Abstract
Multisystem inflammatory syndrome in children (MIS-C) is a rare but severe disease temporarily related to SARS-CoV-2. We aimed to describe the epidemiological, clinical, and laboratory findings of all MIS-C cases diagnosed in children < 18 years old in Catalonia (Spain) to study their trend throughout the pandemic. This was a multicenter ambispective observational cohort study (April 2020–April 2022). Data were obtained from the COVID-19 Catalan surveillance system and from all hospitals in Catalonia. We analyzed MIS-C cases regarding SARS-CoV-2 variants for demographics, symptoms, severity, monthly MIS-C incidence, ratio between MIS-C and accumulated COVID-19 cases, and associated rate ratios (RR). Among 555,848 SARS-CoV-2 infections, 152 children were diagnosed with MIS-C. The monthly MIS-C incidence was 4.1 (95% CI: 3.4–4.8) per 1,000,000 people, and 273 (95% CI: 230–316) per 1,000,000 SARS-CoV-2 infections (i.e., one case per 3,700 SARS-CoV-2 infections). During the Omicron period, the MIS-C RR was 8.2 (95% CI: 5.7–11.7) per 1,000,000 SARS-CoV-2 infections, which was significantly lower (p < 0.001) than that for previous variant periods in all age groups. The median [IQR] age of MIS-C was 8 [4–11] years, 62.5% male, and 80.2% without comorbidities. Common symptoms were gastrointestinal findings (88.2%) and fever > 39 °C (81.6%); nearly 40% had an abnormal echocardiography, and 7% had coronary aneurysm. Clinical manifestations and laboratory data were not different throughout the variant periods (p > 0.05).
Conclusion: The RR between MIS-C cases and SARS-CoV-2 infections was significantly lower in the Omicron period for all age groups, including those not vaccinated, suggesting that the variant could be the main factor for this shift in the MISC trend. Regardless of variant type, the patients had similar phenotypes and severity throughout the pandemic.
What is Known: |
• Before our study, only two publications investigated the incidence of MIS-C regarding SARS-CoV-2 variants in Europe, one from Southeast England and another from Denmark. |
What is New: |
• To our knowledge, this is the first study investigating MIS-C incidence in Southern Europe, with the ability to recruit all MIS-C cases in a determined area and analyze the rate ratio for MIS-C among SARS-CoV-2 infections throughout variant periods. |
• We found a lower rate ratio of MISC/infections with SARS-CoV-2 in the Omicron period for all age groups, including those not eligible for vaccination, suggesting that the variant could be the main factor for this shift in the MISC trend. |
Similar content being viewed by others
Introduction
In December 2019, the new SARS-CoV-2 emerged in China. To date, severe COVID-19 in children remains infrequent; most of them are asymptomatic or exhibit mild symptoms during the acute phase of the infection, in contrast to adults [1].
However, SARS-CoV-2 infection has been associated with MIS-C, which develops approximately 2 to 6 weeks after mild or asymptomatic infection, although some cases have also been described during the acute infection [2]. The case definition of MIS-C has been established by different institutions such as the WHO (May 15, 2020) [3], the US CDC (May 14, 2020) [4], and the Royal College of Paediatrics and Child Health in the UK (May 1, 2020) [5]. The clinical features of this syndrome have been widely defined and described in several report series, overlapping features of KD, toxic shock syndrome, bacterial sepsis, and macrophage-activation syndrome [6,7,8,9]. Recently, it has also been described in some patients after vaccination with mRNA vaccines [10].
Even so, differential characteristics have been observed in MIS-C cases, leading more frequently to shock due to myocardial dysfunction [9]. Additionally, children require timely recognition and prompt start of immunosuppressive treatment, such as IVIG, high dose of methylprednisolone and/or IL-1 inhibitor drugs in refractory cases.
The MIS-C incidence among all SARS-CoV-2 infections has been difficult to determine throughout the pandemic, given the high rate of asymptomatic infection in the pediatric population. In Catalonia (Spain), systematic screening in the schools for all the classmates of COVID-19 cases was maintained from September 2020, after the first pandemic wave [11], to January 2022. This protocol enabled the detection of most of the asymptomatic pediatric cases that otherwise would not have been diagnosed [12]. However, whether this risk for MIS-C is sustained with new SARS-CoV-2 variants and vaccination remains unknown.
In this study, we analyzed a large cohort of all consecutive MIS-C cases diagnosed and treated in Catalonia, Spain, since the onset of the pandemic, thanks to the effort of the multidisciplinary COPEDI-CAT network team. We aimed not only to estimate the main MIS-C epidemiological characteristics, but also to describe the clinical and laboratory findings, the type and responses to the treatment administered and the final outcomes of the disease across the different SARS-CoV-2 variant epidemic waves.
Materials and methods
Study design and study population
This is a multicenter ambispective observational cohort study of patients aged < 18 years old in Catalonia (Spain) diagnosed with MIS-C during the period from April 20, 2020, to April 30, 2022. The WHO definition criteria were used for the inclusion of the cases [3]. Children were excluded from this study, according with the MIS-C definition, if the pediatrician attending the case had an alternate probable etiology of the disease such as bacterial infection, toxic shock syndrome, KD, HLH or MAS, rickettsia, viral syndrome, lupus, and vasculitis. They also were excluded if information regarding the MIS-C diagnosis was missing or if another diagnosis was made after hospitalization.
Data sources and data analysis
Epidemiological and clinical data were obtained from the COVID-19 Catalan surveillance system and medical records of all hospitals in Catalonia. Demographic, laboratory and clinical data were collected from hospital patient history and uploaded to the REDCap® database. The phenotype, severity, and incidence of MIS-C were compared in different periods regarding predominant variants (> 50% of random samples) in our location: the Wuhan type April 2020–February 2021 [13], the Alpha February 2021–June 2021 [14], the Delta June 2021–December 2021 [15], and the Omicron December 2021–April 2022. In addition, we used the six epidemiological periods (based on successive waves, independent of variants) defined by the Spanish Public Health authorities to study the evolution of MIS-C cases regarding COVID-19 incidence [16]. MIS-C was divided by age groups regarding vaccination recommendations (0–5 years, 6–11 years, 12–17 years). Vaccination status was verified through searches of national immunization information systems, including children’s vaccine cards. Patients were considered unvaccinated if they had received no doses, partially vaccinated if they had received one dose, and completely vaccinated if they had received two doses (the second dose > 14 days before the MIS-C episode). They were considered immunized if they received one dose with a documented SARS-CoV-2 infection and had a documented SARS-CoV-2 infection.
MIS-C incidence per SARS-CoV-2 infection
Monthly MIS-C incidence rate per 1,000,000 people in all variant-associated periods was estimated by dividing the number of the monthly reported MIS-C cases by the number of < 18 years old people. In addition, we also calculated the rate between MIS-C cases and COVID-19 cases (from − 30 days w.r.t. the MIS-C interval [2]) in each epidemiological wave and variant-associated period. The public health authorities in Catalonia launched a protocol in September 2020 for testing all the exposed children within bubble groups in the schools (contacts of the initial case at school) [11, 12], which was maintained until the end of January 2022, when Omicron was the prevalent variant. All the health districts or regions in Catalonia followed the same protocol, the diagnostic effort in the pediatric population was maintained everywhere throughout the pandemic. Schools remained open between September 2020 and the end of the study period.
Statistical analyses
MIS-C cases during the pandemic were analyzed according to the different SARS-CoV-2 predominant variants. Demographics, clinical findings, and phenotypes concerning organ involvement, hypotension, and need for admission in the PICU, laboratory characteristics, and specific treatment were included in the predominant variant-related analysis. Statistical analyses were performed with R (v4.2.0) and Python (v3.7.13). Quantitative variables are presented as the median and IQR, and categorical variables are presented as the number of children for each category and the corresponding percentage. Fisher’s exact tests were used to compare categorical variables between groups; z-tests were performed for analyzing MIS-C monthly incidence and rate. Kruskal–Wallis test was used to compare groups for quantitative variables. Benjamini and Hochberg correction was used for a more conservative measure of significance for multiple comparisons in results (excluding MIS-C rate and monthly incidence). RR and its 95% CI were computed to compare rates of MIS-C and SARS-CoV-2 infections between variant periods. For all tests, the results were considered significant if p < 0.05. The Alpha type period was excluded from most of the analyses due to the low data volume, and the Wuhan period, prior to 1st July 2020, was excluded from the MIS-C rate analyses because of the low testing capacity at that time.
Results
Epidemiology data
One hundred fifty-two children were recruited, corresponding to the whole number of MIS-C cases during this study period. Regarding the SARS-CoV-2 variant periods, 73, 5, 44, and 30 cases were diagnosed for Wuhan, Alpha, Delta, and Omicron periods, respectively (Table 1).
Demographic data
The median (IQR) age was 8 (4-11) years; 62.5% were male, and 80.2% did not have any comorbidities. COVID-19 was previously diagnosed in 45.4% (69/152) of patients and was mostly asymptomatic. Nearly 60% (91/152) were Caucasian, 15% (23/152) were Hispanic, and 8.6% (13/152) were Black African, among other ethnicities (Table 2). None of them died.
Laboratory evidence of SARS-CoV-2 data
In Fig. 1, we describe the cohort of children regarding the results for serological (SARS-CoV-2 positive IgG) and microbiological tests for SARS-CoV-2, and vaccination against COVID-19. The median [IQR] time between the SARS-CoV-2 infection and the MIS-C diagnosis was 5.1 [4.2–6.3] weeks, with a range of 0.4–14.6 weeks. Overall, 131 (86.2%) tested positive by SARS-CoV-2 IgG, 33 (21.7%) tested positive by SARS-CoV-2 RT-PCR, and 5 (3.3%) tested positive by rapid antigen testing (Fig. 1).
Clinical presentation at admission
Fever at admission was the main clinical finding (150/152, 98.7%), with a median (IQR) of 4 (3-6) days before the MIS-C diagnosis, and 81.6% had > 39 °C. Two children had no fever at admission: a 6-year-old child testing positive for SARS-CoV-2 IgG serology at admission with a positive PCR for SARS-CoV-2 five weeks earlier, with a hyperacute ischemic infarction in the deep vascular territory of the left medial cerebral artery; and a 6-month-old child diagnosed with myocarditis (ejection fraction < 30%) testing positive for SARS-CoV-2 PCR at admission.
Gastrointestinal symptoms (88.2%), and rash or skin lesions (59.2%) were the most common clinical findings; 81 (53.3%) had signs of shock (defined as reduced awareness, hypotension, hyperventilation and fluid administration), and 68 (44.7%) were admitted to the PICU. Regardless of the SARS-CoV-2 variant, patients had similar severity profile (Table 2).
Laboratory and echocardiography findings
Thrombocytopenia (< 150 × 109/L) was observed in 92 (60.5%) patients, and lymphopenia (< 1.0 × 109/L) in 75 (49.3%) cases. Acute phase reactants were markedly elevated for all the patients, as well as evidence of coagulopathy (Table 2). ALT was normal in 91 children (59.9%) and elevated > 250 UI/L in only four children. 81 children (53.3%) showed clinical features of cardiovascular involvement. Pro-BNP was higher than 2000 pg/mL in 65 (42.8%) patients. Echocardiography was abnormal in 60 children (39.5%), 22 with ventricular dysfunction (14.5%), and 11 with coronary aneurysm (7.2%).
Treatment and early treatment response
Specific treatments administered to the MIS-C cases are described in Table 3. Most of them (147/152; 96.7%) received immune modulating treatment (5.5% only IGIV, 12.3% only steroids and 82.2% combination of IGIV and steroids). Steroids were administered initially at 2 mg/kg, 1 mg/kg and mega-doses in 89/138 (64.5%), 28/138 (20.3%), and 19/138 (13.8%) children, respectively. Five patients did not receive specific treatment as they were diagnosed at the beginning of the pandemic when protocols were not well defined. We did not find any significant differences in the treatment administered across the SARS-CoV-2 variant periods. Anakinra (anti-IL-1) was administered to only one patient with refractory MIS-C.
Association between vaccination and MIS-C
Among the 152 children with MIS-C, only 11 (7.2%) were vaccinated against SARS-CoV-2 before the diagnosis: 5 with a single dose and 6 fully vaccinated; 8/11 (72.7%) received the vaccine < 12 weeks before the onset of MIS-C [Supplementary Information (SI)]. Forty-nine (32.2%) patients were vaccinated after the diagnosis of MIS-C taking into account the Catalan public health authorities recommendations.
Estimated monthly MIS-C incidence per 1,000,000 people
There are 1,361,915 children (< 18 years) in Catalonia. Since the beginning of the pandemic until 30th April 2022, the mean monthly MIS-C incidence was 4.1 cases per 1,000,000 people (95% CI: 3.5–4.7), significantly lower in females throughout the study period (p = 0.006). Additionally, the incidence was significantly higher for children aged 0–4 and 5–11 years (5.0 and 4.9 per 1,000,000 people, respectively) than for adolescents aged 12–17 years (2.6 per 1,000,000) (Fig. 2 and Table 1).
MIS-C rate per 1,000,000 SARS-CoV-2 infections
During the study period 555,848 children were diagnosed with SARS-CoV-2 infection. The MIS-C rate per 1,000,000 SARS-CoV-2 infections was 273 (95% CI: 230–316) (i.e., one case per 3,700 SARS-CoV-2 infections). The MIS-C rate by age groups was: 456 (95% CI: 336–619) among children aged 0–4 years, 305 (95% CI: 243–383) among 5–11 years, and 166 (95% CI: 120–228) among 12–17 years (Table 1). There were significant differences between children aged 0–4 and 5–11 (p = 0.048), 0–4 and 12–17 (p < 0.001), and 5–11 and 12–17 (p = 0.003) (Table 1).
There was not a statistically significant difference between males and females, except for the Omicron period where a predominance on male was documented (p = 0.02) (Table 1).
Comparison of the incidence and rates of MIS-C between different variants and waves
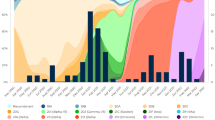
No significant differences in monthly MIS-C incidence per 1,000,000 people were observed between the variant periods (p = 0.68). There was a significantly lower RR of MIS-C in the Omicron period compared to the Wuhan or Delta periods, for all the age groups and sexes (Table 1). The 95% CI confirms the significant differences of the sixth wave, mostly corresponding to the Omicron period, compared to the second, third and fifth waves. The first and fourth pandemic waves were not included in this analysis due to the lack of diagnostic tests and very low MIS-C incidence, respectively. Figure 3 a and b show the MIS-C rate throughout the different waves, by sex and age group.
MIS-C rate (× 10.6) in log scale according to the six epidemiological periods for COVID-19 pandemic. A shows the distribution of MIS-C by epidemiologic period and gender (whole population (all), female, male) and B shows the distribution of MIS-C by epidemiologic period and age-groups (whole population (all), 0–4, 5–11, and 12–17 years)
Discussion
To our knowledge, this is the first study investigating MIS-C incidence in Southern Europe, with the ability to recruit all the MIS-C cases in a determined area, and analyze the RR for MIS-C among SARS-CoV-2 infections throughout variant periods. We found a lower RR of MISC/infections with SARS-CoV-2 in the Omicron period for all age groups, including those not eligible for vaccination.
Overall, we diagnosed 152 MIS-C cases among 1,361,915 children in Catalonia, with a monthly MIS-C incidence of 4.1 cases per 1,000,000 people (95% CI: 3.5–4.7). Incidence was significantly lower in females and adolescents (12–17 years old), even before the initiation of vaccination in this age group, but no differences were observed across the SARS-CoV-2 variant periods. This data confirms that MIS-C is a very rare complication of SARS-CoV-2 infection, even though our incidence is higher than the one reported in Liguria (Italy) [17], but like the US [18] and Sweden [19].
We analyzed the incidence rate for MIS-C among the previously diagnosed SARS-CoV-2 infections. Although this variable is affected by the infection diagnostic capacity, the sustained surveillance protocols in the schools, with systematic screening of all classmates each time a positive case was detected, guaranteed a sustained diagnostic effort among the pediatric population between September 2020 and January 2022 [11, 12]. Omicron showed the significantly lowest MIS-C incidence rate compared to the high number of infected children in all age groups in that period. Moreover, the diagnostic effort was lower after the end of January 2022 because of the relaxation in the surveillance and quarantine measures in the schools [Supplementary Information (SI)], as seen with the increase in the positivity rate, so the MIS-C rate during the Omicron period is likely overestimated because the denominator might be much higher than the reported. This would entail even higher RR in pre-Omicron variant periods than the ones we estimated (Table 1). Possible hypotheses to explain this shift include the Omicron variant itself due to a better viral clearance secondary to stronger immune responses during acute SARS-CoV-2 infection and, subsequently, less persistence in sanctuaries [20]. Other potential explanations include the high-prevalence of SARS-CoV-2 infection among the general population after two years of pandemic, or the high percentage of people who were fully vaccinated against SARS-CoV-2, among other unknown factors. Regarding the variant factor, our results are in concordance with a South African study performed during the Omicron period [21], and with another study conducted in South East England [22]. However, a recent report from the CDC found that MIS-C cases continued to be an important complication of SARS-CoV-2 infection during the last variant-periods [23].
Our current understanding of the immunopathology of SARS-CoV-2 and MIS-C is growing, but still limited. COVID-19 mRNA vaccine immunogenicity and effectiveness are well established in adolescents, and the preventive effect of vaccination on the incidence of MIS-C has been already described [24, 25]. A recent population-based cohort study in Denmark demonstrated the effectiveness of COVID-19 vaccination in preventing this complication during the Delta and Omicron period [26]. On the other hand, very few MIS-C cases have been reported following COVID-19 vaccination [27]. Therefore, the optimal SARS-CoV-2 vaccine strategy for patients with a history of MIS-C remains unclear, as demonstrated in a recent survey conducted by pediatricians from different countries [28]. In our study, only eight patients had been vaccinated against COVID-19 in the previous 12 weeks before the MIS-C episode compared to 102 unvaccinated MIS-C cases that were eligible for vaccination due to their age. None of the vaccinated differed from the unvaccinated regarding the associated risk factors for severity. On the other hand, our study includes 49 children vaccinated against COVID-19, 37 with a single dose and 12 fully vaccinated, after the MIS-C episode. None of them had a relapse of MIS-C after vaccination.
In our cohort of MIS-C cases, Black African were 8.6% in contrast to the proportion of these people living in Catalonia (1.3%) [29]. This is in concordance with previous reports from Europe [30] and the US [18], suggesting an increased susceptibility of this population to present MIS-C after COVID-19.
Clinical manifestations and laboratory findings in our cohort were similar to those previously reported. We did not find any significant differences across the SARS-CoV-2 variants (Table 2 and SI), in contrast to a study conducted in Israel where a decrease of severity of MISC-S was observed during the Omicron period [31].
Our cohort was mainly treated with a combination of IVIG and steroids, with no significant difference in the treatment of MIS-C throughout the variant periods. None of the patients in our cohort died during the acute phase or the follow-up.
This study has some limitations. Firstly, we conducted an ambispective study, so the first MIS-C cases were recruited retrospectively and, consequently, some cases could have misdiagnosed as KD in the first months. Accordingly, we did not include the pre-July 2020 cases in the MIS-C rate analysis. Secondly, a cautious interpretation of the results during the Alpha wave is needed due the small numbers of patients reported in this period; therefore, this period was neither included in the same analysis. Thirdly, we were unable to determine the exact time between SARS-CoV-2 infection and MIS-C diagnosis in more than 50% of the cases (Table 2), and the information regarding the sequencing of the variants of those MIS-C cases with a confirmed previous SARS-CoV-2 infection. However, we consider that this limitation does not affect the main outcomes of our study, since it was taken into account in our analysis. We defined the rate in each period instead of the rate per variant, using a 4-week period between the infection and the MIS-C case. All variants achieved prevalence rates higher than 90%, which supports the appropriate classification of most episodes. In addition, the analysis per epidemiological wave confirms the variant-related findings. We performed the same analysis using a delay of 5 weeks, according to the median delay in our study, and the results were similar. In fact, the percentage of cases in the boundaries between periods, which could potentially be assigned to a different period if the delay was changed, was small.
Our study is the first to compare the clinical presentation and laboratory findings through the different variant periods, and vaccination status in children. Regardless of variant period, the patients had similar phenotypes concerning severity and type of treatment. We estimated a lower MIS-C rate per SARS-CoV-2 infection for all age-groups, including those not vaccinated, during the Omicron period, suggesting that the variant could be the main factor for this shift in the MIS-C trend. Acquisition of knowledge on the MIS-C regarding different SARS-CoV-2 variants is crucial for estimating MIS-C hospitalizations in the future, and in the debate on COVID-19 vaccination of children.
Data availability
It's attached the dataset we used for this study, the link is the following: https://drive.google.com/file/d/1peba_EYJ5Ia6qbuxveXdL6oFk96mx69T/view?ts=63e63893
Abbreviations
- ALT:
-
Alanine aminotransferase
- CDC:
-
Centers for Disease Control and Prevention
- CI:
-
Confidence interval
- CMV:
-
Cytomegalovirus infection
- COPEDI-CAT:
-
Pediatric COVID-19 research network in Catalonia
- COVID-19:
-
Coronavirus disease 2019
- EBV:
-
Epstein Barr virus
- ECMO:
-
Extracorporeal membrane oxygenation
- HLH:
-
Hemophagocytic lymphohistiocytosis
- IgG:
-
Immunoglobulin G
- IL-1:
-
Interleukin-1
- IMV:
-
Invasive mechanical ventilation
- IQR:
-
Interquartile range
- IVIG:
-
Intravenous immunoglobulin
- KD:
-
Kawasaki disease
- L:
-
Liter
- MAS:
-
Macrophage activation syndrome
- MIS-C:
-
Multisystem inflammatory syndrome in children
- NIV:
-
Non-invasive ventilation
- p :
-
p-Value for statistical significance
- RCPCH:
-
Royal College of Paediatrics and Child Health
- pg/ml:
-
Picograms per milliliter
- PICU:
-
Pediatric intensive care unit
- Pro-BNP:
-
N-terminal prohormone of brain natriuretic peptide
- REDCap©:
-
Research Electronic Data Capture
- RR:
-
Rate ratio
- RT-PCR:
-
Reverse transcriptase polymerase chain reaction
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- UI/L:
-
International units per liter
- UK:
-
United Kingdom
- US:
-
United States
- WHO:
-
World Health Organization
- w.r.t.:
-
With regard to
References
Dong Y, Mo X, Hu Y et al (2020) Epidemiology of COVID-19 Among Children in China. Pediatrics 145(6):e20200702. https://doi.org/10.1542/peds.2020-0702
Moraleda C, Serna-Pascual M, Soriano-Arandes A et al (2021) Multi-inflammatory Syndrome in Children Related to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Spain. Clin Infect Dis 72(9):e397–e401. https://doi.org/10.1093/cid/ciaa1042
Multisystem Inflammatory Syndrome in children and adolescents with COVID-19. WHO/2019-nCoV/Sci_Brief/Multisystem_Syndrome_Children/2020.1
Dufort EM, Koumans EH, Chow EJ et al (2020) New York State and Centers for Disease Control and Prevention Multisystem Inflammatory Syndrome in Children Investigation Team. Multisystem inflammatory syndrome in children in New York State. N Engl J Med 383(4):347–358
Royal College of Paediatrics and Child Health. Guidance paediatric multisystem inflammatory syndrome temporally associated with COVID-19 (PIMS). Available from: https://www.rcpch.ac.uk/resources/guidance-paediatric-multisystem inflammatory-syndrome-temporally-associated-covid-19-pims
Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P (2020) Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 395(10237):1607–1608. https://doi.org/10.1016/S0140-6736(20)31094-1
Verdoni L, Mazza A, Gervasoni A et al (2020) An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 395(10239):1771–1778. https://doi.org/10.1016/S0140-6736(20)31103-X
Pino R, Izurieta AC, Ríos-Barnés M et al (2020) Correspondence on: ‘Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort’ by Pouletty et al. Ann Rheum Dis. 2020 Aug 5:annrheumdis-2020–218538. https://doi.org/10.1136/annrheumdis-2020-218538
Vogel TP, Top KA, Karatzios C et al (2021) Multisystem inflammatory syndrome in children and adults (MIS-C/A): Case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 39(22):3037–3049. https://doi.org/10.1016/j.vaccine.2021.01.054
Ouldali N, Bagheri H, Salvo F et al (2022) Hyper inflammatory syndrome following COVID-19 mRNA vaccine in children: A national post-authorization pharmacovigilance study. Lancet Reg Health Eur 100393. https://doi.org/10.1016/j.lanepe.2022.100393
Perramon A, Soriano-Arandes A, Pino D et al (2021) Schools as a Framework for COVID-19 Epidemiological Surveillance of Children in Catalonia, Spain: A Population-Based Study. Front Pediatr 9:754744. https://doi.org/10.3389/fped.2021.754744
Pla d’actuació per al curs 2020–2021 per a centers educatius en el marc de la pandèmia per COVID-19. Barcelona: Departament de Salut (2020). http://hdl.handle.net/11351/5235. Accessed 5 Aug 2022
Martró E, Saludes V, Escales A et al (2021) Analysis of the Alpha variant substitution dynamics of SARS-CoV-2 in Catalonia (Spain): contribution of the space. In: Analysis and prediction of COVID-19 for EU-EFTA-UK and other countries. Rep Coll Sit Rep 248:8–15. Available from: https://biocomsc.upc.edu/en/shared/20210625_report_248.pdf
Ciruela P, Sabrià A, Serrano C, Ferrer C, Mendoza S, Mendioroz J (2021) Week Variant Surveillance Report. Public Health Secretariat. Health Department. Generalitat de Catalunya. Week 27 - 2021 (05/07/21–11/07/21). Accessible from: https://canalsalut.gencat.cat/web/.content/_Professionals/Vigilancia_epidemiologica/snmc/variants-genomiques-sars-cov-2/Informe-noves-variants-genomiques.-SARS-CoV-2_Set.-27_Castella.pdf
Ciruela P, Sabrià A, Serrano C, Ferrer C, Mendoza S, Mendioroz J (2021) Week Variant Surveillance Report. Public Health Secretariat. Health Department. Generalitat de Catalunya. Week 01 - 2022 (03/01/22-09/01/22). Accessible from: https://www.google.com/url?q=https://canalsalut.gencat.cat/web/.content/_Professionals/Vigilancia_epidemiologica/snmc/variants-genomiques-sars-cov-2/informe-variants-genomiques-sars-cov-01-01-22-es.pdf
RENAVE (Red Nacional de Vigilancia Epidemiológica), Informe nº 127 (2022) Situación de COVID-19 en España. Surveillance report. Instituto de Salud Carlos III, Gobierno de España. Accessible from: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/INFORMES/Informes
Castagnola E, Mariani M, Sticchi C et al (2022) Incidence rate of MIS-C in paediatrics: A good reason to vaccinate children against SARS-CoV-2. Acta Paediatr 111(1):123–124. https://doi.org/10.1111/apa.16081
Payne AB, Gilani Z, Godfred-Cato S, et al. Incidence of Multisystem Inflammatory Syndrome in Children Among US Persons Infected With SARS-CoV-2. JAMA Netw Open. 2021;4(6):e2116420.https://doi.org/10.1001/jamanetworkopen.2021.16420.
Rhedin S, Lundholm C, Horne AC et al (2022) Risk factors for multisystem inflammatory syndrome in children - A population-based cohort study of over 2 million children. Lancet Regional Health 19:e100443
Brodin P (2022) SARS-CoV-2 infections in children: understanding diverse outcomes. Immunity 55:201–209
Cloete J, Kruger A, Masha M et al (2022) Pediatric hospitalisations due to COVID-19 during the first SARS-CoV-2 Omicron (B.1.1.529) variant wave in South Africa: a multicentre observational study. Lancet Child Adolesc Health 6(5):294–302
Cohen JM, Carter MJ, Ronny Cheung C, Ladhani S (2022) Evelina PIMS-TS Study Group. Lower Risk of Multisystem Inflammatory Syndrome in Children (MIS-C) with the Delta and Omicron variants of SARS-CoV-2. Clin Infect Dis 5:ciac553. https://doi.org/10.1093/cid/ciac553
Miller AD, Yousaf AR, Bornstein E et al (2022) Multisystem inflammatory syndrome in children (MIS-C) during SARS-CoV-2 Delta and Omicron variant circulation - United States, July 2021 - January 2022. Clin Infect Dis 10:ciac471. https://doi.org/10.1093/cid/ciac471. Epub ahead of print
Zambrano LD, Newhams MM, Olson SM et al (2022) Effectiveness of BNT162b2 (Pfizer- BioNTech) mRNA Vaccination Against Multisystem Inflammatory Syndrome in Children Among Persons Aged 12–18 Years - United States, July-December 2021. MMWR Morb Mortal Wkly Rep 71(2):52–58
Levy M, Recher M, Hubert H et al (2022) Multisystem Inflammatory Syndrome in Children by COVID-19 Vaccination Status of Adolescents in France. JAMA 327(3):281–283. https://doi.org/10.1001/jama.2021.23262
Holm M, Espenhain L, Glenthøj J, Schmidt LS, Nordly SB, Hartling UB, Nygaard U (2022) Risk and Phenotype of Multisystem Inflammatory Syndrome in Vaccinated and Unvaccinated Danish Children Before and During the Omicron Wave. JAMA Pediatr 176(8):821–823. https://doi.org/10.1001/jamapediatrics.2022.2206
Karatzios C, Scuccimarri R, Chédeville G, Basfar W, Bullard J, Stein DR (2022) Multisystem Inflammatory Syndrome Following SARS-CoV-2 Vaccination in Two Children. Pediatrics 150(2):e2021055956. https://doi.org/10.1542/peds.2021-055956
Hoste L (2022) MIS-C researchers, Soriano-Arandes A, Buddingh EP, et al. Severe Acute Respiratory Syndrome Coronavirus 2 Vaccination in Children with a History of Multisystem Inflammatory Syndrome in Children: An International Survey. J Pediatr 19:S0022–3476(22)00438–3. https://doi.org/10.1016/j.jpeds.2022.05.028
https://www.idescat.cat/poblacioestrangera/?geo=cat&nac=a&b=11&lang=en. Accessed 5 Aug 2022
Swann OV, Holden KA, Turtle L et al (2020) Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: prospective multicentre observational cohort study. BMJ 370:m3249. https://doi.org/10.1136/bmj.m3249
Levy N, Koppel JH, Kaplan O, Yechiam H, Shahar-Nissan K, Cohen NK, Shavit I (2022) Severity and Incidence of Multisystem Inflammatory Syndrome in Children During 3 SARS-CoV-2 Pandemic Waves in Israel. JAMA. https://doi.org/10.1001/jama.2022.8025. Epub ahead of print. PMID: 35588048
Acknowledgements
To all the COPEDI-CAT researchers for their great and tireless work through the pandemic, to Spider’s Web translation website, and especially to Cristina Roman and Jane Perkins for their English style review of the manuscript.
Funding
This study has received funding for the data analysis from the “Fundació la Marató TV3” with file number 202134–30-31.
Author information
Authors and Affiliations
Contributions
This is a multidisciplinary study with contribution form pediatricians collecting data of their patients, biophysics exploring and analyzing the data, and epidemiologists interpreting and putting the data in context. Conceptualization, R.P., F.P., C.P., and A.S-A; funding acquisition, C.P. and A.S-A.; investigation, data collection, and resources, all authors; methodology, data curation, and data analysis, R.P., J.M.A., C.P., A.P., E.C., F.F., and A.S-A.; writing-original draft preparation, R.P., C.P., J.M.A., and A-S-A.; writing-review and editing, all authors; visualization, all the authors; supervision, R.P., C.P., and A-S-A. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was reviewed and approved by the research ethics committee of the coordinating center (PR(AMI)271/2021). All the participating sites obtained their approval from the respective ethics committees for the collection of data related to the MIS-C cases. This research was based on the agreement established in Regulation 2016/679 of the European Parliament and the Council of Europe of April 27, 2016 on Data Protection, and Organic Law 3/2018 of December 5 on the Protection of Personal Data and the Guarantee of Digital Rights.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Peter de Winter.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pino, R., Antoñanzas, J.M., Paredes-Carmona, F. et al. Multisystem inflammatory syndrome in children and SARS-CoV-2 variants: a two-year ambispective multicentric cohort study in Catalonia, Spain. Eur J Pediatr 182, 1897–1909 (2023). https://doi.org/10.1007/s00431-023-04862-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-023-04862-z