Abstract
Appropriate outcome measures as part of high-quality intervention trials are critical to advancing hospital-to-home transitions for Children with Medical Complexity (CMC). Our aim was to conduct a Delphi study and focus groups to identify a Core Outcome Set (COS) that healthcare professionals and parents consider essential outcomes for future intervention research. The development process consisted of two phases: (1) a three-round Delphi study in which different professionals rated outcomes, previously described in a systematic review, for inclusion in the COS and (2) focus groups with parents of CMC to validate the results of the Delphi study. Forty-five professionals participated in the Delphi study. The response rates were 55%, 57%, and 58% in the three rounds, respectively. In addition to the 24 outcomes from the literature, the participants suggested 12 additional outcomes. The Delphi rounds resulted in the following core outcomes: (1) disease management, (2) child’s quality of life, and (3) impact on the life of families. Two focus groups with seven parents highlighted another core outcome: (4) self-efficacy of parents.
Conclusion: An evidence-informed COS has been developed based on consensus among healthcare professionals and parents. These core outcomes could facilitate standard reporting in future CMC hospital to home transition research. This study facilitated the next step of COS development: selecting the appropriate measurement instruments for every outcome.
What is Known: • Hospital-to-home transition for Children with Medical Complexity is a challenging process. • The use of core outcome sets could improve the quality and consistency of research reporting, ultimately leading to better outcomes for children and families. | |
What is New: • The Core Outcome Set for transitional care for Children with Medical Complexity includes four outcomes: disease management, children’s quality of life, impact on the life of families, and self-efficacy of parents. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Children with medical complexity (CMC) comprise a diverse population with chronic conditions, functional limitations, substantial family-identified service needs, and high healthcare use [1]. CMC have a variety of diagnoses such as congenital heart diseases, cerebral palsy, metabolic diseases, neurological conditions, or epilepsy. Although CMC represents a small proportion of the pediatric population [2, 3], their healthcare utilization is substantial due to frequent emergency department visits and often lengthy and complicated (re)hospitalizations [4,5,6]. CMC are characterized by complex home care since they often require nursing care, medical equipment, advocacy, and frequent inpatient contact with different medical subspecialists [7]. The transition from hospital (where healthcare professionals are responsible for the care) to home (where parents are the most important and responsible caregivers) is a vulnerable process with many challenges and obstacles. Parents have to learn complex nursing care, need support in logistic and coordination issues, and emotional support [8,9,10,11].
Therefore, it is of great importance to guide the hospital-to-home transition carefully. Various interventions have been developed to improve this transition, such as transitional care units [12, 13], telemedicine [14, 15], post-discharge nurse home visits [16], and post-discharge caregiver coaching [17]. Research on the effectiveness of interventions to support hospital-to-home transitions is growing [18,19,20,21]. Most studies reflected on the life impact (the impact on functioning, quality of life, delivery of care, and personal circumstances) and resource use [22]. The most commonly reported outcomes are the self-efficacy of parents, amount of hospital (re)admissions, and length of stay in the hospital [22]. However, outcomes such as out-of-pocket expenses [23], disease management [14], and adverse events [24] have also been investigated. A core outcome may facilitate the comparison of findings in systematic reviews and meta-analyses, which rely on consistent outcome reporting to pool data across studies. To move forward in this field there is a need for consensus on a Core Outcome Set (COS) to systematically develop and evaluate hospital-to-home interventions.
A COS is “an agreed minimum set of outcomes that should be measured and reported in all clinical trials of a specific health condition, trial population, and/or intervention” [25]. A COS is considered as a fundamental list of outcomes and is not meant to restrict researchers from measuring additional outcomes relevant to a specific study [26]. To date, no COS for transitional care of CMC exists. Therefore, building on a previously created overview of outcomes reported in the literature [22], this study aimed to reach a consensus on a set of core outcomes informed by healthcare professionals and parents.
Methods
We executed a three-round Delphi study followed by focus group discussions. The study followed the COMET methodological recommendations [25] and registered the protocol on the COMET database (www.comet-initiative.org/Studies/Details/1899) [27]. The study is reported in line with COS-Standards for Reporting (COS-STAR) guidance [28] and the GRIPP2 short form for Patient and Public Involvement (PPI) [29].
Research ethics
Approval was provided by the Institutional Review Board of the Amsterdam UMC, location AMC, who waived the need for a full ethical review (W20_220#20.007). Participants for the Delphi survey were informed and asked for consent during recruitment and once more in the introduction of the Delphi survey. Parental informed consent was obtained during the focus group discussions.
Parent involvement
Our study involved parents at three distinct levels to ensure the inclusion of the parents’ perspective in the COS. Firstly, a parent caring for a child with medical complexity and active as a representative of a patient organization (B. S.) was part of our research group. The parent representative provided valuable input throughout the study and contributed to the protocol development by advising how to include parents’ perspectives best. Besides participating in research meetings, the parent representative participated in the pilot testing of the questionnaires for the Delphi study. Additionally, questionnaires for the focus groups were pilot tested to make sure the information letter and questions asked were understandable for other parents. Secondly, representatives of parents’ interest groups were invited to participate in the Delphi survey. Finally, we organized a series of focus groups specifically for parents, which allowed them to share their perspectives and experiences related to the research topic.
Delphi study
Participants
Participants were identified through the literature review [22] and recommendations from recognized professionals in the field. Since CMC deal with many healthcare professionals, we aimed to include opinions from different disciplines. Therefore, we selected professionals from different disciplines, e.g., pediatricians, pediatric intensivists, neonatologists, pediatric nurses, specialized pediatric nurses (e.g., nurse practitioner), psychosocial care workers, and allied healthcare professionals, who all were involved in the hospital-to-home transition of CMC. This resulted in a purposive sample of participants, homogeneous in the field of transitional care, but heterogeneous in terms of professional background. To guarantee the perspectives of patients, also representatives of parents’ interest groups participated in the same manner as the professionals. Parents were recruited through our Transitional Care Unit (TCU) Consortium [12]. This consortium is established in the Netherlands to develop a standard of care for the hospital-to-home transition for CMC.
Questionnaires
Questions for the Delphi rounds were based on the preliminary results of our systematic review, where we identified 24 unique outcomes [22]. These outcomes were categorized into domains according to the taxonomy of Dodd et al. [30]. Links to references were given for every outcome. The questionnaires were distributed online through a web application (Castor EDC). Participants were asked to rate each outcome for inclusion in the COS using a five-point Likert scale (strongly disagree, disagree, neutral, agree, strongly agree). Six potential participants (two medical doctors, three nurses, and one researcher) piloted the first questionnaire to assure clarity. It was tested if Castor EDC worked properly, if the questions and answers options were clear, and how long it took to complete the questionnaire. No adjustments were made after the pilot test. The data from the pilot test have not been used in the analysis. The Delphi rounds took place between October 2021 and February 2022. Professionals were given 4 weeks to complete each round. Non-responders received two motivational e-mails after 2 and 4 weeks, and deadlines were communicated to prevent attrition [31]. At least two of the three questionnaires must have been filled in by the same participant to be included in the analyses. Participants who filled in only one of the three Delphi rounds were excluded from the analyses.
Delphi rounds
The questionnaire in round 1 consisted of three parts. Firstly, demographic information of the participants was collected. Secondly, the 24 outcomes were presented according to the aforementioned structure. Thirdly, respondents could propose novel outcomes and add free-text comments. The research group reviewed new suggestions to ensure they represented a new outcome.
The following definitions were used for the consensus threshold: when at least 70% of the professionals rated an outcome with “agree” or “strongly agree,” consensus for inclusion in the COS was reached [25, 32]. Consensus for exclusion in the COS was reached if at least 70% of the professionals rated the corresponding outcome as “disagree” or “strongly disagree”. The outcomes on which consensus was reached were not presented for re-scoring in the next round.
In round 2, the remaining outcomes were listed with the group response results, described as percentages of the professionals’ scores on the 5-point Likert scale. Professionals were asked to re-score these outcomes, as well as the newly suggested outcomes. Again, outcomes that reached consensus based on the cutoff value of 70% were excluded from round 3.
Round 3 consisted of two parts. In the first part, the outcomes that had not reached consensus in the previous rounds were shown, again with the results in percentages. Professionals were asked to re-score the outcomes. The second part was added since, during the previous two Delphi rounds, participants highlighted that it was hard to fill out the questions since all outcomes were considered important in transitional care. Therefore, we presented the outcomes that reached consensus for inclusion in de COS so far, and asked to confirm if they considered the outcome a core outcome (mandatory in all clinical trials regardless the objective of the study) or an important outcome (relevant, but only when related to the aim of a specific study). It was stressed that the core outcomes should be limited to outcomes relevant in all studies.
Focus groups
Semi-structured online focus groups with parents of CMC were organized to validate the results of the Delphi study and to identify missing outcomes important from the parents’ perspective. Twenty-seven parents in the Netherlands who were (1) Dutch-speaking, (2) > 18 years old, and (3) had experience with the hospital-to-home transition were invited to participate. Parents were recruited through the TCU Consortium [12] and the parent representative in our research group (B. S.). Purposeful sampling was used to maximize diversity among the recruited parents. This involved identifying and selecting parents of CMC with various underlying diagnoses.
Once parents agreed upon participation in the study, they received an information letter with all outcomes considered in the Delphi rounds. Demographics of parents were collected using a short online questionnaire. H. H. moderated the focus groups with support of a second researcher (J. M. or M. A.) all experienced in conducting qualitative research such as focus groups and semi-structured interviews. After a personal introduction, parents were asked about their experiences regarding the hospital-to-home transition. Thereafter, the mentioned outcomes were discussed as well as any missing outcomes. During the discussion, we explored which outcomes were core from the perspective of the parents. Interaction and discussion between the participants were encouraged, but the researchers intervened when the debate strayed away from the purpose of the focus group.
Analyses
The results of the Delphi questionnaires were summarized as frequencies and percentages. The level of agreement was expressed in percentages of the same answers to each question. Based on the 70% level of agreement, outcomes that reached consensus were considered important outcomes in rounds 1, 2, and 3 (first part). Inclusion in the COS occurred when at least 70% of the participants rated the outcome as core in the second part of round 3. Response rates were calculated as the number of respondents who completed a Delphi round as a proportion of the total invited group.
The focus groups were recorded, and three researchers (H. H., J. M., M. A.) replayed and summarized the transcripts independently. The researchers discussed their notes and looked for recurring topics or issues that were discussed by multiple participants to reach a consensus on the core outcomes mentioned by parents. Finally, the results of the focus groups were discussed in the research group until consensus on the final COS was reached.
Results
Delphi study
Participants
In total, 110 professionals were invited to participate in the Delphi study, of whom 67 consented to participate. A total of 45 out of 67 (67%) filled out at least two questionnaires. Of the 45 participants, most lived in the Netherlands (73.3%), followed by Canada (13.3%) and the USA (13.3%). The sample consisted of different healthcare disciplines and three parent representatives. The characteristics of the respondents are summarized in Table 1.
Delphi rounds
Of the group of 67 professionals, 37 (55%) responded in the first round, 38 (57%) in the second round, and 39 (58%) in the third round. Twenty-nine professionals (43%) completed all three questionnaires.
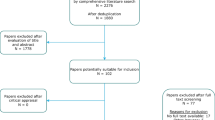
In round 1, consensus was reached on 13 outcomes considered important. No outcomes were excluded. The professionals suggested 12 additional outcomes. Round 2 included the 11 outcomes that remained from the first round for re-rating, along with the 12 additional suggested outcomes. Consensus was reached on eight important outcomes. Again, no outcomes were excluded. In round 3, professionals reached a consensus on three more important outcomes and no outcomes were excluded. A flowchart of the study is presented in Fig. 1. The results of the three Delphi rounds are summarized in Table 2.
As mentioned in the “Methods” section, in the second part of round 3 a distinction was made between a core and important outcome. This resulted in three core outcomes: (1) disease management, (2) child’s quality of life, and (3) impact on the life of the family. See Table 2.
Focus groups
Two online focus groups of approximately 1 h were held in March 2022. Of the 27 parents invited, nine did not respond, eight declined participation, and three did not show up on the focus group day. In total, seven parents participated in the focus groups. Characteristics of parents are presented in Supplement 1. In the focus groups, we went through all the outcomes that had already been mentioned in the information letter point by point. Parents agreed on the importance of the outcomes “child’s quality of life” and “impact on the life of the family”. The priority for the parents was the child’s comfort, and it was emphasized that the child’s well-being affected the whole family, including siblings. In addition, all parents stated that the self-efficacy of parents to provide the needed care for their child was crucial. Parents explained that even though they were trained in the hospital to care for the child, they often felt insecure without healthcare professionals nearby. Therefore, this outcome was added to the list of core outcomes.
Final core outcome set
The final set includes four core outcomes: (1) disease management, (2) child’s quality of life, (3) impact on the life of families, and (4) self-efficacy of parents. The COS development process resulted in 20 additional important outcomes. Results are summarized in Table 3 and descriptions of the core outcomes are presented in Supplement 2.
Discussion
This study resulted in a COS with four core outcomes to be used in all research programs evaluating transitional care for CMC: (1) disease management, (2) child’s quality of life, (3) impact on the life of families, and (4) self-efficacy of parents. These four outcomes underpin that attention should be paid to biomedical aspects of the child, as well as psychological and social factors of the child and its family.
Comparison with previous research
The core outcome “disease management” is used with variability of applied outcomes, such as physical development [33], physical health needs [34], weight on standard growth curve [35], and well-controlled disease [34]. This is in line with other literature where parents prioritize their desire to support their child’s growth, weight gain, and physical development [11]. However, this outcome is reported in only 8% of studies investigating the hospital-to-home transition for CMC [22]. This might be explained by the heterogeneity of diagnoses and syndromes of CMC. In addition, disease management can be operationalized by resource use such as “hospital re-admissions” and “visits to the emergency department”.
The core outcome child’s quality of life is widely acknowledged as an important outcome in many research areas [36,37,38,39]. The outcome is also included in several pediatric COSs for chronic diseases, facilitating comparisons across studies [40,41,42]. Surprisingly, systematic reviews on CMC health outcomes showed that child’s quality of life is measured in very few studies [22, 43]. An explanation could be that studies are designed to acquire data with as little burden as possible for parents. Since the quality of life of CMC can only be obtained by parental proxy measures, it might not have been incorporated in studies to avoid burden for the parents. However, recently a vision paper was published, placing quality of life of CMC and their families central when developing services to provide CMC and their families a life of dignity, autonomy, and independence [44].
Consensus on the core outcome impact on the life of the family echoes prior findings showing the importance that caregivers place on the needs of not only the patient, but also the family members [9, 44,45,46]. The impact of a child’s critical illness and hospital admission on family members may be profound as they may experience psychosocial sequelae too [47]. Family members’ responses may, in turn, influence the outcomes of child survivors following pediatric critical illness [48, 49]. In our systematic review, only one study took the family into account [50], and one study reported on out-of-the-pocket expenditures specifically [22]. A possible explanation could be that the impact of living with a CMC on the family is more explored in qualitative studies. Furthermore, it is a broad concept and might overlap with outcomes identified as important, for example, “impact on the life of siblings” and “post-traumatic stress symptoms in parents”.
The fourth core outcome is “self-efficacy of parents”. This outcome was found in 19% of the intervention studies regarding transitional care [22]. In the studies several terms were used, such as self-confidence [51] and parents’ beliefs in their caregiving skills [52]. To avoid confusion, they were gathered under the outcome self-efficacy of parents, meaning the confidence of parents in their capabilities to manage their child’s demands adequately [51]. The importance of this outcome is congruent with the review of Peer et al. that identified self-efficacy as a key factor in determining how well parents of CMC cope with their situation [53]. Another study recommends developing and testing strategies promoting parents’ self-efficacy to maximize quality of life and improve health outcomes in CMC [54]. Lastly, qualitative studies on transitional care for CMC support the consensus on the core outcome self-efficacy of parents [11, 55].
Core versus important outcome
During the development of this COS participants noted that all outcomes were considered important, and the difference between core and important was difficult to make. Therefore, in the last Delphi round, we explained once more the idea of a COS, and explicitly asked to choose between important or core for every outcome. This resulted in three core outcomes and 20 important outcomes as preliminary results and input for the focus groups. The number of outcomes included in a COS can vary depending on the specific condition or intervention being studied. Williamson et al. stated that the number of outcomes included in COSes for pediatric clinical trials ranged from 3 to 70, with a median of 15 outcomes per COS [27]. Although there is no consensus regarding the ideal number of core outcomes in a COS, it is very likely that fewer outcomes make the implementation more feasible. An explanation for the many important outcomes might be that transitional care is a complex, multi-faceted process that can be evaluated on many different aspects.
Parent involvement
A strength of this study is the involvement of CMC parents in the research team and focus groups. No children were involved since their diagnoses often accompany developmental delay and intellectual disability. The importance of patients’ involvement in research is acknowledged, but not yet common practice in the COS development [56]. However, the effort must be made because the mismatch between researchers and patients in research priorities and outcome selection has proven to result in considerable research waste [57]. In this study, parents in the focus groups had a crucial contribution in the final COS, as they identified the outcome self-efficacy of parents as core which differed from the professionals.
Limitations
Our findings should be interpreted with several caveats in mind. First, the number of healthcare professionals and parents was unbalanced. In line with other studies, we experienced challenges in obtaining a representative sample of parents [56, 58, 59]. Secondly, parents were all female with the Dutch nationality, making it impossible to consider cultural differences. Thirdly, healthcare professionals from only three high-income countries were included, limiting the COS’ generalizability. Fourthly, not all participants completed all three Delphi rounds. Although this is common in Delphi studies [60], it is uncertain to what degree this influenced the consensus procedure. Finally, in round 3 of the Delphi, consensus was found on three more outcomes: compliance of children, e.g., follow-up appointments; post-traumatic stress symptoms in parents; and the experiences of parents with the multidisciplinary transitional care. For these outcomes, participants were not asked to choose between important or core. However, parents did not mention these outcomes in the focus groups, so we decided not to include them in the final COS.
Future directions
Further implementation of the COS would be facilitated by recommendations for feasible and validated measurement tools for each core outcome [61]. Additionally, future work should be used to address the timing of the evaluation moments for each of the core outcomes. Until then, we encourage researchers to give detailed descriptions of the used measurement tools and timing of outcome assessment. An important final step will be the broad dissemination and implementation of the COS. It is important to note that this COS is not intended to restrict researchers from measuring additional outcomes that are deemed relevant to their specific study. Furthermore, as the field continues to evolve, regular updates of COS are crucial in the future. These updates ensure that COS remain comprehensive, reflective of current knowledge, and adaptable to emerging advancements.
Conclusion
Using well-established methods, we present the COS transitional care for CMC with four core outcomes: disease management, child’s quality of life, impact on the life of the family, and self-efficacy of parents. These core outcomes could facilitate standard reporting in future research of CMC hospital to home transition.
Data availability
The data that support the findings of this study are available from the corresponding author (JM), upon reasonable request.
Abbreviations
- CMC:
-
Children with Medical Complexity
- COS:
-
Core Outcome Set
- COMET:
-
Core Outcome Measure in Effectiveness Trials
- COS-STAR:
-
Core Outcome Set–Standards for Reporting Statement
References
Cohen E, Kuo DZ, Agrawal R, Berry JG, Bhagat SK, Simon TD, Srivastava R (2011) Children with medical complexity: an emerging population for clinical and research initiatives. Pediatrics 127(3):529–538. https://doi.org/10.1542/peds.2010-0910
Kuo DZ, Cohen E, Agrawal R, Berry JG, Casey PH (2011) A national profile of caregiver challenges among more medically complex children with special health care needs. Arch Pediatr Adolesc Med 165(11):1020–1026
Simon TD, Berry J, Feudtner C, Stone BL, Sheng X, Bratton SL, Dean JM, Srivastava R (2010) Children with complex chronic conditions in inpatient hospital settings in the United States. Pediatrics 126(4):647–655
Cohen E, Berry JG, Camacho X, Anderson G, Wodchis W, Guttmann A (2012) Patterns and costs of health care use of children with medical complexity. Pediatrics 130(6):e1463–e1470. https://doi.org/10.1542/peds.2012-0175
Gold JM, Hall M, Shah SS et al (2016) Long length of hospital stay in children with medical complexity. J Hosp Med 11(11):750–756. https://doi.org/10.1002/jhm.2633
Namachivayam P, Shann F, Shekerdemian L, Taylor A, van Sloten I, Delzoppo C, Daffey C, Butt W (2010) Three decades of pediatric intensive care: who was admitted, what happened in intensive care, and what happened afterward. Pediatr Crit Care Med 11(5):549–555. https://doi.org/10.1097/PCC.0b013e3181ce7427
Berry JG, Agrawal RK, Cohen E, Kuo DZ (2013) The landscape of medical care for children with medical complexity
Nageswaran S, Sebesta MR, Golden SL (2020) Transitioning children with medical complexity from hospital to home health care: implications for hospital-based clinicians. Hosp Pediatr 10(8):657–662. https://doi.org/10.1542/hpeds.2020-0068
Ronan S, Brown M, Marsh L (2020) Parents’ experiences of transition from hospital to home of a child with complex health needs: a systematic literature review. J Clin Nurs 29(17–18):3222–3235. https://doi.org/10.1111/jocn.15396
Seppänen A-V, Sauvegrain P, Draper ES et al (2021) Parents’ ratings of post-discharge healthcare for their children born very preterm and their suggestions for improvement: a European cohort study. Pediatr Res 89(4):1004–1012. https://doi.org/10.1038/s41390-020-01120-y
Leyenaar JK, O’Brien ER, Leslie LK, Lindenauer PK, Mangione-Smith RM (2017) Families’ priorities regarding hospital-to-home transitions for children with medical complexity. Pediatrics 139(1). https://doi.org/10.1542/peds.2016-1581
TCU Consortium (2022). Available from: https://tcuconsortium.nl/
Het Jeroen Pit Huis (2022). Available from: https://hetjeroenpithuis.nl/
Mosquera RA, Avritscher EBC, Pedroza C et al (2021) Telemedicine for children with medical complexity: a randomized clinical trial. Pediatrics 148(3). https://doi.org/10.1542/peds.2021-050400
Looman WS, Antolick M, Cady RG, Lunos SA, Garwick AE, Finkelstein SM (2015) Effects of a telehealth care coordination intervention on perceptions of health care by caregivers of children with medical complexity: a randomized controlled trial. J Pediatr Health Care 29(4):352–363. https://doi.org/10.1016/j.pedhc.2015.01.007
Auger KA, Simmons JM, Tubbs-Cooley HL et al (2018) Postdischarge nurse home visits and reuse: the Hospital to Home Outcomes (H2O) trial. Pediatrics 142(1). https://doi.org/10.1542/peds.2017-3919
Coller RJ, Klitzner TS, Lerner CF et al (2018) Complex care hospital use and postdischarge coaching: a randomized controlled trial. Pediatrics 142(2). https://doi.org/10.1542/peds.2017-4278
Conkol KJ, Martinez-Strengel A, Coller RJ, Bergman DA, Whelan E-M (2020) Pediatric hospitalists’ lessons learned from an innovation award to improve care for children with medical complexity. Hosp Pediatr 10(8):694–701. https://doi.org/10.1542/hpeds.2020-0069
Williams LJ, Waller K, Chenoweth RP, Ersig AL (2021) Stakeholder perspectives: communication, care coordination, and transitions in care for children with medical complexity. J Special Pedia Nursing 26(1):e12314. https://doi.org/10.1111/jspn.12314
Pordes E, Gordon J, Sanders LM, Cohen E (2018) Models of care delivery for children with medical complexity. Pediatrics 141(Supplement_3):S212-S223. https://doi.org/10.1542/peds.2017-1284F
Hamline MY, Speier RL, Dai Vu P, Tancredi D, Broman AR, Rasmussen LN, Tullius BP, Shaikh U, Li S-TT (2018) Hospital-to-home interventions, use, and satisfaction: a meta-analysis. Pediatrics 142(5). https://doi.org/10.1542/peds.2018-0442
de Lange A, Alsem MW, Haspels HN, van Karnebeek CD, van Woensel JBM, Jamaluding FSE, Maaskant JM (2023) Hospital-to-home transitions for Children with Medical Complexity: Part 1 - a systematic review of reported outcomes. Eur J Pediatr. Accepted
Breen C, Altman L, Ging J, Deverell M, Woolfenden S, Zurynski Y (2018) Significant reductions in tertiary hospital encounters and less travel for families after implementation of Paediatric Care Coordination in Australia. BMC Health Serv Res 18(1):1–10
Moreno L, Peck JL (2020) Nurse practitioner-led telehealth to improve outpatient pediatric tracheostomy management in South Texas. J Pediatr Health Care 34(3):246–255
Kirkham JJ, Davis K, Altman DG, Blazeby JM, Clarke M, Tunis S, Williamson PR (2017) Core outcome Set-STAndards for development: the COS-STAD recommendations. PLoS Med 14(11):e1002447. https://doi.org/10.1371/journal.pmed.1002447
Williamson P, Altman D, Blazeby J, Clarke M, Gargon E (2012) Driving up the quality and relevance of research through the use of agreed core outcomes. SAGE Publications Sage UK: London, England. p. 1–2. https://doi.org/10.1258/jhsrp.2011.011131
Williamson PR, Altman DG, Bagley H et al (2017) The COMET handbook: version 1.0. Trials 18(3):1–50. https://doi.org/10.1186/s13063-017-1978-4
Kirkham JJ, Gorst S, Altman DG et al (2016) Core outcome set–STAndards for reporting: the COS-STAR statement. PLoS Med 13(10):e1002148. https://doi.org/10.1371/journal.pmed.1002148
Staniszewska S, Brett J, Simera I et al (2017) GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ 358. https://doi.org/10.1136/bmj.j3453
Dodd S, Clarke M, Becker L, Mavergames C, Fish R, Williamson PR (2018) A taxonomy has been developed for outcomes in medical research to help improve knowledge discovery. J Clin Epidemiol 96:84–92. https://doi.org/10.1016/j.jclinepi.2017.12.020
Edwards PJ, Roberts I, Clarke MJ, DiGuiseppi C, Wentz R, Kwan I, Cooper R, Felix LM, Pratap S (2009) Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev (3). https://doi.org/10.1002/14651858.MR000008.pub4
Williamson PR, Altman DG, Blazeby JM, Clarke M, Devane D, Gargon E, Tugwell P (2012) Developing core outcome sets for clinical trials: issues to consider. Trials 13(1):1–8
Tolomeo CT, Major NE, Szondy MV, Bazzy-Asaad A (2017) Standardizing care and parental training to improve training duration, referral frequency, and length of stay: our quality improvement project experience. J Pediatr Nurs 32:72–79. https://doi.org/10.1016/j.pedn.2016.10.004
Caliskan Yilmaz M, Ozsoy SA (2010) Effectiveness of a discharge-planning program and home visits for meeting the physical care needs of children with cancer. Support Care Cancer 18(2):243–253. https://doi.org/10.1007/s00520-009-0650-2
Noritz G, Madden M, Roldan D, Wheeler TA, Conkol K, Brilli RJ, Barnard J, Gleeson S (2017) A population intervention to improve outcomes in children with medical complexity. Pediatrics 139(1). https://doi.org/10.1542/peds.2015-3076
Germain N, Aballéa S, Toumi M (2019) Measuring health-related quality of life in young children: how far have we come? Journal of Market Access & Health Policy 7(1):1618661. https://doi.org/10.1080/20016689.2019.1618661
Killien EY, Loftis LL, Clark JD et al (2021) Health-related quality of life outcome measures for children surviving critical care: a scoping review. Qual Life Res 30(12):3383–3394. https://doi.org/10.1007/s11136-021-02928-9
Hordijk JA, Verbruggen SC, Buysse CM, Utens EM, Joosten KF, Dulfer K (2022) Neurocognitive functioning and health-related quality of life of children after pediatric intensive care admission: a systematic review. Qual Life Res p. 1–14. https://doi.org/10.1007/s11136-022-03124-z
Killien EY, Rivara FP, Dervan LA, Smith MB, Watson RS (2022) Components of health-related quality of life most affected following pediatric critical illness. Crit Care Med 50(1):e20–e30. https://doi.org/10.1097/CCM.0000000000005230
Joachim KC, Farid-Kapadia M, Butcher NJ et al (2020) Core outcome set for children with neurological impairment and tube feeding. Dev Med Child Neurol 62(2):201–206. https://doi.org/10.1111/dmcn.14326
Allin BSR, Hall NJ, Ross AR, Marven SS, Kurinczuk JJ, Knight M (2019) Development of a gastroschisis core outcome set. Arch Dis Child Fetal Neonatal Ed 104(1):F76–F82. https://doi.org/10.1136/archdischild-2017-314560
Pugliese M, Tingley K, Chow A et al (2021) Core outcome sets for medium-chain Acyl-CoA dehydrogenase deficiency and phenylketonuria. Pediatrics 148(2). https://doi.org/10.1542/peds.2020-037747
Barnert ES, Coller RJ, Nelson BB et al (2019) Key population health outcomes for children with medical complexity: a systematic review. Matern Child Health J 23(9):1167–1176. https://doi.org/10.1007/s10995-019-02752-1
Coleman CL, Morrison M, Perkins SK, Brosco JP, Schor EL (2022) Quality of life and well-being for children and youth with special health care needs and their families: a vision for the future. Pediatrics 149(Supplement 7). https://doi.org/10.1542/peds.2021-056150G
Manning JC, Pinto NP, Rennick JE, Colville G, Curley MA (2018) Conceptualizing post intensive care syndrome in children—the PICS-p framework. Pediatr Crit Care Med 19(4):298–300. https://doi.org/10.1097/PCC.0000000000001476
Lindstrom K, Cady R, Bushaw A (2020) Family-centered care for children with medical complexity: a goal-planning initiative. Nurse Pract 45(8):49–55. https://doi.org/10.1097/01.NPR.0000681796.57869.a0
Nelson LP, Gold JI (2012) Posttraumatic stress disorder in children and their parents following admission to the pediatric intensive care unit: a review. Pediatr Crit Care Med 13(3):338–347. https://doi.org/10.1097/PCC.0b013e3182196a8f
Colville G, Pierce C (2012) Patterns of post-traumatic stress symptoms in families after paediatric intensive care. Intensive Care Med 38(9):1523–1531. https://doi.org/10.1007/s00134-012-2612-2
Poh P-F, Carey MC, Lee JH, Manning JC, Latour JM (2022) Impact of ethnicity on parental health outcomes and experiences after paediatric intensive care unit discharge: a mixed-methods systematic review. Eur J Pedia p. 1–13. https://doi.org/10.1007/s00431-022-04595-5
Donnelly S, Shaw E, Timoney P, Foca M, Hametz P (2020) Parents’ assessment of an advanced-practice nurse and care coordination assistant model medical care coordination program for children with medical complexity. J Pediatr Health Care 34(4):325–332. https://doi.org/10.1016/j.pedhc.2020.01.007
Vance AJ, Brandon DH (2017) Delineating among parenting confidence, parenting self-efficacy and competence. ANS Adv Nurs Sci 40(4):E18. https://doi.org/10.1097/ANS.0000000000000179
Berry JG, Hall M, Hall DE, Kuo DZ, Cohen E, Agrawal R, Mandl KD, Clifton H, Neff J (2013) Inpatient growth and resource use in 28 children’s hospitals: a longitudinal, multi-institutional study. JAMA Pediatr 167(2):170–177
Peer JW, Hillman SB (2014) Stress and resilience for parents of children with intellectual and developmental disabilities: a review of key factors and recommendations for practitioners. J Policy Pract Intellect Disabil 11(2):92–98. https://doi.org/10.1111/jppi.12072
Bravo L, Killela MK, Reyes BL, Santos KMB, Torres V, Huang C-C, Jacob E (2020) Self-management, self-efficacy, and health-related quality of life in children with chronic illness and medical complexity. J Pediatr Health Care 34(4):304–314. https://doi.org/10.1016/j.pedhc.2019.11.009
Desai AD, Durkin LK, Jacob-Files EA, Mangione-Smith R (2016) Caregiver perceptions of hospital to home transitions according to medical complexity: a qualitative study. Acad Pediatr 16(2):136–144. https://doi.org/10.1016/j.acap.2015.08.003
Gargon E, Williamson PR, Young B (2017) Improving core outcome set development: qualitative interviews with developers provided pointers to inform guidance. J Clin Epidemiol 86:140–152. https://doi.org/10.1016/j.jclinepi.2017.04.024
Chalmers I, Glasziou P (2009) Avoidable waste in the production and reporting of research evidence. Lancet 374(9683):86–89. https://doi.org/10.1016/S0140-6736(09)60329-9
Biggane AM, Williamson PR, Ravaud P, Young B (2019) Participating in core outcome set development via Delphi surveys: qualitative interviews provide pointers to inform guidance. BMJ Open 9(11):e032338. https://doi.org/10.1136/bmjopen-2019-032338
Vanderhout SM, Smith M, Pallone N et al (2021) Patient and family engagement in the development of core outcome sets for two rare chronic diseases in children. Res Involv Engagem 7(1):1–8. https://doi.org/10.1186/s40900-021-00304-y
Sinha IP, Smyth RL, Williamson PR (2011) Using the Delphi technique to determine which outcomes to measure in clinical trials: recommendations for the future based on a systematic review of existing studies. PLoS Med 8(1):e1000393. https://doi.org/10.1371/journal.pmed.1000393
Prinsen CA, Vohra S, Rose MR, Boers M, Tugwell P, Clarke M, Williamson PR, Terwee CB (2016) How to select outcome measurement instruments for outcomes included in a “Core Outcome Set”—a practical guideline. Trials 17(1):1–10. https://doi.org/10.1186/s13063-016-1555-2
Acknowledgements
We would like to thank the members of the Transitional Care Unit Consortium in the Netherlands and colleagues from BC Children’s Hospital, Canada for supporting the recruitment of participants. Also, we would like to thank all participants for their contribution to the Delphi study. Furthermore, we thank the parents, who took part in the focus groups, for sharing their experiences.
Funding
This project is made possible by the Foundation “Steun Emma Kinderziekenhuis” and The Netherlands Organization for Health Research and Development (ZonMw; 845008701). Both funders had no role in the design and conduct of the study.
Author information
Authors and Affiliations
Contributions
Heleen Haspels designed the data collection instruments, collected data, carried out the initial analyses, drafted the initial manuscript, and reviewed and revised the manuscript. Annemieke de Lange and Mattijs Alsem designed the data collection instruments, collected data, carried out the initial analyses, and revised the manuscript. Bettina Sandbergen co-designed the study by advising how to include parents’ perspective best and reviewed and revised the manuscript. Clara van Karnebeek, Job van Woensel, Karolijn Dulfer, Matthijs de Hoog, and Koen Joosten conceptualized and co-designed the study and reviewed and revised the manuscript. Jolanda Maaskant conceptualized and designed the study, coordinated and supervised the data collection and analyses, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval
The Institutional Review Board of the Amsterdam UMC location AMC waived the need for ethical approval (W20_220 #20.007).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable to this manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Peter de Winter
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haspels, H.N., de Lange, A.A., Alsem, M.W. et al. Hospital-to-home transitions for children with medical complexity: part 2—a core outcome set. Eur J Pediatr 182, 3833–3843 (2023). https://doi.org/10.1007/s00431-023-05049-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-023-05049-2