant. The studied variables, including flow rate (1 ± 0.02) and methanol content (79% ± 1), were tested. Retention time and peak area were recorded in Table S3 upon these minor changes to indicate the robustness of the developed method.
Clinical evaluation of patients
The patients in this study are 11 males and 13 females, with ages ranging from 2.5 months to 12 years old. Their demographics and the laboratory values at baseline and after therapy with ceftriaxone are listed in Table 1.
Nine patients administer ceftriaxone because of meningitis (37.5%), six patients because of pneumonia (25%), three patients because of urinary tract infection (12.5%), one patient because of meningitis and gastroenteritis (≈ 4.167%), one patient because of peritonitis (≈ 4.167%) and one patient because of infective endocarditis (≈ 4.167%). Other associated medical conditions and the observed drug-drug interactions are recorded in Table S4.
The duration of treatment with ceftriaxone ranges from 5 to 21 days, with a median of 10 days. Only one patient was treated with ceftriaxone for more than 14 days, while the others received the drug for a duration equal to or less than 14 days.
All patients had a significant difference in total bilirubin values at baseline and after treatment, although within the normal range with a p-value of < 0.0001 and 95% CI from − 0.1607 to − 0.08017 and r of 0.8349. Also, liver enzymes, including ALT and AST, are mildly elevated with p-values of < 0.0001 and 95% CI values from − 6.409 to − 4.225 and from − 12.55 to − 5.144 and r of 0.9802 and 0.8678, respectively (Figure S2) (Table S1).
Pharmacokinetics
In the case of total ceftriaxone concentration, none of the observed values were below the limit of quantification (BLOQ). All the observed concentration values for the total ceftriaxone were found to be within the lower limit of quantification (LLOQ) and did not require any method for imputation of BLOQ data.
In the case of free ceftriaxone concentration, observed data related to two subjects exhibited BLOQ values. These BLOQ values were replaced with half the value of LLOQ as per Beal’s M5 method for imputation of BLOQ data (Tables 2 and 3).
Compartmental model diagnostics
The concentration–time curves for total and free ceftriaxone fit the population one-compartmental model. Phoenix software collected data demonstrates low values of Akaike information criterion (AIC) (191.35, 524.63) and Bayesian information criterion (BIC) (202.73, 536.01) for free and total ceftriaxone, respectively, in population one-compartmental model compared to those in population two-compartmental model (AIC = 200.85, 528.15 and BIC = 221.34, 548.64) Table 4.
The excellent agreement between observed and predicted concentrations for population and individuals demonstrated that the one-compartment model could precisely represent the concentration–time courses of total and unbound ceftriaxone in 24 pediatric patients (Fig. 2A–B), (Figs. S3, S4, S5 and S6).
By plotting the residuals (difference between observed and predicted concentrations) against standard normal quantiles, the data fall very close to the diagonal line, meaning that the residuals are normally distributed (Fig. 2C–D).
Pharmacokinetic parameters results
Regarding the non-compartmental analysis results, all pharmacokinetic parameters obtained by phoenix software are illustrated in Table 2. Furthermore, exposure parameters including Cmax, Co, AUC AND AUCM are listed in Table 3.
Correlation between renal function and the free fraction of ceftriaxone
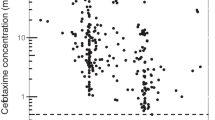
The clearance of free ceftriaxone for all patients has a good correlation with the renal function of the patients(eGFR) [r2 = 0.7252, the regression equation that represents the clearance of the free ceftriaxone was Cl (ml/min/1.73 m2) = 3.9465 × eGFR(ml/min/1.73 m2) – 7.2258] while the clearance of total ceftriaxone for all patients has poor correlation with the renal function of the patients (eGFR) (r2 = 2 × 10−5) (Fig. 3).
Discussion
Many analytical techniques determine the plasma concentration of ceftriaxone. One utilizes high-performance liquid chromatography with column Hypersil 250 × 4.6 mm and a mobile phase consisting of a mixture of tetra butyl ammonium hydroxide buffer and acetonitrile in a ratio of (70:30) [13]. Another study utilizes high-performance liquid chromatography with UV detection with octadecyl silica (ODS) column (Hydrosphere 150 × 4.6 mm, 5 μm) in an isocratic elution system with mobile phase A composed of methanol and 10 mM phosphoric acid (25:75, v/v) mixture solution and mobile phase B composed of methanol and water (80:20, v/v) mixture solution with detection at 280 nm [17]. Another study utilizes an ultra-high performance liquid chromatography with UV–visible detection C18 (2.1 × 100 mm; 1.8 μm) column protected by a VanGuard guard column. Both were placed into the column oven and maintained at 40 °C. The mobile phase was composed of a mixture of methanol and ammonium acetate 20 mM (21:79, v/v) with detection at 260 nm [14]. This study utilized this method with some modifications, as discussed in the Methods part.
In this observational study, we describe the pharmacokinetics of total and unbound ceftriaxone with clinical evaluation in 24 pediatric patients. Data for total and free ceftriaxone were best fit on a one-compartment model, based on lower values for both AIC and BIC values from one-compartment model compared to those from two-compartment model, with linear clearance of the free ceftriaxone. Some previous studies show data for free and total ceftriaxone fitted on a two-compartment model [18,19,20]. Other studies show data for ceftriaxone best fit on one compartment model, which agrees with our research [5].
As demonstrated in the results, all patients have a trough concentration of total ceftriaxone above the range of 0.5 to 2 ug/ml, which is adequate to achieve ceftriaxone’s target activity against most susceptible organisms. Patients with hypoalbuminemia (6 patients in our study) show an elevated free fraction up to 0.55 with a high volume of distribution of free ceftriaxone up to 30 L with a relatively long elimination half-life of about 10–15 h (except for patients with good kidney function show shorter elimination half-life of about 4 h and high drug clearance). These patients have eGFR of around 60 ml/min/1.73 m2 with relatively low clearance of total and free ceftriaxone attributed to a somewhat reduced kidney function (except for patients 3 and 4, in which the kidney function is perfect).
As in previous studies, not only bilirubin and albumin concentrations have an impact on the free fraction, but also the critical illness can do, as most of our patients were admitted to intensive care unit contributing to such elevated free fractions up to 0.6, which led to changes in the volume of distribution and clearance of the drug as discussed [9].
Furthermore, the critical illness has great impact on the augmented renal function supporting this the use of Bedside Schwartz Formula as mentioned at "Patients and Methods" section which consider the constant value to adjust the effect of critical illness to kidney function. In this study, the use of the Bedside Schwartz formula allowed for a more accurate estimation of renal function in critically ill patients and ensured that the impact of critical illness on drug clearance was appropriately accounted for. This is an important consideration in the management of critically ill patients, as accurate dosing of medications like ceftriaxone is crucial for achieving optimal therapeutic outcomes. This is obviously observed in patients 3, 4, 6, 13, 16 and 22 for whom the augmented renal function was clear [21].
Most adverse effects precipitated by ceftriaxone, especially in pediatrics, occur due to its inappropriate use [22]. Although none of the patients developed hyperbilirubinemia, the total bilirubin increased compared to baseline values. This increase is mild, but this can support the implication of ceftriaxone in cholestasis, the possibility of gallstone development, and the risk of ceftriaxone-induced cholestatic hepatitis. One of the adverse effects of ceftriaxone is cholestasis which can lead to biliary calculi and may lead to cholestatic hepatitis. Total bilirubin concentrations and liver enzymes, including ALT and AST, were mildly increased after treatment in these patients. The test which was performed is T-test from which changes were significant. After running exposure–response analysis there wasn’t any correlation observed suggesting that these changes are not concentration-dependent. A published case report showed a case of a 5-year-old boy presented with ceftriaxone-induced cholestatic hepatitis [23].
In the present study, no patients develop gallbladder stones after treatment but are still at risk. So, we warn the health care providers in our sitting that treatment with ceftriaxone requires monitoring for cholestasis and gallbladder stones to avoid this problem in patients. Around 50% of our patients have impaired renal function, which contributes to reduced protein binding by unknown mechanisms. This may be due to waste products accumulated in the body that may displace ceftriaxone from its binding sites.
In many previous studies, the drug’s protein binding changes have little clinical importance. This can be illustrated by a condition that causes an acute increase in the free fraction causing the excess of the drug to be eliminated keeping a steady fraction of the drug. But this depends on kidney function, and in such patients with impaired kidney function, accumulation of ceftriaxone occurs, leading to undesirable effects of the drug. One has been reported as discussed, so dosage adjustment was necessary [24].
Regarding drug-drug interaction screening in our patients, only two pharmacodynamic interactions have been reported in patient number 1. One of them is serious between ceftriaxone and enoxaparin, leading to increased prothrombin activity as a synergistic effect. The second pharmacodynamic interaction is minor between ceftriaxone and furosemide, leading to additive nephrotoxicity. From these interactions, the second one added a negative effect on renal function as observed (eGFR < 30 ml/min/1.73 m2) also affecting the clearance of ceftriaxone (reduced CL = 412.42 ml/h).
The covariate analysis was already done, and found that only body weight has a significant effect as covariate (p = 015, r2 = 0.4) on clearance of ceftriaxone in all patients together with. But, on the other hand, we conduct this study on different ages with different clinical indications for ceftriaxone and administering different doses ranging from 50 to 100 mg/kg in order to evaluate the PK of ceftriaxone individually and evaluate the cases clinically with possible drug-drug interaction or another associated medical conditions. This could be appropriate for future population PK of ceftriaxone focusing on one specific clinical indication (as pneumonia, or meningitis) with fixed doses to run a such covariate analysis.
Limitations of this study involve a small sample size, although determined by IRB, and this is attributed to a small number of patients admitted to the hospital who fit the inclusion criteria; a large-scale clinical trial is required in different areas worldwide utilizing more advanced techniques for protein binding study which is not possible for us due to financial issues. Also, regarding sampling time, we faced such a problem due to ethical issues only specific time during the day, which is fixed according to hospital regulations without the chance of obtaining samples at different time intervals from the last dose.
Conclusion
Data for total and free ceftriaxone were best fit on a one-compartment model with linear clearance of the free ceftriaxone. The current dosing regimen of ceftriaxone (50 to 100 mg/kg) provides appropriate pathogen exposure in most critically ill pediatric patients. Total bilirubin concentration was within the normal range, and an increase in total bilirubin after treatment with ceftriaxone was observed. Also, liver enzymes, including ALT and AST, are mildly increased. So, liver function tests and total bilirubin monitoring are necessary during treatment with ceftriaxone, especially in pediatric patients and those administered the ceftriaxone for a long duration, more than 5 days, or use another agent in patients with high baseline values to avoid the development of cholestasis. Also, large-scale multi-center pharmacokinetic studies involving different ethnicity from the pediatric population are recommended to report various variabilities in ceftriaxone pharmacokinetics which affect its therapeutic outcomes and the possibility of the emergence of its undesirable effects.
Data availability
Data supporting results in this study including chromatograms generated by HPLC apparatus, data for direct and total bilirubin, associated clinical conditions & drug-drug interactions, HPLC Method Validation results, individual plots for observed and predicted concentrations of ceftriaxone generated by Phoenix Winnonlin Program software were included in the Supplementary file. Other data required will be available on request from the corresponding author.
Notes
Doses were determined by the health care provider according to the actual body weight.
Abbreviations
- 95% CI :
-
95% Confidence interval
- AIC:
-
Akaike information criterion
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- AUCall:
-
Total area under curve from 0-infinity
- AUMC:
-
Area under moment curve.
- BLOQ:
-
Below the limit of quantification
- BIC:
-
Bayesian information criterion
- C0:
-
Initial concentration
- CL:
-
Clearance
- Cmax:
-
Maximum concentration observed
- eGFR:
-
Estimated glomerular filtration rate
- EIPICO:
-
Egyptian International Pharmaceutical Industries Company
- EUCAST:
-
European Committee on antimicrobial susceptibility testing
- FDA:
-
Food and Drug Administration
- fT:
-
Free fraction of the drug
- HPLC:
-
High-performance liquid chromatography
- IRB:
-
Institutional Review Board
- LLOQ:
-
Lower limit of quantification
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantification
- MIC:
-
Minimum inhibitory concentration
- mM:
-
Millimole
- NCA:
-
Non compartmental analysis
- PBPs:
-
Penicillin-binding proteins
- RSD%:
-
Relative standard deviation
- S:
-
The slope
- Scr :
-
Serum creatinine
- SD:
-
Standard deviation
- Vss :
-
The volume of distribution at steady state
References
JM, Flower RJ, Henderson G, Rang HP, Loke YK, MacEwan D (2020) Rang & Dale's pharmacology, antibacterial drugs chapter. Elsevir. p. 661–77
Ranganathan N, Iyer KT (2022) Beta lactam. Elsevier, Comprehensive pharmacology, pp 3–63
Bennett JE, Dolin R, Blaser MJ (2019) Douglas, and Bennett's principles and practice of infectious diseases E-book: Elsevier Health Sciences.
Drug Information Provided by Lexi-Comp [Internet]. Available from: https://www.merckmanuals.com/
Khan MW, Wang Y-K, Wu Y-E, Tang B-H, Kan M, Shi H-Y et al (2020) Population pharmacokinetics and dose optimization of ceftriaxone for children with community-acquired pneumonia. Eur J Clin Pharmacol 76(11):1547–1556
Tsai D, Lipman J, Roberts JA (2015) Pharmacokinetic/pharmacodynamic considerations for the optimization of antimicrobial delivery in the critically ill. Curr Opin Crit Care 21(5):412–420
Roberts JA, Kirkpatrick CM, Roberts MS, Robertson TA, Dalley AJ, Lipman J (2009) Meropenem dosing in critically ill patients with sepsis and without renal dysfunction: intermittent bolus versus continuous administration? Monte Carlo dosing simulations and subcutaneous tissue distribution. J Antimicrob Chemother 64(1):142–150
FDA Drug label for ROCEPHIN® (ceftriaxone sodium) Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2004/50585s057,50624s027lbl.pdf
Schleibinger M, Steinbach CL, Töpper C, Kratzer A, Liebchen U, Kees F et al (2015) Protein binding characteristics and pharmacokinetics of ceftriaxone in intensive care unit patients. Br J Clin Pharmacol 80(3):525–533
WE Hoy , Hughson , GR Singh, R Douglas-Denton, Bertram J (2006) Reduced nephron number and glomerulomegaly in Australian Aborigines: a group at high risk for renal disease and hypertension. Kidney Int 10:70:104
Mahajan, Anubha, et al (2016) Trans-ethnic fine mapping highlights kidney-function genes linked to salt sensitivity. The American Journal of Human Genetics 99.3: 636–646.)
Fabian, J et al (2022) Measurement of kidney function in Malawi, South Africa, and Uganda: a multicentre cohort study. The Lancet Global Health 10.8: e1159-e1169
Sharma VD, Singla A, Chaudhary M, Taneja M (2016) Population pharmacokinetics of fixed dose combination of ceftriaxone and sulbactam in healthy and infected subjects. AAPS PharmSciTech 17(5):1192–1203
Campos ML, de Moura AJ, dos Santos ME, Oliveira JA, Hussni CA, Peccinini RG (2017) Ceftriaxone pharmacokinetics by new simple and sensitive ultra-high-performance liquid chromatography method. Diagn Microbiol Infect Dis 88(1):95–99
IHT Guideline, (2005) Validation of analytical procedures: text and methodology. Q2 (R1), 1 (20), 05
Food and Drug Administration (2001) Center for Drug Evaluation and Research 1:124–129
Kotani A, Hirai J, Hamada Y, Fujita J, Hakamata H (2019) Determination of ceftriaxone concentration in human cerebrospinal fluid by high-performance liquid chromatography with UV detection. J Chromatogr B 1124:161–164
Garot D, Respaud R, Lanotte P, Simon N, Mercier E, Ehrmann S et al (2011) Population pharmacokinetics of ceftriaxone in critically ill septic patients: a reappraisal. Br J Clin Pharmacol 72(5):758–767
Hartman SJ, Upadhyay PJ, Hagedoorn NN, Mathôt RA, Moll HA, Van Der Flier M et al (2021) Current ceftriaxone dose recommendations are adequate for most critically ill children: results of a population pharmacokinetic modeling and simulation study. Clin Pharmacokinet 60:1361–1372
Tang Girdwood S, Dong M, Tang P, Stoneman E, Jones R, Yunger T et al (2022) Population pharmacokinetic modeling of total and free ceftriaxone in critically ill children and young adults and Monte Carlo simulations support twice daily dosing for target attainment. Antimicrob Agents Chemother 66(1):e01427-e1521
Smeets, Nori JL, Michiel F. Schreuder, and Saskia N. de Wildt (2022) Correspondence to: Reliability of glomerular filtration rate estimating formulas compared to iohexol plasma clearance in critically ill children. Eur J Pediatr 181.12: 4227–4227
Ren X, Liu D, Ding N, Huang K, Xiong Y, Du G et al (2012) Safety evaluation of cephalosporins based on utilization and adverse drug events: analysis of two databases in China. Expert Opin Drug Saf 11(5):689–697
Castellazzi ML, Agostoni CV, Palella J, Civeriati D, Marchisio P, Nebbia G (2022) Ceftriaxone-induced cholestatic hepatitis in a child: a case report and a review of the literature. Front Pediatr 10:2150
Benet LZ, Hoener B-A (2002) Changes in plasma protein binding have little clinical relevance. Mosby, Inc 11830 Westline Industrial Dr, St Louis, MO 63146–3318 USA; p. 115–21
Acknowledgements
The authors would like to thank all patients who participated in this study and their parents, Dr. Eslam Hamed (Department of Pharmacology, Faculty of Veterinary Medicine, Zagazig University, Egypt) and Dr. Esraa A. Hamed (Laboratory Assistant at Faculty of Pharmacy, Zagazig University, Egypt) for their technical help in this work.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Mohamed W. Eldougdoug contributes to the conception and design of the study, acquisition of data, drug analysis, interpretation of pharmacokinetic data, and drafting of the manuscript. Doaa M. Youssef contributes to the conception and design of the study, clinical evaluation of cases to be included, and revising the manuscript for important intellectual content. Amal S. El-Shal contributes to the conception and design of the study, clinical evaluation of cases to be included, and revising the manuscript for important intellectual content. Yasmine Ahmed Sharaf contributes to drug analysis and revises the manuscript for important intellectual content. Sridivya Raparla contributes to analysis and interpretation of ceftriaxone pharmacokinetics from the obtained data. Bhaskara R. Jasti contributes to analysis and interpretation of ceftriaxone pharmacokinetics from the obtained data. Hanan M. Elnahas contributes to the conception and design of the study, interpretation of pharmacokinetic data and revising the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Ethics approval
The ethics committee represented by the Institutional Review Board (IRB) at the Faculty of Medicine, Zagazig University, gave us approval for collecting blood samples from pediatric patients opportunistically during the duration of treatment (Approval Number; ZU-IRB#6070/26/4/2020). The sample was taken as a comprehensive sample due to the rare attendance of cases (4 cases or less/month), so the committee determined the sample to be 24 cases.
Consent to participate
Parents of pediatric patients in this study signed two consent forms previously determined by the IRB committee. The first one (ZU-IRB #: FORM 2) is for initial acceptance to participate in this study. The second one (ZU-IRB #: FORM 3) is for acceptance to obtain blood samples from the patients for this study.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Peter de Winter
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eldougdoug, M.W., Youssef, D.M., El-Shal, A.S. et al. Evaluation of ceftriaxone pharmacokinetics in hospitalized Egyptian pediatric patients. Eur J Pediatr 182, 4407–4420 (2023). https://doi.org/10.1007/s00431-023-05091-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-023-05091-0