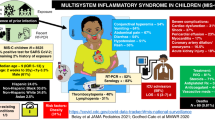
Abstract
Recently, a new pattern of multisystem inflammatory syndrome following an infection with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has emerged globally. The initial cases were described in the adult population followed by sporadic cases in the pediatric population also. By the end of 2020, similar reports were recognised in the neonatal age group. The purpose of this study was to systematically review clinical characteristics, laboratory parameters, treatment, and outcomes of neonates with multisystem inflammatory syndrome in neonates (MIS-N). A systematic review was conducted after registering with PROSPERO and electronic databases including MEDLINE, EMBASE, PubMed, SCOPUS, Google Scholar, and Web of Science were searched from January 1st 2020 till September 30th 2022. A total of 27 studies describing 104 neonates were analysed. The mean gestation age and birth weight was 35.9 ± 3.3 weeks and 2255.7 ± 783.7 g respectively. A large proportion (91.3%) of the reported cases belonged to the South-East Asian region. The median age of presentation was 2 days (range: 1–28 days) with cardiovascular system being the predominant system involved in 83.65% followed by respiratory (64.42%). Fever was noted in only 20.2%. Commonly elevated inflammatory markers were IL-6 in 86.7% and D-dimer in 81.1%. Echocardiographic evaluation suggested ventricular dysfunction in 35.8% and dilated coronary arteries in 28.3%. Evidence of SARS-CoV-2 antibodies (IgG or IgM) was seen in 95.9% neonates and evidence of maternal SARS-CoV-2 infection, either as history of COVID infection or positive antigen or antibody test, was noted in 100% of the cases. Early MIS-N was reported in 58 (55.8%) cases, late MIS-N in 28 (26.9%), and 18 cases (17.3%) did not report the timing of presentation. There was a statistically increased proportion of preterm infants (67.2%, p < 0.001), and a trend towards increased low birth weight infants, in the early MIS-N group when compared to the infants with late MIS-N. Fever (39.3%), central nervous system (50%), and gastrointestinal manifestations (57.1%) were significantly higher in the late MIS-N group (p = 0.03, 0.02, 0.01 respectively). The anti-inflammatory agents used for the treatment of MIS-N included steroids 80.8% which were given for a median of 10 (range 3–35) days and IVIg in 79.2% with a median of 2 (range 1–5) doses. The outcomes were available for 98 cases, of whom 8 (8.2%) died during treatment in hospital and 90 (91.8%) were successfully discharged home.
Conclusion: MIS-N has a predilection for late preterm males with predominant cardiovascular involvement. The diagnosis is challenging in neonatal period due to overlap with neonatal morbidities and a high risk of suspicion is warranted, especially in presence of supportive maternal and neonatal clinical history. The major limitation of the review was inclusion of case reports and case series, and highlights need of global registries for MIS-N.
What is Known: |
• A new pattern of multisystem inflammatory syndrome following SARS-CoV-2 infection has emerged in adult population with sporadic cases now being reported in neonates. |
What is New: |
• MIS-N is an emerging condition with a heterogeneous spectrum and has a predilection for late preterm male infants. Cardiovascular system is the predominant system involved followed by respiratory, however fever remains an uncommon presentation unlike other age-groups. There are two subtypes based on timing of presentation, with early MIS-N being reported more in preterm and low-birth weight infants. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronavirus disease (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has rapidly evolved over the last 3 years into a pandemic involving all age groups [1,2,3]. A new pattern of multisystem inflammatory syndrome following SARS-CoV-2 infection emerged in the adult population with sporadic pediatric cases also [4, 5]. By the end of 2020, similar clinical patterns were being recognised in the neonatal age group [6].
There remains a lack of uniformity in definitions of multisystem inflammatory syndrome in neonates (MIS-N), and most have been extrapolated from multisystem inflammatory syndrome in children (MIS-C). There is a wide clinical spectrum and management practices vary across the globe [7]. MIS-N appears to be linked to development of hyperinflammatory response following a perinatal or postnatal COVID-19 exposure [6, 7]. A recent systematic review suggested cardiovascular system being the most commonly involved organ, manifesting with cardiac dysfunction, arrhythmia, dilated coronaries, pericardial effusion, persistent pulmonary hypertension (PPHN), and intracardiac thrombus. Additionally, neonates exhibited a rise in the inflammatory markers such as c-reactive protein (CRP), ferritin, and procalcitonin [8]. Management strategies commonly used included anti-inflammatory therapies primarily intravenous immunoglobulin and corticosteroids, along with supportive interventions [9].
Two systematic reviews have analysed the clinical features and management strategies of published cases of MIS-N till October 2021 [8, 9]. As there has been an increase in reported cases since then, we aim to systematically review the demographic profile, clinical presentation, laboratory abnormalities, and treatment of MIS-N. We also describe the cases based on their timing of presentation, namely early and late MIS-N.
Methodology
We aimed to systematically review the reports on MIS-N to analyse the demographic characteristics, clinical manifestations, laboratory abnormalities, therapeutic interventions, and outcomes.
Protocol registration
The protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) with the registration number CRD42022321114.
Search strategy
Electronic databases including MEDLINE, EMBASE, PubMed, SCOPUS, Google Scholar, and Web of Science were searched. Additionally, pre-print repositories (BioRxiv and MedRxiv) and reference list of included studies were taken as additional sources. All publications in English language from January 1st 2020 till September 30th 2022 were reviewed. The combination of the following keywords was used as the search strategy for literature search in the various databases: Population [“neonates” OR”newborn” OR “neonatal” OR “infant” OR “preterm” OR “premature” OR “low birth weight” OR “very low birth weight” OR “small gestational age” OR “extremely low birth weight” OR “baby” OR “very preterm” OR “extremely preterm”] AND Virus [“sars-cov-2” OR “covid” OR “covid-19″ OR “coronavirus” OR “novel coronavirus” OR “severe acute respiratory syndrome coronavirus 2″] AND Condition [(“multisystem” OR “multisystemic” OR “multisystems”) AND (“inflammatories” OR “inflammatory”) AND (“syndrom” OR “syndromal” OR “syndromally” OR “syndrome” OR “syndromes” OR “syndromic” OR “syndroms”)]. Studies such as case reports, case series, observational (prospective or retrospective), brief communications, and letters to editors that incorporated details of neonates with MIS-N were included. Studies without access to full-text version, not in English language, or no patient data were excluded.
Study selection
The abstracts of the citations obtained from the initial broad search were read independently by reviewers DM, MG, PI, and SK to identify potentially eligible studies. Full-text articles of these studies were obtained and assessed for eligibility by reviewers DM, MG, PI, and SK independently, using the pre-defined eligibility criteria. Any differences in the opinions were resolved by a discussion amongst the group members and suggestions from the senior authors (RN, AA) to reach a consensus. To prevent data duplications multiple publications of the same study were excluded.
MIS-N definition
The World Health Organization (WHO) and Centres for Disease Control (CDC) have published MIS-C diagnostic criteria [10, 11]. However, the diagnostic criteria for MIS-N remain unclear and are evolving. Hence for this review, we adapted a diagnostic criterion considering the CDC and WHO recommendations for the definition of MIS-C, with modifications based upon current understanding of the immunological process of the disease in neonatal period along with the available literature on pathophysiologic mechanisms and clinical spectrum in neonates.
The neonates were categorised as MIS-N if they fulfilled the following definition:
-
1.
Onset of symptoms from birth till 28 days of life.
-
2.
Fever and/or with features suggestive of ≥ 2 organ system involvement (as fever is relatively uncommon in neonatal period).
-
3.
Laboratory evidence of elevated inflammatory markers (CRP, procalcitonin, ESR, LDH, D-dimer, IL-6, ferritin, fibrinogen)
-
4.
Evidence of SARS-CoV-2:
-
(a)
Neonate: Presence of SARS-CoV-2 antibodies (either IgG or IgM) in the neonate and a negative SARS-CoV-2 antigen test during the presentation to rule out an active COVID-19 infection.
-
(b)
Mother: During pregnancy or peripartum period any history of COVID-19 infection or positive SARS-CoV-2 antigen or SARS-CoV-2 antibodies serology positive (IgG or IgM).
-
(a)
-
5.
No alternative diagnosis given to explain the clinical features.
Based on the above definition, the neonates were classified as most likely, possible, and unlikely MIS-N. The neonates who fulfilled all the criteria for the definition were termed as “most likely MIS-N”. The neonates who presented with a high suspicion for MIS-N but could not fulfil all the criteria were termed as “possible MIS-N” if no alternative diagnosis was suggested, or “unlikely MIS-N” if an alternative diagnosis was available. All MIS-N cases were stratified as early or late onset depending upon their time of presentation. The neonates who presented within the first 3 days of life were termed as early, and those who presented beyond 3 days till 28 days of life were termed as late MIS-N.
Data extraction
Reviewers DM and MG independently extracted the data and discrepancies during the data extraction process were resolved by group discussion. The data collection included the name of first author, year, journal, country, study design, number of cases, gestational age (GA), birth weight (BW), sex distribution, mode of delivery, apgar scores, and clinical symptoms. Investigations included blood counts, inflammatory markers, cardiac biomarkers, coagulation profile, maternal and neonatal serology for SARS-CoV-2 infection, electrocardiogram, ultrasound findings, and echocardiographic evaluation. The treatment details recorded comprised ventilation type and duration, inotrope requirement and duration, steroids, antibiotics, aspirin, intravenous immunoglobulin, and other supportive and symptomatic therapies. The length of stay and outcomes were also noted. All data was recorded into MS Excel spreadsheet in a pre-defined manner.
Risk of bias assessment
The Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA) checklist was used to guide the data extraction process. The study quality was assessed by quality assessment tools published by JBI Critical Appraisal Checklist for case reports and case series.
Data analysis
Data analysis was performed using SPSS statistics software v24 (IBM corporation).
Results
A total of 1761 records were identified from MEDLINE, EMBASE, PubMed, SCOPUS, Google Scholar, and Web of Science from January 1st 2020 till September 30th 2022. After removing 985 duplicates, 776 studies left were screened on title and abstract. After screening, 740 articles were excluded due to various reasons such as editorial or review articles, studies in paediatric population, not relevant, non-English literature, and articles with no patient data. Thirty-six full-text articles were assessed for eligibility, of which 27 studies were included in qualitative synthesis. Nine studies were excluded as seven did not meet inclusion criteria and two were review articles (Fig. 1). We have summarised data from 27 studies, which reported 104 cases of MIS-N in Table 1. Of the studies included, 17 were case reports, 7 were case series, and 3 were cross-sectional observational studies. (12 – 38) There was a regional variation in the reports with the largest proportion of neonates with MIS-N being from India (93) followed by USA (3), UAE (2), and Iran (2). Other cases were described from Italy (1), Thailand (1), Bangladesh (1), and Netherlands (1).
Demographic features and clinical characteristics
In the 27 studies included, the majority were males (60.2%) with a mean GA of 35.9 ± 3.3 weeks and a mean BW of 2255.7 ± 783.7 g. Most were late preterm (34.9%) and term (44.7%), with 59.2% low birth weight and 34.4% small for gestational age, as depicted in Table 2. A large proportion (91.3%) of the reported cases belonged to the South-East Asian region. The median age of presentation was 2 days (range 1 to 28 days). Early MIS-N was reported in 58 (55.8%), late MIS-N in 28 (26.9%), and 18 cases (17.3%) did not report the age at presentation and were not classified.
Cardiovascular system was the predominant system involved in 83.65%, which included shock, arrhythmia and PPHN, echocardiographic abnormalities and raised cardiac biomarkers, followed by respiratory (64.42%). Common symptoms reported were respiratory distress (61.5%), shock (53.9%), lethargy (24%), and coagulopathy (24%). Fever, though an important diagnostic criterion for MIS-C, [10, 11] was noted in only 20.2% (Table 3).
Diagnosis and classification of MIS-N
Raised inflammatory markers were noted in majority of the cases, with the commonest being IL-6 in 13/15 (86.7%) and D-dimer in 77/95 (81.1%) (Table 4). Echocardiographic evaluation suggested ventricular dysfunction in 29/81 (35.8%) and dilated coronary arteries in 28/99 (28.3%) neonates. Evidence of SARS-CoV-2 antibodies (IgG or IgM) were seen in 93/97 (95.9%) neonates and evidence of maternal SARS-CoV-2 infection, either as history of COVID-19 or positive antigen or antibody test, was noted in 93/93 (100%) cases. Of the 22 cases which provided details on maternal vaccination, none of the mothers had received it.
Based on the definition, a diagnosis of “most likely MIS-N” was considered in 55/104 (52.9%), “possible MIS-N” in 41/104 (39.4%), and “unlikely MIS-N” in 8/104 (7.7%). The cases of unlikely MIS-N did not report any alternative diagnosis for the clinical presentation.
Management and outcomes
A majority of the neonates 69/104 (63.3%) received any form of ventilatory support, while 35/104 (33.7%) reported the need for inotropes. The anti-inflammatory agents used included steroids [84/104 (80.8%)] which were given for a median of 10 (6.5, 14) days and intravenous immunoglobulin (IVIg) [80/104 (79.2%)] with a median of 2 (2, 2) doses. The steroids used were methylprednisolone [32 (38.09%)], prednisolone [4 (4.76%)], combination of methylprednisolone and prednisolone [10 (11.90%)], dexamethasone [22 (26.19%)], hydrocortisone [4 (4.76%)], and it was not specified in 12 (14.29%) neonates.
Additional therapies included antibiotics, aspirin, heparin, and diuretics (Table 5). None of the studies reported the use of anakinra and tocilizumab. The median duration of hospital stay was 13 days, ranging from 2 to 86 days. Data regarding the outcomes was available for 98 cases, of whom 8 (8.2%) died during treatment in hospital and 90 (91.8%) were successfully discharged home.
Early and late MIS-N
There was a statistically increased proportion of preterm infants (67.2%, p < 0.001) in the early compared to late MIS-N. Fever (39.3%), central nervous system (50%), and gastrointestinal manifestations (57.1%) were significantly higher in the late MIS-N (p = 0.03, 0.02, 0.01 respectively), while haematological were more frequent in the early MIS-N (41.4%, p < 0.001). There was a significant increase in the requirement of respiratory support (72.4%, p = 0.001), steroids (84.5%, p = 0.03), and IVIg (86.2%, p < 0.001) in early MIS-N. Outcomes between the two groups showed no statistically significant difference (Table 6).
Discussion
MIS-N is an inflammatory syndrome in neonates with multisystem involvement, raised inflammatory markers, and serological evidence of antibodies against SARS-CoV-2. In our review of 27 studies, which included 104 cases, we found a male predominance compared to a nearly equal distribution in previous reviews. Preterm neonates comprised a slightly larger proportion, a finding similar to other reviews [7, 8]. This could be due to a significant overlap in the symptomatology of MIS-N with preterm morbidities.
The pathophysiology and exact natural history of MIS-N are not clearly understood. It could be due to direct damage by the antibodies or secondary to the inflammation following an altered immune response [6]. The role of epigenetics and contributions of specific genetic loci in the distinctive exaggerated inflammatory response seen in the neonates are possible, as illustrated in a large retrospective cohort of cases of MIS in children [39]. The interval between COVID-19 and onset of MIS-C has been reported to be around 27 days (interquartile range: 21–36 days) [7]. Hence, the cases reported early in the neonatal period are likely to be secondary to maternal infection acquired during pregnancy leading to a fetal inflammatory response following transfer of maternal antibodies, or due to transplacental transfer of virus to the foetus mounting an endogenous altered immune response. A third possibility is immune dysregulation secondary to postnatal acquired infection. In the current review, we found a positive serology for IgG SARS-CoV-2 antibodies in 95.9% and IgM in 12.4% neonates. In terms of maternal serology, most women were positive for IgG (98.2%) and IgM was positive in 38.9% and nearly half also had a positive antigen test. With up scaling of COVID-19 vaccination in pregnancy, the possibility of maternal IgG being positive following vaccination needs to be considered. Molloy et al. have described testing for the specific subtypes such as anti-nucleocapsid antibodies (post-infection) and anti-spike protein (post-infection or post-vaccination) antibodies as a probable solution to distinguish between the two [7]. In our review, only 22 cases (21.2%) reported details of maternal vaccination, of which none of the mothers had received vaccination. Hence, it is important to document the maternal vaccination to prevent a misdiagnosis of MIS-N.
The most commonly involved system was cardiovascular in 83.65% with clinical manifestations such as shock, arrhythmia and PPHN, echocardiographic abnormalities, and raised cardiac biomarkers. This was similar to a recent review, where nearly two-third of the neonates had cardiac dysfunction, arrhythmia, PPHN, or dilated coronaries. Interestingly, they reported fever in only 17 neonates (36.2%), which was comparable to our finding of fever in 20.2% [8]. This parallels the findings of De Rose et al. where incidence of fever was less in MIS-N (18.2%) than MIS-C (84.4%) [9]. Fever, though considered a mandatory criterion for diagnosis of MIS in children and adults, was not reported in the majority of the newborns, necessitating the need for a separate diagnostic criterion for MIS-N. A key concern while making a diagnosis of MIS-N in any neonate is to ensure exclusion of neonatal COVID-19 as a diagnosis, as there are many overlapping clinical features and thorough investigations should be done to distinguish between the two [40].
Majority of published cases have reported an array of inflammatory markers to establish the diagnosis in suspect cases [12, 13, 26, 29]. However, it seems important to identify the diagnostic accuracy of these markers and use those with a high sensitivity. Additional testing should be guided by the organ system involvement [41]. In light of predominant cardiovascular involvement, an echocardiography should be considered while treating MIS-N and clinicians managing these cases should utilise point of care ultrasound to guide treatment, especially of the critically ill neonates to evaluate the cardiac function and intravascular volume status [42].
The current management strategies for MIS-N have been extrapolated from the MIS-C, as there are no globally accepted management protocols for MIS-N. The recommended use of steroids and intravenous immunoglobulin (IVIg) has their basis in the immune-mediated hyperinflammatory pathogenesis [43,44,45]. In our review, we found 80% were treated with IVIg, and an equal proportion with steroids, nearly half of which were methylprednisolone. De Luca et al. in their review discussed successful management of MIS-N with use of steroids especially dexamethasone and hydrocortisone and IVIg [46]. A recent meta-analysis of 756 cases of MIS in the age group less than 21 years compared the treatment outcomes of IVIg alone vs IVIg and steroids. The combination therapy decreased the risk of persistence of fever. Interestingly, the combination did not significantly reduce the risk of left ventricular dysfunction and need for inotropic support, which are predominant morbidities of MIS-N. This highlights the need to re-evaluate the efficacy of use of individual agents and combination therapy in the neonatal population [44].
A striking pattern of antibiotics use in nearly 50% of the included neonates points towards a potential overuse of empiric antibiotics. The use of antibiotics needs to be guided by the principles of antibiotic stewardship and restricting use is essential to prevent drug resistance. The widespread use of antibiotics could be due to overlap with symptoms of sepsis along with raised CRP, procalcitonin, and IL-6 levels, which are also key biomarkers for diagnosing sepsis. We found nearly one-third of the neonates with MIS-N to have received aspirin and heparin. The use of aspirin has been recommended in all cases of mild MIS-C to improve platelet count and to prevent thrombosis in presence of coronary artery aneurysms. Additionally, heparin has been recommended for use in moderate-severe MIS-C cases with raised D-dimers (> 5 ULN) and low ejection fraction (< 35%) to prevent thrombosis [47]. The use of these agents in neonates should be considered only in indicated cases.
Interestingly, we found a nearly seven times higher likelihood of prematurity in early MIS-N as compared to late MIS-N. This draws parallel to an increased incidence of preterm births in pregnant women affected by COVID-19. Allotey et al. in their living systematic review and meta-analysis found a nearly threefold increase in preterm births; however, most were attributable to medical induction for maternal COVID-19 [48]. Hence, an in utero exposure to SARS-CoV-2 could possibly lead to premature birth and initiate an inflammatory cascade leading to early MIS-N. However, at this stage, it is difficult to determine whether it is truly a cause-effect relationship or merely an association. Fever was more in late MIS-N, with median onset on 11 days pointing to a similarity to pathophysiologic mechanisms of MIS-C. Ventilation, systemic steroids, and IVIg were found to be used more in early MIS-N with a lower mortality rate than late MIS-N reflecting a possible overlap with preterm morbidities in early MIS-N.
Mortality in MIS-N was 9–11% in previous reviews [8, 9]. However, current review with nearly double the number of cases found a slightly lower mortality rate (8.2%). These are significantly higher than mortality in MIS-C which range between 0.8 and 1.9% [7, 9]. The reasons include a probable reporting bias for the critical neonates with MIS-N in literature or a possible variation in immunological response in two age groups, which needs to be further elucidated. Moreover, there is likely to be underdiagnoses of mild cases, as clinicians often keep a low risk of suspicion and attribute to prematurity-related complications. Partnering of neonatologists and paediatricians who have managed more cases of MIS, while making the diagnosis of MIS-N in suspected neonates who present with unusual clinical course or sudden deterioration, especially in areas of high viral circulation during pregnancy can possibly prevent over diagnosis and improve neonatal outcomes of MIS-N [49].
The strengths of the present review are inclusion of a larger number of cases and relevance considering increasing global burden of MIS-N (Supplementary Fig. 1). Along with a detailed analysis, we have additionally distinguished between the early and late MIS-N, with the latter mirroring the MIS-C and hence highlighting a need for delineation of separate guidelines for the two. The major limitation is inclusion of case reports and case series, as they form the current predominant scientific literature for MIS-N. This limits the level of evidence to low. Larger global data registries are required to strengthen the understanding of the natural history and improve the diagnostic criteria and management. A long-term follow-up of MIS-N is mandated to determine the delayed effects.
Conclusion
MIS-N is an emerging condition in neonatal population with a predilection for late preterm males and commonly leading to cardiovascular involvement. The diagnosis is challenging due to overlap with neonatal morbidities and a high risk of suspicion is warranted, especially in presence of supportive maternal and neonatal clinical history. There remains a strong need to develop guidelines to optimise clinical practice of diagnosing and treating MIS-N globally.
Availability of data and material
N/A.
Code availability
N/A.
Abbreviations
- BW:
-
Birth weight
- CDC:
-
Centres for Disease Control
- COVID-19:
-
Coronavirus disease
- CRP:
-
C-reactive protein
- GA:
-
Gestation age
- IVIg:
-
Intravenous immunoglobulin
- MIS-C:
-
Multisystem inflammatory syndrome in children
- MIS-N:
-
Multisystem inflammatory syndrome in neonates
- PPHN:
-
Persistent pulmonary hypertension of newborn
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus-2
- WHO:
-
World Health Organization
References
WHO (2020) COVID-19 - China. Available from: https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-causechina/en/
Worldometer. COVID-19 coronavirus pandemic. Available from: https://www.worldometers.info/coronavirus. Accessed 15 Dec 2022
Di Toro F, Gjoka M, Di Lorenzo G, De Santo D, De Seta F, Maso G et al (2021) Impact of COVID-19 on maternal and neonatal outcomes: a systematic review and meta-analysis. Clin Microbiol Infect 27(1):36–46
Kunal S, Ish P, Sakthivel P, Malhotra N, Gupta K (2022) The emerging threat of multisystem inflammatory syndrome in adults (MIS-A) in COVID-19: a systematic review. Heart Lung 54:7–18
Morris SB, Schwartz NG, Patel P, Abbo L, Beauchamps L, Balan S et al (2020) Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection — United Kingdom and United States, March–August 2020. MMWR Morb Mortal Wkly Rep 69(40):1450–1456
Nakra N, Blumberg D, Herrera-Guerra A, Lakshminrusimha S (2020) Multi-system inflammatory syndrome in children (MIS-C) following SARS-CoV-2 infection: review of clinical presentation: Hypothetical pathogenesis, and proposed management. Children 7(7):69
Molloy EJ, Nakra N, Gale C, Dimitriades VR, Lakshminrusimha S (2022) Multisystem inflammatory syndrome in children (MIS-C) and neonates (MIS-N) associated with COVID-19: optimizing definition and management. Pediatr Res
Shaiba LA, More K, Hadid A, Almaghrabi R, Al Marri M, Alnamnakani M et al (2022) Multisystemic inflammatory syndrome in neonates: a systematic review. Neonatology 119(4):405–417
De Rose DU, Pugnaloni F, Calì M, Ronci S, Caoci S, Maddaloni C et al (2022) Multisystem inflammatory syndrome in neonates born to mothers with SARS-CoV-2 infection (MIS-N) and in neonates and infants younger than 6 months with acquired COVID-19 (MIS-C): a systematic review. Viruses 14(4):750
WHO (2020) Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19. Available from: https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19
CDC. Multisystem inflammatory syndrome (MIS). Available from: https://www.cdc.gov/mis/mis-c/hcp/index.html. Accessed 1 May 2022
More K, Aiyer S, Goti A, Parikh M, Sheikh S, Patel G et al (2022) Multisystem inflammatory syndrome in neonates (MIS-N) associated with SARS-CoV2 infection: a case series. Eur J Pediatr 181(5):1883–1898
Pawar R, Gavade V, Patil N, Mali V, Girwalkar A, Tarkasband V et al (2021) Neonatal multisystem inflammatory syndrome (MIS-N) associated with prenatal maternal SARS-CoV-2: a case series. Children 8(7):572
Agrawal G, Wazir S, Arora A, Sethi SK (2021) Multisystem inflammatory syndrome in a neonate masquerading as surgical abdomen. BMJ Case Rep 14(10):e246579
Malek A, Khadga M, Zahid MN, Mojib S, Debnath R, Khan S et al (2022) Multisystem inflammatory syndrome of a neonate from a COVID-19-infected mother: a case report. Cureus
Divekar AA, Patamasucon P, Benjamin JS (2021) Presumptive neonatal multisystem inflammatory syndrome in children associated with coronavirus disease 2019. Am J Perinatol 38(6):632–636
Kappanayil M, Balan S, Alawani S, Mohanty S, Leeladharan SP, Gangadharan S et al (2021) Multisystem inflammatory syndrome in a neonate, temporally associated with prenatal exposure to SARS-CoV-2: a case report. Lancet Child Adolesc Health 5(4):304–308
Hashiq N, Nigade R, Amith K, Kurane AB (2021) Multisystem inflammatory syndrome in a neonate, temporally associated with prenatal exposure to SARS-COV-2: Case series. I J Paediatr Geriatrics 4(1):148–150
Shah SA (2022) COVID-19 related potential multisystem inflammatory syndrome (MIS-N). Cytokines. J Contemp Med Pract 4(3).
Shanker V, Chaudhary M, Shanker P (2021) Antenatal SARS-COV-2 exposure leading to multisystem inflammatory syndrome (MIS-N) presenting with neonatal encephalopathy. J Clin Pediatr Neonatol 1(3):41–44
Arun S, Cherian TG, Philip C (2022) Multisystem inflammatory syndrome in a neonate with severe hemophilia-a diagnostic challenge in COVID times: a case report. BMC Pediatr 22(1):1–4
Bakhle A, Sreekumar K, Baracho B, Sardessai S, Silveira MP (2022) Cavitary lung lesions in a neonate: potential manifestation of COVID-19 related multisystem inflammatory syndrome. Pediatr Pulmonol 57(1):311–314
Shaiba LA, Hadid A, Altirkawi KA, Bakheet HM, Alherz AM, Hussain SA et al (2021) Case report: neonatal multi-system inflammatory syndrome associated with SARS-CoV-2 exposure in two cases from Saudi Arabia. Front Pediatr 242
Voddapelli SK, Murki S, Rao VP (2022) Neonatal multisystem inflammatory syndrome (MIS-N) presenting as necrotizing enterocolitis and cardiac dysfunction. Indian Pediatr 59(6):502
Amonkar PS, Gavhane JB, Kharche SN, Kadam SS, Bhusare DB (2021) Aortic thrombosis in a neonate with COVID-19-related fetal inflammatory response syndrome requiring amputation of the leg: a case report. Paediatr Int Child Health 41(3):211–216
Balleda L, Pasupula S, Kolla S, Thimmapuram CR (2022) Clinical profile, laboratory parameters, management and outcomes of newborns with multisystem inflammatory syndrome (mis-n) due to transplacental transfer of SARS-CoV 2 antibodies: a study from a tertiary care institute. J Clin Neonatol 11(2):65
Schoenmakers S, Snijder P, Verdijk RM, Kuiken T, Kamphuis SS, Koopman LP et al (2021) Severe acute respiratory syndrome coronavirus 2 placental infection and inflammation leading to fetal distress and neonatal multi-organ failure in an asymptomatic woman. J Pediatr Infect Dis 10(5):556–561
Shinde MD, Khamkar AM, Pote PD, Suryawanshi PB (2022) Fetal inflammatory response syndrome associated with SARS-CoV-2 exposure in utero. Pediatric Oncall 19(4)
Chaudhuri M, Tomar M, Gaonkar S, Rastogi A, Shenoi A (2022) Pilot study analyzing combination of point‑of‑care echocardiography and clinical correlation in unveiling cryptic multi‑inflammatory syndrome in neonates during coronavirus disease 2019 pandemic. Journal of the Indian Academy of Echocardiography and Cardiovascular Imaging XX(XX):2
Saeedi M, Mirnia K, Sangsari R, Makan ZJ, Ziaee V (2021) Neonatal multi-system inflammatory syndrome associated with Covid-19 exposure in two cases from Iran
Gupta P, B SA, Tamatam PR, Dhulipudi B, Vardhelli V, Deshabhotla S et al (2022) Neonatal multisystem inflammatory syndrome (MIS-N) associated with maternal SARS-CoV-2 exposure. Indian J Pediatr
Diwakar K, Gupta B, Uddin M, Sharma A, Jhajra S (2022) Multisystem inflammatory syndrome with persistent neutropenia in neonate exposed to SARS-CoV-2 virus: a case report and review of literature. Journal of Neonatal-Perinatal Medicine 15(2):373–377
Costa S, Delogu AB, Bottoni A, Purcaro V, D'Andrea V, Paladini A et al (2022) COVID-19-associated multisystem inflammatory syndrome in a neonate with atypical coronary artery involvement. Am J Perinatol
Tambekar HJ, Ashtekar SD, Mirgunde SP, Khot S, Mane S (2022) Neonatal multisystem inflammatory syndrome associated with prenatal maternal SARS-CoV-2 exposure: a case series. International Journal of Contemporary Pediatrics 9(4):381
Borkotoky RK, Barua PB, Paul SP, Heaton PA (2021) COVID-19-related potential multisystem inflammatory syndrome in childhood in a neonate presenting as persistent pulmonary hypertension of the newborn. Pediatr Infect Dis J 40(4):e162–e164
McCarty KL, Tucker M, Lee G, Pandey V (2021) Fetal inflammatory response syndrome associated with maternal SARS-CoV-2 infection. Pediatrics 147(4)
Sojisirikul N, Lapphra K, Ngerncham S, Charuvanij S, Durongpisitkul K, Curlin ME et al (2022) Neonatal multisystem inflammatory syndrome (MIS-N): the first case report in Thailand. COVID 2(9):1265–1269
Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J et al (2020) Multisystem inflammatory syndrome in children in New York State. N Engl J Med 383(4):347–358
Davalos V, García-Prieto CA, Ferrer G, Aguilera-Albesa S, Valencia-Ramos J, Rodríguez-Palmero A, Ruiz M, Planas-Serra L, Jordan I, Alegría I, Flores-Perez P (2022) Epigenetic profiling linked to multisystem inflammatory syndrome in children (MIS-C): a multicenter, retrospective study. EClinicalMedicine 1(50):101515
Raschetti R, Vivanti AJ, Vauloup-Fellous C et al (2020) Synthesis and systematic review of reported neonatal SARS-CoV-2 infections. Nat Commun 5164(11)
Broad J, Forman J, Brighouse J, Sobande A, McIntosh A, Watterson C et al (2021) Post-COVID-19 paediatric inflammatory multisystem syndrome: association of ethnicity, key worker and socioeconomic status with risk and severity. Arch Dis Child 106(12):1218–1225
Singh Y, Tissot C, Fraga MV, Yousef N, Cortes RG, Lopez J, Sanchez-de-Toledo J, Brierley J, Colunga JM, Raffaj D, Da Cruz E, Durand P, Kenderessy P, Lang HJ, Nishisaki A, Kneyber MC, Tissieres P, Conlon TW, De Luca D (2020) International evidence-based guidelines on Point of Care Ultrasound (POCUS) for critically ill neonates and children issued by the POCUS Working Group of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC). Crit Care 24(1):65
The Lancet COVID-19 Commission: preparing for COVID-19 part III: planning, protocols, and policy guidelines for paediatrics 2021. Available from: https://static1.squarespace.com/static/5ef3652ab722df11fcb2ba5d/t/60c503cf7f8fc60da0abf98f/1623524303975/Paediatric+expert+panel+paper.pdf. Accessed 20 Sept 2022.
Rauniyar R, Mishra A, Kharel S, Giri S, Rauniyar R, Yadav S et al (2022) IVIG plus glucocorticoids versus IVIG alone in multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19: a systematic review and meta-analysis. Can J Infect Dis Med Microbiol 2022:1–7
Tagarro A, Domínguez-Rodríguez S, Mesa JM, Epalza C, Grasa C, Iglesias-Bouzas MI, Fernández-Cooke E, Calvo C, Villaverde S, Torres-Fernández D, Méndez-Echevarria A (2023) Treatments for multi-system inflammatory syndrome in children—discharge, fever, and second-line therapies. Eur J Pediatr 182(1):461–466
De Luca D, Vauloup-Fellous C, Benachi A, Masturzo B, Manzoni P, Vivanti A (2022) The essentials about neonatal severe acute respiratory syndrome coronavirus 2 infection and coronavirus disease: a narrative review. Am J Perinatol 10
Henderson LA, Canna SW, Friedman KG, Gorelik M, Lapidus SK, Bassiri H et al (2022) American College of Rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS–CoV‐2 and hyperinflammation in pediatric COVID‐19: Version 3. Arthritis Rheumatol 74(4)
Allotey J, Fernandez S, Bonet M, Stallings E, Yap M, Kew T, Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease, et al (2019) in pregnancy: living systematic review and meta-analysis. BMJ 2020:m3320
Buonsenso D (2022) Partnerships in the comprehensive management of multisystem inflammatory syndrome in neonates (MIS-N): translating 2 years of pediatric practice to the neonatal wards. Am J Perinatol 21
Author information
Authors and Affiliations
Contributions
DM and MG conceptualised and designed the study, conducted literature search, collected data, drafted the initial manuscript, and reviewed and revised the manuscript. AH, RN, PI, and SK conceptualised and designed the study, coordinated and supervised data collection, and critically reviewed and revised the manuscript for important intellectual content and finalised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethical approval
The study was conducted after registering with PROSPERO.
Consent to participate
N/A.
Consent for publication
N/A.
Conflicts of interest
The authors declare no competing interests.
Additional information
Communicated by Daniele De Luca.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mascarenhas, D., Goyal, M., Haribalakrishna, A. et al. Multisystem inflammatory syndrome in neonates (MIS-N): a systematic review. Eur J Pediatr 182, 2283–2298 (2023). https://doi.org/10.1007/s00431-023-04906-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-023-04906-4