Abstract
Pediatric tuberculosis is a major cause of mortality and morbidity in children due to high transmission, poor diagnostic tools, and various respiratory diseases mimicking TB. Identifying risk factors will provide evidence for clinicians to strongly relate their diagnosis to the associated pathology. Studies were retrieved from PubMed, Embase, and Google Scholar, systematically reviewed, and meta-analyzed for various risk factors and their association with pediatric TB. Meta-analysis depicted that four out of eleven risk factors were significant—contact with known TB cases (OR 6.42 [3.85,10.71]), exposure to smoke (OR 2.61 [1.24, 5.51]), overcrowding in the houses (OR 2.29 [1.04, 5.03]), and, poor household conditions (OR 2.65 [1.38, 5.09]). Although significant odds ratio estimates were obtained, we observed heterogeneity in the studies included.
Conclusion: The study findings demand the constant screening of risk factors such as contact with known TB cases, exposure to smoke, overcrowding, and, poor household conditions for the development of pediatric TB.
What is Known: • Knowledge of the risk factors of a disease is of utmost importance in the planning and institution of its control measures. Well-established risk factors in the occurrence of TB in the pediatric group are HIV positivity, older age and close contact with a known case of TB. | |
What is New: • In addition to what is already known; this review and meta-analysis has identified exposure to indoor smoking, overcrowding and poor household conditions as important risk factors for developing pediatric TB. • Implications of the study: The findings highlight that in addition to routine contact screening for the pediatric group, the children living in poor household conditions and getting exposed to passive indoor smoking demand more attention to prevent the development of pediatric TB. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, the prevention and management of childhood tuberculosis (TB) have posed to be a major challenge to clinicians, academicians, and program managers for decades. Programmatically, TB in children has failed to gain enough momentum despite its high mortality and morbidity because the transmission rate from children is low due to the paucibacillary state [1]. The lack of newer TB diagnostic tools that are simple and feasible to implement in the field adds up to the existing problem. The incidence of pediatric TB is the direct measure and a proxy indicator for the transmission of TB in the community [1, 2].
The Global TB Report 2021 states that 8.75% of the total treated cases of TB during 2018–2022 were children (3.5 out of 40 million) signifying that the transmission rate is relatively high in the community [3]. Hence, the risk factors leading to the development of TB disease have gained focus over the years. Children bear severe forms of TB in comparison to adults [4, 5]. Since no tests are available to measure the progression of the disease, the associated risk factors can be advantageous to speculate the disease.
Contact with known adult TB cases is known as an important risk factor in the case of both children and adults, whereas other risk factors are less explored in the pediatric population [6, 7]. The Global TB Report 2020 suggests that 86% of newly detected pediatric cases occurred in 30 high-burden countries [8, 9]. Thus, this study is focused on comprising research data published in these high-burden countries listed by the World Health Organization (WHO).
To address the knowledge gaps, data were pooled from studies published from January 1990 to June 2022 and systematically reviewed to determine the association of various risk factors with the development of TB among the pediatric population.
Methods
This systematic review protocol was registered at the International Prospective Register of Systematic Reviews (PROSPERO) (identifier: CRD42022315722), and the study was conducted and reported as per the Meta-analysis of Observational Studies in Epidemiology (MOOSE checklist) [10]. The MOOSE checklist is provided as supplement copy 1.
Search strategy and selection criteria
We searched electronic databases like MEDLINE (through PubMed), Embase, and Google Scholar for the published research articles, limited to the English language from January 1990 to June 2022, reporting the risk factors for tuberculosis in children less than 19 years old.
The search strategy was developed with combinations of keywords like pediatric, tuberculosis, risk factors, and the list of high TB burden countries along with their synonyms, Boolean operators, and truncations as per the databases.
In the PubMed, we used the search term “pediatric*”[All Fields] OR “pediatrics”[MeSH Terms] OR “paediatric*”[All Fields] OR “child*”[All Fields] OR “child”[MeSH Terms] OR “adolescent”[MeSH Terms] OR “adolescen*”[All Fields] OR “infant*”[All Fields] OR “infant”[MeSH Terms] OR “infant, newborn”[MeSH Terms] OR “newborn*”[All Fields] “tuberculosis”[All Fields] OR “tuberculosis”[MeSH Terms] OR “tuberculosis”[All Fields] OR “tuberculosis”[MeSH Terms] OR “TB”[All Fields] OR “Latent TB”[All Fields] OR “latent tuberculosis”[MeSH Terms] OR “Koch’s Disease”[All Fields] OR “mycobacterium infections”[MeSH Terms] OR “Mycobacterium tuberculosis”[MeSH Terms] OR “Mycobacterium tuberculosis”[All Fields] “risk factor*”[All Fields] OR “risk factors”[MeSH Terms] OR “socioeconomic factor*”[All Fields] OR “social determinants of health”[MeSH Terms] OR “social determinant*”[All Fields] OR “risk determinant*”[All Fields] OR “risk predictor*”[All Fields] OR “epidemiologic factor*”[All Fields] OR “epidemiologic factors”[MeSH Terms] OR “risk assessment*”[All Fields].
A similar search strategy was used for Embase and Google Scholar, which is provided in supplement copy 2.
Screening and selection
The research articles were imported into the Rayyan website (https://www.rayyan.ai/) and duplicates were removed. Two investigators independently screened the title and abstract of the articles based on the following inclusion and exclusion criteria.
Inclusion criteria
We included case–control, cohort, and cross-sectional studies with the pediatric population less than 19 years old and reported risk factor analysis for TB.
Exclusion criteria
Reviews and conference abstracts were excluded. Study outcomes focusing on the adult population and pediatric cases with previously known contact with TB were excluded.
Subsequently, the authors reviewed full-text versions of selected records. The reasons for article exclusion were recorded, and potential disagreements were specified to be resolved by the involvement of a third investigator (BNS).
Data extraction
The data extracted from the included articles were entered into a standardized excel sheet. The data sheet included variables such as author, year of publication, study design, the country where the study was conducted, study group, sample size, and significant risk factors.
Quality assessment
Quality assessment was done by the Newcastle–Ottawa scale to check the methodological quality of the selected articles [11]. Studies scoring ≥ 7 points were considered high quality, 5–6 were medium quality, and ≤ 4 was low quality.
Data analysis
Meta-analysis of the studies was depicted as forest plots to estimate the overall odds ratio (OR). Heterogeneity among studies was evaluated using the Galbraith plot and I2 statistic categorized as follows: < 30% not important, 30–50% moderate, 50–75% substantial, and 75–100% considerable. If significant heterogeneity was identified among studies, further examination of the individual studies was conducted, and random effects models (Dersimonian-Laird method) were used to obtain the summary odds ratio estimates. Otherwise, fixed effects models were used (Mantel–Haenszel method). Publication bias was assessed by the Egger regression asymmetry test and funnel plots. The meta-analytic software program used was Stata 17.0 BE-Basic (S. No. 301706309069).
Results
Search results
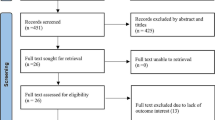
Based on the keyword search in the database of PubMed, Embase, and Google Scholar, we obtained 3231 articles. Of these, 3056 articles were screened for titles and abstracts after excluding 175 duplicates. The studies were listed based on our study inclusion and exclusion criteria; 31 studies were subjected to full-text screening after which 14 eligible studies were included for analysis. Figure 1 illustrates the PRISMA flow chart (https://prisma-statement.org/prismastatement/flowdiagram.aspx) of studies retrieved and the selection process through various stages.
Characteristics of the studies
Characteristics of included studies are shown in Table 1. All the included studies in this review belong to the eight high TB burden countries: South Africa, Nigeria, Uganda, Brazil, China, the Philippines, Bangladesh, and India. Among these fourteen articles, seven were case–control studies, along with five cross-sectional and two cohort studies. The study population ranged from 0 to 19 years old with the most common age group between 10 and 18 years, and the detection of latent TB infection or TB disease was the aim of most of the articles. Either questionnaire or interview was used as a data collection tool for the studies included. The significant risk factors in the individual study are tabulated.
Quality assessment
Quality assessment of the included study methodology was carried out for both cohort and case–control study designs. The quality of cross-sectional studies could not be assessed due to the non-availability of the standardized tool. Criteria for assessing the quality of studies are included in supplement copy 2.
Cohort studies
Of the two cohort studies assessed for quality, one each was of high and medium quality.
Case–control studies
Out of the seven studies assessed for quality, 71 percent of studies (05 nos.) were high quality, and two studies are of medium quality.
Risk factors
Among the 14 studies included, the significant risk factors identified were (Table 2) TB contacts (12/14), living conditions (11/14), child health and well-being (07/14), older age (07/14), socio-demographic factors (05/14), socio-economic status (04/14), education (03/14), and food insecurity (02/14). Contact with a known TB case is the most common risk factor among the studies, and food insecurity is the least common risk factor. Contact with TB cases, poor living conditions, child health, and older age have occurred in 50% or more studies whereas socio-demographic factors, socio-economic status, educational status, and food insecurity have occurred in less than 50% of the studies.
Interpretation of study findings
Case-control studies (Table 3)
Risk factors that are common in at least two of the studies were selected to calculate the pooled odds ratio. The following factors showed significance when pooled odds ratio at 95% confidence interval was calculated—household contact 6.199 (4.836–7.946), child’s education 0.5293 (0.4018–0.6974), father’s education 0.7607 (0.6158–0.9396), father’s occupation or income 1.349 (1.08–1.684), HIV positivity 22.81 (8.01–64.97), passive smoking 2.352 (1.694–3.265), exposure to indoor gases 2.062 (1.566–2.715), household condition 2.51 (1.966–3.204), less number of rooms 2.755 (1.961–3.87), overcrowding 2.414 (1.898–3.07), no adequate ventilation 2.366 (1.349–4.149), and kitchen position 2.153 (1.611–2.876).
Cohort studies
Out of the 14 studies selected for review, 2 studies were cohort study designs. Bunyasi et al. [15] reported 48.5% of LTBI in comparison to the prevalence a decade ago which was 43.8%. Mumpe-Mwanja et al. [16] reported 16.1% LTBI. The average LTBI prevalence among these studies was 36.1%.
Cross-sectional studies
-
Mzembe et al. [12] reported a 23% MTB infection prevalence using a QFT assay in rural South Africa.
-
Gatchalian et al. [13] reported 6.5% of TST positivity and 0.3% had active clinical TB disease in the Philippines.
-
Attah et al. [5] reported 32% of definitive TB cases in Nigeria.
-
Hu et al. [19] reported that 4.7% had positive T-SPOT in Shanghai, China.
-
Mahomed et al. [23] reported that 55.2% had positive TST tests and 50.9% had positive QFT in South Africa.
Meta-analysis
We considered eleven factors for the meta-analysis; out of these four risk factors are household TB contact OR 6.42 (3.85, 10.71), exposure to smoke OR 2.61(1.24, 5.51), poor housing condition OR 2.29 (1.04, 5.03), and crowding OR 2.65 (1.38, 5.09) which showed overall significance for developing TB (refer to forest plot in Figs. 2, 3, 4 and 5 and supplement copy 2 for additional forest plots).
Heterogeneity
Galbraith plots were used to assess the heterogeneity of the study, which showed significant heterogeneity. Most of the included studies in this review showed inconsistency; ten out of the eleven factors considered for meta-analysis show heterogeneity above 50%. Since moderate to considerable values of heterogeneity are observed, the study finding will not be conclusive (refer to Galbraith plots in Figs. 2, 3, 4, and 5 and supplement copy 2 for additional Galbraith plots).
Publication bias
Funnel plots were used to assess publication bias, despite four factors being overall significant but do not appear to contain symmetric data. Few studies fall out of the confidence interval range as well (refer to funnel plots in Figs. 2, 3, 4, and 5 and supplement copy 2 for additional funnel plots).
Discussion
Our study focused on the risk factors associated with TB among the pediatric population. Fourteen studies matched our inclusion criteria. Seven studies were included to analyze the pooled odds ratio.
The studies were categorized based on the year of publication, and the characteristics of each study were tabulated. The significant risk factors of all the studies were grouped under a broad category, and the frequency of each risk factor that occurred in all the studies was recorded. The quality of the studies was checked by the Newcastle–Ottawa scale where most of the studies included in our study are good quality. The odds ratio was calculated for the risk factors of the case–control studies, where thirteen out of the nineteen factors considered for the analysis were significant.
The risk factors that appeared to be significant in the calculated pooled odds ratio are described in detail below:
-
1.
Gender
In our review, the odds of developing TB in males compared to females were non-significant. Two included studies (Mumpe-Mwanja et al. and Stevens et al.) reported males as the risk factor, whereas the finding of our study is contradicting. Also, very few studies had evaluated gender as a risk factor in childhood TB [25, 26]. Gender can be considered a risk factor only after the child attains puberty since sexual hormones play an important role in immunological dimorphism [27]. It is well established in adults that males have a higher risk of TB as they encounter the outside world more often as the breadwinner of the family [25].
-
2.
Household contact
The odds of household contact developing TB, when compared to no contact or external contact, were found to be five times higher in our review. Twelve out of the fourteen studies had adult case exposure as the risk factor, but the exposure varied from household and non-household. Hence, we analyzed the known TB case exposure in two categories household contact and non-household contact. A study conducted by Jaganath et al. in Uganda showed a 10% TB prevalence among child contacts [28]. Low detection in contacts might be due to low screening. In a similar study conducted by Laghari et al. in India, only 9.3% of contacts were screened [29]. Our earlier research findings have also highlighted the need for constant screening for early diagnosis of pediatric TB cases [30].
-
3.
Child’s education
According to our findings, the odds of school-going children developing TB are insignificant compared to the children not attending school. Children going to school and/or with higher educational qualifications had lower chances of acquiring the disease since they might have a better knowledge of disease transmission and causes of the diseases like TB. Two studies (two articles published by Karim et al.) reported that the incidence of TB in children who attended school till higher classes are relatively low compared to children who skipped school or discontinued during primary education. A study conducted in Malawi reported that 90% of the children know about the TB disease and its transmission while a lower proportion of children were aware of the symptoms [31]. Children should be encouraged to attend school and continue their higher education which will increase disease awareness.
-
4.
Father’s occupation/income
The odds of children with low-income/lower socio-economic households have a 0.3 times higher risk of developing TB compared to the children with stable-income households, as per our review. As described, TB is a disease in poor people, and the occurrence of the disease is directly impacted by the socio-economic status of the family, which rationalized the high burden of TB in low-middle-income countries. Better socio-economic status indirectly caters to the nutritional and healthcare requirements of the child. Consumption of nutritious food at a young age will help in the overall development of the child. Providing protein-rich food through government programs can solve the problem of malnutrition.
-
5.
Passive smoking and exposure to indoor air pollution
We observed that the odds of children exposed to tobacco smoke and indoor air pollution had two times higher risk of developing TB compared to the children not being exposed. Previous studies have concluded that both passive exposure and active exposure to tobacco smoke are associated with TB disease [32]. Smoking is prevalent in most developing countries; hence, exposure to tobacco smoke can be of great concern as smoking leads to the downregulation of macrophage TNF-α in the lungs [32, 33]. A systematic review published by Patra et al. reported that more than a threefold increased risk of secondhand smoke (SHS) is associated with active TB in children [33]. Toxic particulate matter produced from the burning of biomass induces inflammation in the lungs causing respiratory tract-related infections and asthma. Parents should be counselled about the harmful effects of smoke on their child’s health. Those willing to quit can be sent to rehabilitation centers. In the case where quitting is not an option, smoking inside houses or around children should be avoided. Cooking outside the home if no separate kitchen or use of LPG will reduce the risk of exposure to harmful gases.
-
6.
HIV status
As per our review, the odds of children living with HIV have a twenty-one times higher chance of developing TB when compared to children with no history of HIV. Dodd et al. concluded that HIV is a potent risk factor for childhood TB, and early diagnosis of HIV with anti-retroviral therapy (ART) initiation could reduce the risk of developing TB [34].
-
7.
Household condition
The odds of children living in poor household conditions had two times higher risk of developing TB when compared to a normal household as per our study. A cross-sectional study conducted in Thailand to determine the association between environmental factors and TB infection showed that children living in crowded households were five times more likely to have the infection [35]. It is better to suggest the patient be isolated in a well-ventilated room; hence, exposure to other family members will be reduced.
Conclusion
Contact with known TB cases, exposure to smoke, overcrowding in the houses, and poor household conditions are the significant risk factors for pediatric TB. The public health implications of these findings suggest we keep a constant watch for these factors and focus our intervention accordingly, to reach the goal of TB elimination by 2030.
Data availability
All data supporting the findings of this study are available within the paper and its supplementary Information.
References
Carvalho AC, Kritski AL (2022) What is the global burden of tuberculosis among children? Lancet Glob Health 10(2):e159–e160
Jenkins HE, Yuen CM, Rodriguez CA, Nathavitharana RR, McLaughlin MM, Donald P, Marais BJ, Becerra MC (2017) Mortality in children diagnosed with tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis 17(3):285–295
WHO TB report- 2021 page- 04 accessed on 02th June, 2022. https://www.who.int/teams/global-tuberculosis-programme/tb-reports
WHO TB report- 2012 page- 14 accessed on 02th June, 2022. https://apps.who.int/iris/bitstream/handle/10665/75938/9789241564502_eng.pdf?sequence=1
Attah C, Oguche S, Egah D, Ishaya T, Banwat M, Adgidzi A (2018) Risk factors associated with paediatric tuberculosis in an endemic setting. Alexandria Journal of Medicine 54(4):403–409
Nelson JL, Moreno A, Orcau A, Altet N, Martínez-Roig A, Cayla JA, Casals M, Millet JP, Moraga-Llop F (2010) Childhood Tuberculosis Working Group of Barcelona. Transmission of childhood tuberculosis: risk factors associated with an unidentified index case and outbreak evolution in Barcelona (1987–2007). Ped Infect Dis J 29(9):876–9
Gessner BD, Weiss NS, Nolan CM (1998) Risk factors for pediatric tuberculosis infection and disease after household exposure to adult index cases in Alaska. J Pediatr 132(3):509–513
WHO TB fact sheet accessed on 11th July 2022. https://www.who.int/news-room/fact-sheets/detail/tuberculosis
WHO global lists of high burden countries for tuberculosis (TB), TB/HIV and multidrug/rifampicin-resistant TB (MDR/RR-TB), 2021–2025 accessed on 11th July 2022. https://cdn.who.int/media/docs/default-source/hq-tuberculosis/who_globalhbcliststb_2021-2025_backgrounddocument.pdf?sfvrsn=f6b854c2_9
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283(15):2008–2012
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P (2000) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
Mzembe T, Lessells R, Karat AS, Randera-Rees S, Edwards A, Khan P, Tomita A, Tanser F, Baisley K, Grant AD (2021) Prevalence and risk factors for mycobacterium tuberculosis infection among adolescents in rural South Africa. InOpen forum infectious diseases (Vol. 8, No. 1, p. ofaa520). US: Oxford University Press
Gatchalian SR, Agathis NT, Castillo-Carandang NT, Gunter SM, Murray KO, Mandalakas AM (2020) Design and evaluation of risk assessment tools to identify pediatric tuberculosis infection in Bohol, the Philippines, a low–HIV-and high–TB-burden setting. Am J Trop Med Hyg 103(5):1818
Jafta N, Jeena PM, Barregard L, Naidoo RN (2019) Association of childhood pulmonary tuberculosis with exposure to indoor air pollution: a case control study. BMC Public Health 19(1):1–1
Bunyasi EW, Geldenhuys H, Mulenga H, Shenje J, Luabeya AK, Tameris M, Nemes E, Mahomed H, Rozot V, Wood R, Scriba T (2019) Temporal trends in the prevalence of Mycobacterium tuberculosis infection in South African adolescents. Int J Tuberc Lung Dis 23(5):571–578
Mumpe-Mwanja D, Verver S, Yeka A, Etwom A, Waako J, Ssengooba W, Matovu JK, Wanyenze RK, Musoke P, Mayanja-Kizza H (2015) Prevalence and risk factors of latent tuberculosis among adolescents in rural Eastern Uganda. Afr Health Sci 15(3):851–860
Jubulis J, Kinikar A, Ithape M, Khandave M, Dixit S, Hotalkar S, Kulkarni V, Mave V, Gupte N, Kagal A, Jain S (2014) Modifiable risk factors associated with tuberculosis disease in children in Pune, India. Int J Tuberc Lung Dis 18(2):198–204
Stevens H, Ximenes RA, Dantas O, Rodrigues LC (2014) Risk factors for tuberculosis in older children and adolescents: a matched case–control study in Recife. Brazil Emerging themes in epidemiology 11(1):1–7
Hu Y, Zhao Q, Wu L, Wang W, Yuan Z, Xu B (2013) Prevalence of latent tuberculosis infection and its risk factors in schoolchildren and adolescents in Shanghai, China. The European Journal of Public Health 23(6):1064–1069
Karim MR, Rahman MA, Mamun SA, Alam MA, Akhter S (2012) Risk factors of childhood tuberculosis: a case control study from rural Bangladesh. WHO South-East Asia journal of public health 1(1):76–84
Patra S, Sharma S, Behera D (2012) Passive smoking, indoor air pollution and childhood tuberculosis: a case control study. Indian J Tuberc 59(3):151–155
Karim MR, Rahman MA, Mamun SA, Alam MA, Akhter S (2012) What cannot be measured cannot be done; risk factors for childhood tuberculosis: a case control study. Bangladesh Med Res Counc Bull 38(1):27–32
Mahomed H, Hawkridge T, Verver S, Geiter L, Hatherill M, Abrahams DA, Ehrlich R, Hanekom WA, Hussey GD, SATVI Adolescent Study Team (2011) Predictive factors for latent tuberculosis infection among adolescents in a high-burden area in South Africa. Intern J Tubercul Lung Dis 15(3):331–6
Ramachandran RI, Indu PS, Anish TS, Nair S, Lawrence T, Rajasi RS (2011) Determinants of childhood tuberculosis–a case control study among children registered under revised National Tuberculosis Control Programme in a district of South India. Indian J Tuberc 58(4):204–207
Abubakar I, Laundy MT, French CE, Shingadia D (2008) Epidemiology and treatment outcome of childhood tuberculosis in England and Wales: 1999–2006. Arch Dis Child 93(12):1017–1021
Syridou G, Mavrikou M, Amanatidou V, Spyridis N, Prasad P, Papaventsis D, Kanavaki S, Zaoutis T, Tsolia MN (2012) Trends in the epidemiology of childhood tuberculosis in Greece. Int J Tuberc Lung Dis 16(6):749–755
Stival A, Chiappini E, Montagnani C, Orlandini E, Buzzoni C, Galli L, de Martino M (2014) Sexual dimorphism in tuberculosis incidence: children cases compared to adult cases in Tuscany from 1997 to 2011. PLoS ONE 9(9):e105277
Jaganath D, Zalwango S, Okware B, Nsereko M, Kisingo H, Malone L, Lancioni C, Okwera A, Joloba M, Mayanja-Kizza H, Boom WH (2013) Contact investigation for active tuberculosis among child contacts in Uganda. Clin Infect Dis 57(12):1685–1692
Laghari M, Sulaiman SA, Khan AH, Talpur BA, Bhatti Z, Memon N (2019) Contact screening and risk factors for TB among the household contact of children with active TB: a way to find source case and new TB cases. BMC Public Health 19(1):1–10
Chawla K, Burugina Nagaraja S, Siddalingaiah N, Sanju C, Shenoy VP, Kumar U, Das A, Hazra D, Shastri S, Singarajipur A, Reddy RC (2021) Challenges perceived by health care providers for implementation of contact screening and isoniazid chemoprophylaxis in Karnataka, India. Tropical Medicine and Infectious Disease 6(3):167
Nyasulu P, Kambale S, Chirwa T, Umanah T, Singini I, Sikwese S, Banda HT, Banda RP, Chimbali H, Ngwira B, Munthali A (2016) Knowledge and perception about tuberculosis among children attending primary school in Ntcheu District. Malawi Journal of Multidisciplinary Healthcare 9:121
den Boon S, Verver S, Marais BJ, Enarson DA, Lombard CJ, Bateman ED, Irusen E, Jithoo A, Gie RP, Borgdorff MW, Beyers N (2007) Association between passive smoking and infection with Mycobacterium tuberculosis in children. Pediatrics 119(4):734–739
Patra J, Bhatia M, Suraweera W, Morris SK, Patra C, Gupta PC, Jha P (2015) Exposure to second-hand smoke and the risk of tuberculosis in children and adults: a systematic review and meta-analysis of 18 observational studies. PLoS Med 12(6):e1001835
Dodd PJ, Prendergast AJ, Beecroft C, Kampmann B, Seddon J (2017) The impact of HIV and antiretroviral therapy on TB risk in children: a systematic review and meta-analysis. Thorax 72(6):559–575
Tornee S, Kaewkungwal J, Fungladda W, Silachamroon U, Akarasewi P, Sunakorn P (2005) The association between environmental factors and tuberculosis infection among household contacts. Southeast Asian J Trop Med Public Health 36:221–224
Acknowledgements
We would like to acknowledge the Health Science Library, Kasturba Medical College, and Manipal Academy of Higher Education for the help in retrieving full-text articles.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal. No funds, grants, or other support were received to conduct this study.
Author information
Authors and Affiliations
Contributions
NS: Conceptualization, title, abstract screening, full-text article screening, data extraction, and manuscript writing. KC: Conceptualization, study design, and critical analysis. SBN: Study design, statistical analysis, interpretation of data, and manuscript editing. DH: Title and abstract screening, full-text article screening, data extraction, and manuscript editing.
Corresponding author
Ethics declarations
Ethics approval
This is a review article hence ethical approval is not required.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Peter de Winter.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Siddalingaiah, N., Chawla, K., Nagaraja, S.B. et al. Risk factors for the development of tuberculosis among the pediatric population: a systematic review and meta-analysis. Eur J Pediatr 182, 3007–3019 (2023). https://doi.org/10.1007/s00431-023-04988-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-023-04988-0