Abstract
Hybrid closed loop (HCL) systems are the combination of a pump for insulin delivery and a glucose sensor for continuous glucose monitoring. These systems are managed by an algorithm, which delivers insulin on the basis of the interstitial glucose levels. The MiniMed™ 670G system was the first HCL system available for clinical purpose. In this paper, we reviewed the literature about metabolic and psychological outcomes in children, adolescents and young adults with type 1 diabetes treated with MiniMed™ 670G. Only 30 papers responded to the inclusion criteria and thus were considered. All the papers show that the system is safe and effective in managing glucose control. Metabolic outcomes are available up to 12 months of follow-up; longer study period are lacking. This HCL system may improve HbA1c up to 7.1% and time in range up to 73%. The time spent in hypoglycaemia is almost neglectable. Better improvement in blood glucose control is observed in patients with higher HbA1c at HCL system start and larger daily use of auto-mode functionality.
Conclusion: The Medtronic MiniMed™ 670G is safe and well accepted, without any increase in the burden for patients. Some papers report an improvement in the psychological outcomes, but other papers do not confirm this finding. So far, it significantly improves the management of diabetes mellitus in children, adolescents and young adults. Proper training and support by the diabetes team are mandatory. Studies for a period longer than 1 year would be appreciated to better understand the potentiality of this system.
What is Known: • The Medtronic MiniMedTM 670G is a hybrid closed loop system which combines a continuous glucose monitoring sensor with an insulin pump. • It has been the first hybrid closed loop system available for clinical purpose. Adequate training and patients support play a key role in diabetes management. | |
What is New: • The Medtronic MiniMedTM 670G may improve HbA1c and CGM metrics up to 1-year of follow-up, but the improvement appears lower than advanced hybrid closed loop systems. This system is effective to prevent hypoglycaemia. • The psychosocial effects remain less understood in terms of improvement of psychosocial outcomes. The system has been considered to provide flexibility and independence by the patients and their caregivers. The workload required to use this system is perceived as a burden by the patients who decrease the use of auto-mode functionality over time. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nearly four decades ago, the Diabetes Control and Complication Trial showed that intensive insulin treatment is more effective than the standard treatment in improving the blood glucose control [1]. This milestone was a breakthrough in diabetes management. Ever since, clinicians and researchers aimed to develop new strategies to reduce the risk of hypoglycaemia and to manage hyperglycaemia, definitively improving the blood glucose control.
The progressive improvement of insulin pumps and the availability of continuous glucose monitoring (CGM) were both further steps in diabetes management. The uptake of insulin pumps has rapidly increased worldwide, and the possibility that a sensor can transfer by a transmitter the interstitial glucose values directly to the insulin pump additionally increased the adoption of these devices. Companies are working to develop integrated systems, so-called artificial pancreas (AP) or “closed loop” systems, which can automatically manage the glucose values.
The Medtronic MiniMed™ 670G system (Medtronic, Northridge, CA) was the first hybrid closed-loop (HCL) system available on the market for clinical purpose [2]. This system combines the MiniMed™ 670G insulin pump with the Guardian™ 3 CGM glucose sensor, which are managed by an algorithm called SmartGuard™ technology (Medtronic, Northridge, CA) [2]. The algorithm is based on a modified proportional-integral-derivative software [3] that responds to the real-time interstitial glucose values measured by the sensor every 5 min [2]. The algorithm works by increasing, decreasing, or suspending the insulin delivery to obtain the pre-fixed target blood glucose [4]. The HCL functionality, which is named auto mode, targets a glucose value of 120 mg/dL, which can be increased to 150 mg/dL by the patient in the case of physical activity or any other need. The system switches from auto to manual mode for several reasons, but in particular because of missing sensor calibrations (the Guardian™ 3 sensor require 2 calibrations/day), prolonged hyperglycaemia and inconsistent sensor readings. The basal insulin dose is determined by the system on the basis of previous total daily insulin dose and fasting interstitial glucose value, while the actual base dose also takes patients CGM values and active insulin values into account [5]. The parameters to calculate the pre-meal insulin dose (insulin to carbohydrates ratio, insulin sensibility factor and so on) are set up by the patient.
The Medtronic MiniMed™ 670G system has been available for more than 4 years now, and increasing evidence on the clinical and the psychological outcomes has been published during this time. In this manuscript, we review the existing literature about this system to summarize the metabolic and psychological outcomes in children, adolescents and young adults patients.
Methods
This review is reported according to the PRISMA statement for reporting systematic reviews [6].
Search strategy and selection criteria
We searched Medline (PubMed) from inception to 10 August 2022 using the following search terms: MiniMed 670G, hybrid closed loop system. We omitted terms related to type 1 diabetes (T1D) or paediatric age to avoid missing potentially relevant studies. Non-English language literature was excluded while no publication date nor publication status restrictions were imposed.
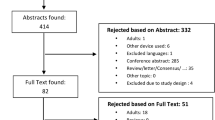
We included all randomised trials, observational studies, retrospective studies and case reports regarding children, adolescents and nonpregnant young adults with T1D that were treated with the Medtronic MiniMed™ 670G in auto mode. Articles regarding all types of inpatient and outpatient setting (normal living conditions, hotels, diabetes camps), prior and during the COVID-19 pandemic, irrespective of duration of intervention, or baseline insulin treatment (multiple daily insulin injections, insulin pumps, with or without CGM) were included (Fig. 1).
Data extraction
Two reviewers (GMS and AG) worked independently and screened all records, excluding duplicates. Initially, records were screened at title and abstract level, and potentially eligible studies were assessed in full text. If multiple records of one study were retrieved, we collected data from all records, and used data from the report with the most recent publication date. Articles reporting no original data (reviews, commentaries, guidelines and editorials) as well as articles that did not provide information on these specific outcomes were excluded. Disagreements between reviewers were resolved by discussion and consensus.
Outcomes
Safety outcomes included severe hypoglycaemia events (glucose level < 54 mg/dl) that required third party assistance and diabetic ketoacidosis (DKA).
The metabolic outcomes were proportion (%) of time when the sensor glucose level was within normoglycaemic range (3.9–10 mmol/L; 70–180 mg/dL; TIR), proportion (%) of time when the sensor glucose level was below normoglycaemic range (< 3.9 mmol/L; < 70 mg/dL; TBR), proportion (%) of time when the sensor glucose level was above normoglycaemic range (> 10.0 mmol/L; > 180 mg/dL, TAR), glycated haemoglobin (HbA1c) while using the Medtronic MiniMed 670G in auto mode, mean sensor glucose (SG) level and coefficient of variation (CV). When available, TIR, TAR and TBR were extracted both for 24- and overnight periods (as defined in each individual study).
Psychological outcomes included fear of hypoglycaemia and sleep quality.
Information that were extracted from each included paper, where provided: (1) characteristics of participants (age, sex, duration of diabetes, HbA1c at baseline and treatment prior study enrolment), inclusion and exclusion criteria in case of trials; (2) type of intervention (switching from multiple daily insulin injections/insulin pump, with or without CGM to the Medtronic MiniMed 670G in auto mode), type of outpatient setting, and follow-up duration; and (3) type of outcome measure (metabolic outcomes such as TIR, HbA1c, TBR, mean SG, CV; psychological outcomes such as fear of hypoglycaemia and sleep quality; safety outcomes such as risk of DKA and severe hypoglycaemic events that required third party assistance).
Patient involvement
No patients were involved in definition of the research question, outcome measures, interpretation and the writing of the results.
Data analysis
Extracted data were evaluated and synthesized using a narrative analysis. Evidence from qualitative studies was synthesized thematically. If data were collected in cohort with a difference age range, we considered only data about children, adolescents and young adults if clearly available.
Results
Characteristics of the included studies
Figure 1 shows the study selection process. The original database search resulted in 734 records from Medline. The first phase of screening excluded 654 records. This process left 80 records to assess for eligibility by screening the full-text articles (we were not able to assess two records for eligibility because full-text was not available, these two records were therefore excluded). The second phase of screening excluded 50 records. This left 30 unique articles that were included in this review (2 randomized controlled trials, 1 randomized trial, 2 randomized crossover trial, 14 observational studies, 5 retrospective studies, 2 observational + retrospective study and 4 case reports) [4, 5, 7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34].
Table 1 shows characteristics of the 30 studies included in the systematic review and their participants at baseline.
Outcomes
Metabolic outcomes
Data from a randomized control study run in a 7-day and 7-night nonstructured camp setting show that the HCL is effective in improving the CGM metrics. However, the results were similar in the control and in the study group, suggesting that the “study effect” and the education may significantly affect the metabolic outcomes [17]. Data about longer follow-up are provided in the following sections.
Data at 3 months. The first report about metabolic outcomes in a large cohort of paediatric patients was provided by Garg et al. [4], who reported that HbA1c significantly dropped from 7.7 to 7.1% after a 3-month study period in 30 adolescents and young adult patients aged 14–21 years. The CV and the standard deviation score of SG were significantly reduced as well, but these goals were reached by a significant increased of the total daily dose of insulin, overall of the pre-meal boluses. The TIR increased from 60.4 to 67.2%, paralleled by a reduction of the TBR (from 4.3 to 2.8%; the decrease was more evident overnight) and of the TAR (from 35.3 to 30.0%). Similar data about the TIR, with an increase from 57 to 65%, and TAR, with a decrease from 2.5 to 2.2%, were reported in real-world setting switching from manual mode to auto mode in the same age group by Stone et al. [30]. The improvement in the metabolic outcomes was irrespective of CGM use before using the Medtronic MiniMed™ 670G [15].
An increase in the total daily dose of insulin was not confirmed by Messer et al. [5]. In their study (31 patients, 14–26 years old), they investigated the setup parameters of the algorithm to provide useful suggestions for clinical practice. The authors showed that the baseline HbA1c of 7.8% decreased by 0.75% and the TIR increased by 14%, without a significant change in the total daily dose of insulin. Interestingly, they showed that a frequent tuning of the carbohydrates to insulin ratio is mandatory in the first month of treatment to reach such goals.
The efficacy of the system was confirmed in 105 younger patients (age 7–13 years), who presented a TBR of 1.9%, a decrease in HbA1c from 7.9 to 7.5%, and an increase in TIR from 56.2 to 65.0%. Again, an increase in the total daily dose, overall of the pre-meal boluses, was described [20].
Data at 6 months. Real-world data in 92 youths aged 2–25 years show that the use of the HCL system declined significantly after 6 months, prompting the authors to highlight the need for patients support and intervention strategies. The use of the auto mode functionality decreased significantly from 65.5% at 1 month to 51.2% at 6 months [12]. Patients who used the auto mode functionality presented a baseline HbA1c of 8.7%, which significantly decreased to 8.2% and 8.4% after 3 and 6 months, respectively. In parallel, the TIR increased from 50.7% at baseline to 58.7% and 56.9% after 3 and 6 months, respectively. The prevalence of hypoglycaemia was neglectable (TBR always < 3%). Interestingly, the patients with higher baseline HbA1c presented a larger decrease at 6 months (from 10.7 to 9.3%), with a decrease by 0.07% in HbA1c for each 10% increase in auto mode use. The effect of time spent in auto mode on glucose control was confirmed by Duffus et al. [19] in a cross-sectional study recruited 96 patients aged between 10 and 21 years after a mean time of 188 day. In this study, each increase of 3.4-h spent in auto mode per day was associated to a reduction in the HbA1c by 0.1% and each 8.6 h per day to an increase of the TIR by 5%.
Data at 12 months. Two studies run in 30 patients (age 10.2 ± 2.4 years) [26] and 111 patients (age 3–16 years) [31] evaluated the effect of this HCL at 1 year with similar findings. The baseline HbA1c dropped from 8.2 and 8.5% to 7.1% [26] and 7.3% [31], respectively. The time spent in auto mode ranged from 85 to 90% at 3 months to 80 to 85% at 1 year of follow-up, higher than previous data at 6 months [12]. The use of the auto mode functionality allowed a significant increase of TIR from 46.9 to 73.4% [26] and from 55.7 to 67.3% [31]. An increase in the total daily dose of insulin was found [26] but not confirmed [31]. Finally, the HCL confirmed to be safe also at 12 months, with a TBR reduction from 5.9 to 3.2% [31].
A reduction about the use of CGM in real world was confirmed in 115 patients younger than 25 years. Its use decreased from 71% at month 1 to 49–55% at month 12, and the TIR from 60.4–63.3% to 53.6–61.3% [13].
Off-label use. Despite the system is approved for patients above 7 years of age, two studies were run in younger children. Forlenza et al. [21] reported a mean HbA1c of 8% at baseline and of 7.5% 3 months later in 46 children, with an increase in TIR from 55.7 to 63.9%, without any change in TBR (3.3% at baseline, 3.2% at 3 months). The time spent in auto mode was higher than what was reported in older patients by previous papers. Salehi et al. described similar results after a mean period of 6.3 months [29]. The data from von dem Berge et al. showed that this system was efficient in managing the blood glucose control in pre-school children as much as in primary school children [33].
Safety: DKA and severe hypoglycemic events
The first data report about the safety of this HCL system was obtained in 124 patients aged 10–75 years (30 adolescents and 94 adults) in a one-arm 3-month study [10]. The authors concluded that the Medtronic MiniMed™ 670G system was associated with few serious or device-related adverse events. De Bock et al. showed that the HCL system is effective to protect against exercise-induced hypoglycaemia. In this in-clinic 4-day study, 8 patients (7 of them were adolescents and 1 was adult) who underwent a 45-min exercise on a stationary bicycle at 55% of their peak rate of oxygen consumption were recruited. None of the 7 adolescent patients experienced exercise-induced hypoglycaemia or nocturnal hypoglycaemia [16]. Eventually, Wood et al. confirmed the safety of the system in 105 patients aged 7–13 years who underwent an in-hospital hypoglycaemia induction protocol of 90 min. During this session, the patients could bike, or walk, or play Nintendo® Wii games, or any other aerobic activities. The hypoglycaemia alert was set at 65 mg/dl, and the “suspend before low” function was able to prevent 80% of the hypoglycaemias in 79 of the patients who experienced a blood glucose value below 65 mg/dl. None of the patients presented severe hypoglycaemia nor rebound hyperglycaemia [34].
Data on the occurrence of severe hypoglycaemia events were available in 9 different studies plus 3 case reports (total of 491 patients; age range 2–21 years) [7, 9,10,11, 20, 21, 23, 24, 26, 29, 31]. Follow-up duration was very different among studies, ranging from 1 to 12 months, with the majority of the studies lasting for about 3 months. Overall, the occurrence of severe hypoglycaemia events while using the Medtronic 670G system in auto mode in an outpatient setting was very low. Nevertheles, Varimo et al. who retrospectively followed 111 patients aged 3 to 16 years for 12 months in an outpatient setting reported one episode of severe nocturnal hypoglycaemia. It occurred in a 15-year-old patient after disconnecting the CGM at bed time for an unknown reason [31].
Finally, in the case report by Dominguez-Riscart et al. [18], the HCL system proved to be effective in blood glucose management and safe to prevent hypoglycaemia in a 9-year-old boy who underwent appendectomy. The authors’ message “take your pump to surgery” can be an interesting suggestion to take into consideration.
No episodes of diabetic ketoacidosis were reported in any of the studies.
Psychological and sleep quality outcomes
Few studies explored psychological and sleep quality outcomes in the paediatric population and youths.
One very short study conducted on 14 adults and 15 adolescents (age 14–40 years) showed a decrease in diabetes management distress and a more positive attitude towards diabetes technology, after a 4–5-day usage of this device, without any change in hypoglycaemia fear [8]. The authors hypothesized that a longer exposure to the HCL technology might have had a bigger impact on this outcome (Table 2).
A longer study (3 months) was conducted by Beato-Víbora et al. on a similar population (58 patients aged 7–63 years starting on the 670G system) [9]. Only adults and adolescents older than 13 years old completed a set of questionnaires that showed that, by the end of the study, diabetes management distress, quality of life, treatment satisfaction and also fear of hypoglycaemia had all improved. Moreover, the percentage of patients with poor sleep quality was reduced from 49 to 40%, suggesting that the better glycaemic control and less glycaemic variability that can be achieved thanks to this HCL system have a positive impact on sleep quality and counteract the negative effects of the system alarms and the finger sticks requirements that the system demands.
On contrast, Cobry et al. reached opposite findings. In an observational study, they evaluated the impact of the Medtronic 670G system on sleep and quality of life in 37 adolescents (aged 10–17 years) and their parents over a 3-month period [14]. During the study, both objective and subjective sleep data were collected through a wrist-worn accelerometer, a sleep diary and the Pittsburgh Sleep Quality Index questionnaire. Results showed that neither adolescents’ nor parents’ sleep characteristics changed significantly pre–post device initiation. Adolescents’ mean total sleep time decreased from 7 h 16 min to 7 h 9 min, while parents’ total sleep time decreased from 6 h 47 min to 6 h 38 min. Also there were no significant differences in most of the survey measures regarding parental and adolescent diabetes distress and hypoglycaemia fear.
Similar psychosocial outcomes were also obtained by Berget et al. in 92 youth (aged 2–25 years) and their primary caregiver during the first 6 months of 670G HCL system usage [12]. Across time, no changes in hypoglycaemia fear or diabetes-related problems were found and 30% of the participants discontinued the HCL by the end of the study.
The study by Abraham et al. is to our knowledge the only one that assessed psychosocial outcomes in a long-term randomized clinical trial [7]. One hundred thirty-five patients between 12 and 25 years of age were randomly assigned to either the control group for conventional therapy (continuous subcutaneous insulin infusion or multiple daily insulin injections with or without CGM) or the intervention group for HCL therapy for a 6-month period. Psychosocial measures were collected by validated questionnaires, and hybrid closed-loop therapy was associated with improved diabetes-specific quality of life and treatment satisfaction compared with conventional therapy. While no change in diabetes distress and fear of hypoglycaemia were observed between groups.
Roberts et al. [28] explored the patients’ and caregivers’ lived experience with a semi-structured interview after 6 months on MiniMed™ 670G. The participants acknowledged the benefits of this system in improving glycaemic outcomes. Interestingly, according to their answers the device provided flexibility and independence.
Discussion
Efficacy and safety play a very important role in the choice of starting an automated insulin delivery system. Nowadays, various HCL systems are available on the market with some differences in terms of physical device, algorithm and glucose sensor, which allow to personalize the choice of the system. Even if the MiniMed™ 670G system has been replaced by MiniMed™ 780G and other closed-loop systems, such as Tandem Control-IQ and DBLG-1 system, we think that reviewing the available may be useful for clinicians and researchers. Our systematic review shows that the MiniMed™ 670G system may improve the metabolic control in paediatric population and youths and it is safe.
Data about the metabolic outcomes in children, adolescents and young adults over the first 12 months on MiniMed™ 670G are available, and most of them were run in patients older than 7 years of age, in keeping with the market authorization. Two studies were run in patients younger than 7 years. No data beyond 12 months of HCL utilization are still available in literature. Short-term data show that the MiniMed™ 670G reduces the HbA1c levels to 7–7.5% and increases TIR at least to 65% [2, 4, 5, 20]. Contrasting data are reported about the daily insulin dose, which is reported increased, in particular as pre-meal boluses [4, 20], or unchanged [5]. Notably, the fine-tuning of the carbohydrates to insulin ratio setup in the first month on HCL plays a key role in the improvement of the glucose control [5]. A consistent decrease in HbA1c levels to 7.1–7.3% is confirmed by data from longer follow-up (1 year), with a TIR of 67–73% [26; 31]. The patients in the retrospective study by Varimo et al. [31] had better baseline control than in other papers commonly reported. The authors attribute these potentially to patients being better at carb counting and consistently taking boluses before meals.
All the studies show that the time spent in hypoglycaemia is about 2–3% irrespective of the study duration, within the recommended clinical targets [35]. As expected, real-life study evaluated cross-sectionally the effect of auto-mode use, showing that longer period of use allows larger improvement in blood glucose control. In particular, HbA1c improves by 0.07% for each 10% increase in auto mode use [12] or by 0.1% for each daily 3.4-h increase of time spent in auto mode [18]. Furthermore, each daily 8.6-h increases the TIR by 5% [18].
Behind the data showing the effectiveness of this HCL in improving the blood glucose control, some data from the same research group [12, 13] show data that the use of CGM may decrease over time, reducing the time spent in auto mode. These papers highlight the need for appropriate training programs and support by the diabetes team, to motivate the patients to use CGM and the auto mode functionality as much as possible to take advantages from the system.
Data about off-label patients deserve some comments. Patients younger than 7 years old have special needs, overall in consideration of the hypoglycaemia unawareness. In these patients, the system is safe in preventing hypoglycaemia and yields HbA1c level of 7.5% with a TIR above 60% [21, 29]. Even at this age, the MiniMed™ 670G represents a reliable tool to get good glucose control, as much as in older patients [33]. This system proved to be efficient also during the COVID-19 pandemics, when the physical activity was restricted. There is evidence that the blood glucose control did not worsen and improved in the patients who continued physical activity during this period [32]. The time spent in hypoglycaemia is always lower than 3% [4, 5, 20].
Different authors highlighted the key role of the education in diabetes management and of the training in the use of devices. Interestingly, virtual training programs can be provided to optimize the time spent by physicians for education of the patients [25, 27].
Safety of hybrid closed-loop systems can be assessed through the incidence of diabetes-related potential life-threatening adverse events such as diabetic ketoacidosis, and severe hypoglycaemia events requiring third party assistance. Data about safety come from studies with different follow-up duration, up to 12 months. Overall, the occurrence of severe hypoglycaemia events while using the Medtronic 670G system in auto mode in an outpatient setting was very low [31]. It is to note that in their paper, Varimo et al. [31] reported a better baseline control than other authors. The result seems to suggest that the system works safely and rather improves the low glucose exposure to patients who demonstrate good therapy adherence.
Interestingly, the MiniMed™ 670G system may prevent from hypoglycaemia even during perioperative procedure [18]. Our systematic revision also showed that no episodes of diabetic ketoacidosis were reported in any of the studies. These data confirm the safety of Medtronic 670G system in auto mode while used in the home setting.
Other automated insulin delivery systems are available for clinical purpose, and advanced HCL (AHCL) systems are largely used nowadays. Data from short-term randomized clinical trials (MiniMed™ 670G HCL versus MiniMed™ 780G AHCL) in 113 adolescents and young adults [36] and 60 children and adults [37] show that results about the blood glucose control favoured the AHCL system. The auto mode exits with the AHCL system were lower, and thus, the closed-loop usage was higher. Furthermore, data from more than 4000 users of MiniMed™ 780G showed that TIR was 76% and closed-loop usage 94% in real-life setting, with only 1 auto mode exit per week and 3.4 fingerpricks per day over a mean follow-up of 54 days [38]. Real-world data from more than 12,000 paediatric and adult users confirmed these results over a 6-month follow-up period [39]. All these findings suggest that usability is improved in this system.
The Control-IQ AHCL system improved TIR of 11% in 101 patients, 6–13 years old, after 4 months [40] and in 168 patients older than 14 years [41], with significant reductions in HbA1c and TBR [40] as compared to sensor-augmented pump (SAP) in randomized control trials. Median closed-loop usage was 90% or above. No severe hypoglycaemia or DKA events were reported in the paediatric study [40]. Improvement in TIR was confirmed in children by the 12-week extension study [42]. Real-world retrospective data from more than 9000 users older than 6 years (80% having type 1 diabetes) showed a closed-loop use of 94%, with TIR of 74% and TBR of 1%, stable over a 1-year follow-up [43].
Data from the randomized control trial by Kariyawasam et al. [44] comparing the Diabeloop system versus SAP over 6 weeks in 17 patients (6–12 years old) showed that TIR improved from 59% with control to 66% with close loop, without any DKA or hypoglycaemia. The closed-loop usage was 99%.
Studies comparing the CamAPS FX system to SAP showed more favourable outcomes in AHCL users across all CGM metrics, with an increase in TIR of 11% in the 12-week study in 86 children, adolescents and adults older than 6 years (HbA1c > 58 mmol/mol) [45] and of 15% in the 6-month study in 133 patients aged 6–18 years (HbA1c > 53 mmol/mol) [46]. There were no severe hypoglycaemia events and one DKA event [45] in the AHCL group due to infusion set failure. This AHCL system has been more effective in improving the blood glucose control than SAP also in 74 children aged 1 to 7 years over 4 months, being TIR increased by 9% [47].
Few data and no randomized clinical trials are available about the Omnipod 5 system, available in the USA since early 2022. This a HCL system, not a AHCL, and a study in 235 users (111 between 6 and 18 years old) showed a closed-loop usage of 96% at 3 months [48]. One hypoglycaemia event, due to delayed eating after a pre-prandial bolus, and one DKA event, due to infusion site failure, occurred in the paediatric group. TIR and TBR were 68% and 1.8%, respectively, in the paediatric group.
Besides the benefits of the MiniMed™ 670G system, the major concern is the decrease in auto mode use over time with a high rate of dropouts. Berger et al. [12] showed that the use of HCL functionality declined from 66% at 1 month of use to 51% at 6 months and that 30% of the patients discontinued auto mode use after 6 months of MiniMed™ 670G. The workload required to use HCL was the main reason for HCL discontinuation [49]. Similar results were reported by Lal et al. [50], who observed a decline in auto mode use after 1 year of HCL system in 79 patients aged 9–61 years. Twenty-six of them (32.9%) discontinued HCL by 12 months because of sensor issues (62%), problems obtaining supplies (12%), hypoglycaemia fear (12%), multiple daily injection preference (8%) and sports (8%). No data are provided only for children and adolescents.
The decline in fingerstick calibration over time leads to a decrease in the use of CGM use and thus to a decrease in HCL use. Furthermore, system alarms burden the patients especially overnight. The result is that auto mode exits and frequent alarms may prompt the users to drop out HCL functionality. All these findings support the key role of education in diabetes management and of the training in the use of devices. The patients need proper education about technology and appropriate support from healthcare providers, and rights expectations should be set during training and follow-up.
The emotional burden of living with T1D is extensive, and people commonly report psychological distress regarding the practical aspects of diabetes management and the fear of bad outcomes such as severe hypoglycaemia. Overtime, diabetes technologies have progressed considerably, and the MiniMed™ 670G could provide an opportunity not only for improved glycaemic control, but also for enhanced quality of life among youth with T1D and their caregivers. Results are however conflicting, and whether or not this device has improved psychosocial outcomes has not yet been clearly established.
Although the MiniMed™ 670G has improved glycaemic control, its psychosocial effects remain less understood and whether or not this device has improved psychosocial outcomes in youth with T1D and their caregivers has not yet been clearly established. What, to this day, can be undoubtedly inferred, is that its use is not linked to an increase of diabetes perceived burden, even in preschool patients. Interestingly, the system has been considered to provide flexibility and independence by the patients and their caregivers [28].
Finally, the treatment cost of MiniMed™ 670G (and in general the cost of insulin pump and continuous glucose monitoring) is higher than that of multiple daily injections. However, recent findings showed that over patient lifetimes, the incremental clinical benefits associated with the use of 670G is likely to be cost-effective relative to the continued use of insulin pump in people with type 1 diabetes, particularly for those with a fear of hypoglycaemia or poor baseline glycaemic control [51]. Therefore, given the positive effects of pumps and continuous glucose monitoring on type 1 diabetes health outcomes, it is possible that short-term costs are offset by future savings.
Strengths and limitations of study
This is the first systematic review on the metabolic, safety and psychological outcomes of the MiniMed™ 670G in children, adolescents and young adults. Several limitations must be considered when interpreting the results of this work. First, different kinds of studies were included (both clinical trials and observational studies and case reports), but most of them were observational ones with potential bias (for instance, observational studies are likely only to report success, while those patients who stopped using this system are often not included in this kind of study). However, evidence from randomized clinical trials, real-world studies on 670 pumps and their effects on glycaaemic on safety outcomes are a helpful method for evaluating its safety and effectiveness. Second, most trials had a small sample size, limiting the precision of our effect estimates. Furthermore, references 23 to 27 report data from the same study group. Third, the age range of the population varies among studies and included patients beyond the paediatric age; therefore, we cannot perform a systematic review focused only on paediatric patients. Moreover, as a possible limitation, this systematic review was restricted to English language, potentially limiting the generalizability of the findings to English literature and reducing the number of patients including in this data analysis.
Implication and conclusion
Our systematic review has shown that MiniMed™ 670G Hybrid Closed-Loop System is an efficacious and safe treatment approach for children and adolescents with type 1 diabetes, leading to increased time in near normoglycaemic range, and reduced time in hypoglycaemia and hyperglycaemia, without increasing the risk of severe hypoglycaemic events and secondary DKA. In spite of the improved outcomes, more aggressive and advanced HCL systems are required to keep on increasing time in range values and decrease the therapy withdrawal. Currently, multiple HCL systems have been developed by different companies and commercially available in many countries. Their clinical implication, safety and cost-effectiveness, and long-term efficacy in paediatric population are under investigation.
References
Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Eng J Med 329(14):977–986. https://doi.org/10.1056/NEJM199309303291401
Stone JY, Haviland N, Bailey TS (2018) Review of a commercially available hybrid closed-loop insulin-delivery system in the treatment of Type 1 diabetes. Ther Deliv 9(2):77–87. https://doi.org/10.4155/tde-2017-0099
Ruiz JL, Sherr JL, Cengiz E, Carria L, Roy A, Voskanyan G, Tamborlane WV, Weinzimer SA (2012) Effect of insulin feedback on closed-loop glucose control: a crossover study. J Diabetes Sci Technol 6(5):1123–1130. https://doi.org/10.1177/193229681200600517
Garg SK, Weinzimer SA, Tamborlane WV, Buckingham BA, Bode BW, Bailey TS, Brazg RL, Ilany J, Slover RH, Anderson SM, Bergenstal RM, Grosman B, Roy A, Cordero TL, Shin J, Lee SW, Kaufman FR (2017) Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 19(3):155–163. https://doi.org/10.1089/dia.2016.0421
Messer LH, Forlenza GP, Sherr JL, Wadwa RP, Buckingham BA, Weinzimer SA, Maahs DM, Slover RH (2018) Optimizing hybrid closed-loop therapy in adolescents and emerging adults using the MiniMed 670G system. Diabetes Care 41(4):789–796. https://doi.org/10.2337/dc17-1682
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 151(4):W65-94. https://doi.org/10.7326/0003-4819-151-4-200908180-00136
Abraham MB, de Bock M, Smith GJ, Dart J, Fairchild JM, King BR, Ambler GR, Cameron FJ, McAuley SA, Keech AC, Jenkins A, Davis EA, O’Neal DN, Jones TW, Australian Juvenile Diabetes Research Fund Closed-Loop Research group (2021) Effect of a hybrid closed-loop system on glycemic and psychosocial outcomes in children and adolescents with type 1 diabetes: a randomized clinical trial. JAMA Pediatr 175(12):1227–1235. https://doi.org/10.1001/jamapediatrics.2021.3965
Adams RN, Tanenbaum ML, Hanes SJ, Ambrosino JM, Ly TT, Maahs DM, Naranjo D, Walders-Abramson N, Weinzimer SA, Buckingham BA, Hood KK (2018) Psychosocial and human factors during a trial of a hybrid closed loop system for type 1 diabetes management. Diabetes Technol Ther 20(10):648–653. https://doi.org/10.1089/dia.2018.0174
Beato-Víbora Pilar I, Gallego-Gamero F, Lázaro-Martín L, Romero-Pérez MdM, Arroyo-Díez FJ (2020) Prospective analysis of the impact of commercialized hybrid closed-loop system on glycemic control, glycemic variability, and patient-related outcomes in children and adults: a focus on superiority over predictive low-glucose suspend technology. Diabetes Technol Ther 22(12):912–919. https://doi.org/10.1089/dia.2019.0400
Bergenstal RM, Garg S, Weinzimer SA, Buckingham BA, Bode BW, Tamborlane WV, Kaufman FR (2016) Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA 316(13):1407–1408. https://doi.org/10.1001/jama.2016.11708
Bergenstal RM, Nimri R, Beck RW, Criego A, Laffel L, Schatz D, Battelino T, Danne T, Weinzimer SA, Sibayan J, Johnson ML, Bailey RJ, Calhoun P, Carlson A, Isganaitis E, Bello R, Albanese-O’Neill A, Dovc K, Biester T, Weyman K, Hood K, Phillip M, for the FLAIR Study Group (2021) A comparison of two hybrid closed-loop systems in adolescents and young adults with type 1 diabetes (FLAIR): a multicentre, randomised, crossover trial. Lancet 397(10270):208–219
Berget C, Messer LH, Vigers T, Frohnert BI, Pyle L, Wadwa RP, Driscoll KA, Forlenza GP (2020) Six months of hybrid closed loop in the real-world: an evaluation of children and young adults using the 670G system. Pediatr Diabetes 21(2):310–318. https://doi.org/10.1111/pedi.12962
Berget C, Akturk HK, Messer LH, Vigers T, Pyle L, Snell-Bergeon J, Driscoll KA, Forlenza GP (2021) Real-world performance of hybrid closed loop in youth, young adults, adults and older adults with type 1 diabetes: Identifying a clinical target for hybrid closed-loop use. Diabetes Obes Metab 23(9):2048–2057. https://doi.org/10.1111/dom.14441
Cobry EC, Hamburger E, Jaser SS (2020) Impact of the hybrid closed-loop system on sleep and quality of life in youth with type 1 diabetes and their parents. Diabetes Technol Ther 22(11):794–800. https://doi.org/10.1089/dia.2020.0057
Cordero TL, Garg SK, Brazg R, Bailey TS, Shin J, Lee SW, Kaufman FR (2017) The effect of prior continuous glucose monitoring use on glycemic outcomes in the pivotal trial of the MiniMed™ 670G Hybrid closed-loop system. Diabetes Technol Ther 19(12):749–752. https://doi.org/10.1089/dia.2017.0208
de Bock M, Dart J, Roy A, Davey R, Soon W, Berthold C, Retterath A, Grosman B, Kurtz N, Davis E, Jones T (2017) Exploration of the performance of a hybrid closed loop insulin delivery algorithm that includes insulin delivery limits designed to protect against hypoglycemia. J Diabetes Sci Technol 11(1):68–73. https://doi.org/10.1177/1932296816668876
de Bock M, Dart J, Hancock M, Smith G, Davis EA, Jones TW (2018) Performance of Medtronic hybrid closed-loop iterations. results from a randomized trial in adolescents with type 1 diabetes. Diabetes Technol Ther 20(10):693–697. https://doi.org/10.1089/dia.2018.0161
Dominguez-Riscart J, Buero-Fernandez N, Garcia-Zarzuela A, Marmolejo-Franco FA, Perez-Guerrero AC, Lechuga-Sancho AM (2021) Hybrid closed-loop system achieves optimal perioperative glycemia in a boy with type 1 diabetes: a case report. Front Pediatr 9:625390. https://doi.org/10.3389/fped.2021.625390
Duffus SH, Ta’ani ZA, Slaughter JC, Niswender KD, Gregory JM (2020) Increased proportion of time in hybrid closed-loop “Auto Mode” is associated with improved glycaemic control for adolescent and young patients with adult type 1 diabetes using the MiniMed 670G insulin pump. Diabetes Obes Metab 22(4):688–693. https://doi.org/10.1111/dom.13912
Forlenza GP, Pinhas-Hamiel O, Liljenquist DR, Shulman DI, Bailey TS, Bode BW, Wood MA, Buckingham BA, Kaiserman KB, Shin J, Huang S, Lee SW, Kaufman FR (2019) Safety evaluation of the MiniMed 670G system in children 7–13 years of age with type 1 diabetes. Diabetes Technol Ther 21(1):11–19. https://doi.org/10.1089/dia.2018.0264
Forlenza GP, Ekhlaspour L, DiMeglio LA, Fox LA, Rodriguez H, Shulman DI, Kaiserman KB, Liljenquist DR, Shin J, Lee SW, Buckingham BA (2022) Glycemic outcomes of children 2–6 years of age with type 1 diabetes during the pediatric MiniMed™ 670G system trial. Pediatr Diabetes 23(3):324–329. https://doi.org/10.1111/pedi.13312
Nally LM, Wagner J, Sherr J, Tichy E, Weyman K, Ginley MK, Zajac K, Desousa M, Shabanova V, Petry NM, Tamborlane WV, Van Name M (2021) A Pilot study of youth with type 1 diabetes initiating use of a hybrid closed-loop system while receiving a behavioral economics intervention. Endocr Pract 27(6):545–551. https://doi.org/10.1016/j.eprac.2020.11.017
Petrovski G, Al Khalaf F, Hussain K, Campbell J (2018) Optimizing a hybrid closed loop system in type 1 diabetes: a case report. Diabetes Ther 9:2173–2177. https://doi.org/10.1007/s13300-018-0473-6
Petrovski G, Al Khalaf F, Campbell J, Hussain K, Fisher H, Umer F (2020) Glucose control during Ramadan fasting in a teenager with type 1 diabetes on MiniMed 670G hybrid closed-loop system. Acta Diabetol 57(1):105–107. https://doi.org/10.1007/s00592-019-01414-6
Petrovski G, Khalaf FA, Campbel J, Fisher H, Umer F, Hussain K (2020) 10-Day structured initiation protocol from multiple daily injection to hybrid closed-loop system in children and adolescents with type 1 diabetes. Acta Diabetol 57(6):681–687. https://doi.org/10.1007/s00592-019-01472-w
Petrovski G, Al Khalaf F, Campbell J, Umer F, Almajaly D, Hamdan M, Hussain K (2021) One-year experience of hybrid closed-loop system in children and adolescents with type 1 diabetes previously treated with multiple daily injections: drivers to successful outcomes. Acta Diabetol 58(2):207–213. https://doi.org/10.1007/s00592-020-01607-4
Petrovski G, Campbell J, Almajali D, Al Khalaf F, Hussain K (2021) Successful initiation of hybrid closed-loop system using virtual pump training program in a teenager with type 1 diabetes previously treated with multiple daily injections. J Diabetes Sci Technol 15(6):1394–1398. https://doi.org/10.1177/1932296820950753
Roberts A, Fried L, Dart J, de Bock M, Fairchild J, King B, Ambler GR, Cameron F, McAuley SA, Keech AC, Jenkins A, O Neal DN, Davis EA, Jones TW, Abraham MB, Australian JDRF Closed Loop Research Group (2022) Hybrid closed-loop therapy with a first-generation system increases confidence and independence in diabetes management in youth with type 1 diabetes. Diabet Med 39(9):e14907. https://doi.org/10.1111/dme.14907
Salehi P, Roberts AJ, Kim GJ (2019) Efficacy and safety of real-life usage of MiniMed 670G automode in children with type 1 diabetes less than 7 years old. Diabetes Technol Ther 21(8):448–451. https://doi.org/10.1089/dia.2019.0123
Stone PM, Agrawal P, Chen X, Liu M, Shin J, Cordero TL, Kaufman FR (2018) Retrospective analysis of 3-month real-world glucose data after the MiniMed 670G system commercial launch. Diabetes Technol Ther 20(10):689–692. https://doi.org/10.1089/dia.2018.0202
Varimo T, Pulkkinen MA, Hakonen E, Hero M, Miettinen PJ, Tuomaala AK (2021) First year on commercial hybrid closed-loop system-experience on 111 children and adolescents with type 1 diabetes. Pediatr Diabetes 22(6):909–915. https://doi.org/10.1111/pedi.13235
Tornese G, Ceconi V, Monasta L, Carletti C, Faleschini E, Barbi E (2020) Glycemic control in type 1 diabetes mellitus during covid-19 quarantine and the role of in-home physical activity. Diabetes Technol Ther 22(6):462–467. https://doi.org/10.1089/dia.2020.0169
von dem Berge T, Remus K, Biester S, Reschke F, Klusmeier B, Adolph K, Holtdirk A, Thomas A, Kordonouri O, Danne T, Biester T (2022) In-home use of a hybrid closed loop achieves time-in-range targets in preschoolers and school children: Results from a randomized, controlled, crossover trial. Diabetes Obes Metab 24(7):1319–1327. https://doi.org/10.1111/dom.14706
Wood MA, Shulman DI, Forlenza GP, Bode BW, Pinhas-Hamiel O, Buckingham BA, Kaiserman KB, Liljenquist DR, Bailey TS, Shin J, Huang S, Chen X, Cordero TL, Lee SW, Kaufman FR (2018) In-clinic evaluation of the MiniMed 670G system “suspend before low” feature in children with type 1 diabetes. Diabetes Technol Ther 20(11):731–737. https://doi.org/10.1089/dia.2018.0209
Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, Bosi E, Buckingham BA, Cefalu WT, Close KL, Cobelli C, Dassau E, DeVries JH, Donaghue KC, Dovc K, Doyle FJ 3rd, Garg S, Grunberger G, Heller S, Heinemann L, Hirsch IB, Hovorka R, Jia W, Kordonouri O, Kovatchev B, Kowalski A, Laffel L, Levine B, Mayorov A, Mathieu C, Murphy HR, Nimri R, Nørgaard K, Parkin CG, Renard E, Rodbard D, Saboo B, Schatz D, Stoner K, Urakami T, Weinzimer SA, Phillip M (2019) Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 42(8):1593–1603. https://doi.org/10.2337/dci19-0028
Bergenstal RM, Nimri R, Beck RW, Criego A, Laffel L, Schatz D, Battelino T, Danne T, Weinzimer SA, Sibayan J, Johnson ML, Bailey RJ, Calhoun P, Carlson A, Isganaitis E, Bello R, Albanese-O'Neill A, Dovc K, Biester T, Weyman K, Hood K, Phillip M, FLAIR Study Group (2021) A comparison of two hybrid closed-loop systems in adolescents and young adults with type 1 diabetes (FLAIR): a multicentre, randomised, crossover trial. Lancet 16;397(10270):208–219. https://doi.org/10.1016/S0140-6736(20)32514-9
Collyns OJ, Meier RA, Betts ZL, Chan DSH, Frampton C, Frewen CM, Hewapathirana NM, Jones SD, Roy A, Grosman B, Kurtz N, Shin J, Vigersky RA, Wheeler BJ, de Bock MI (2021) Improved glycemic outcomes with medtronic minimed advanced hybrid closed-loop delivery: results from a randomized crossover trial comparing automated insulin delivery with predictive low glucose suspend in people with type 1 diabetes. Diabetes Care 44(4):969–975. https://doi.org/10.2337/dc20-2250
Silva JD, Lepore G, Battelino T, Arrieta A, Castañeda J, Grossman B, Shin J, Cohen O (2022) Real-world performance of the MiniMed™ 780G System: first report of outcomes from 4120 users. Diabetes Technol Ther 24(2):113–119. https://doi.org/10.1089/dia.2021.0203
Arrieta A, Battelino T, Scaramuzza AE, Da Silva J, Castañeda J, Cordero TL, Shin J, Cohen O (2022) Comparison of MiniMed 780G system performance in users aged younger and older than 15 years: evidence from 12 870 real-world users. Diabetes Obes Metab 24(7):1370–1379. https://doi.org/10.1111/dom.14714
Brown SA, Kovatchev BP, Raghinaru D, Lum JW, Buckingham BA, Kudva YC, Laffel LM, Levy CJ, Pinsker JE, Wadwa RP, Dassau E, Doyle FJ 3rd, Anderson SM, Church MM, Dadlani V, Ekhlaspour L, Forlenza GP, Isganaitis E, Lam DW, Kollman C, Beck RW, iDCL Trial Research Group (2019) Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 381(18):1707–1717. https://doi.org/10.1056/NEJMoa1907863
Breton MD, Kanapka LG, Beck RW, Ekhlaspour L, Forlenza GP, Cengiz E, Schoelwer M, Ruedy KJ, Jost E, Carria L, Emory E, Hsu LJ, Oliveri M, Kollman CC, Dokken BB, Weinzimer SA, DeBoer MD, Buckingham BA, Cherñavvsky D, Wadwa RP; iDCL Trial Research Group (2020) A randomized trial of closed-loop control in children with type 1 diabetes. N Engl J Med 383(9):836–845. https://doi.org/10.1056/NEJMoa2004736
Kanapka LG, Wadwa RP, Breton MD, Ruedy KJ, Ekhlaspour L, Forlenza GP, Cengiz E, Schoelwer MJ, Jost E, Carria L, Emory E, Hsu LJ, Weinzimer SA, DeBoer MD, Buckingham BA, Oliveri M, Kollman C, Dokken BB, Cherñavvsky D, Beck RW; iDCL Trial Research Group (2021) Extended use of the control-IQ closed-loop control system in children with type 1 diabetes. Diabetes Care 44(2):473–478. https://doi.org/10.2337/dc20-1729
Breton MD, Kovatchev BP (2021) One year real-world use of the control-IQ advanced hybrid closed-loop technology. Diabetes Technol Ther 23(9):601–608. https://doi.org/10.1089/dia.2021.0097
Kariyawasam D, Morin C, Casteels K, Le Tallec C, Sfez A, Godot C, Huneker E, Garrec N, Benhamou PY, Polak M, Charpentier G, Franc S, Beltrand J (2022) Hybrid closed-loop insulin delivery versus sensor-augmented pump therapy in children aged 6–12 years: a randomised, controlled, cross-over, non-inferiority trial. Lancet Digit Health 4(3):e158–e168. https://doi.org/10.1016/S2589-7500(21)00271-5
Ware J, Boughton CK, Allen JM, Wilinska ME, Tauschmann M, Denvir L, Thankamony A, Campbell FM, Wadwa RP, Buckingham BA, Davis N, DiMeglio LA, Mauras N, Besser REJ, Ghatak A, Weinzimer SA, Hood KK, Fox DS, Kanapka L, Kollman C, Sibayan J, Beck RW, Hovorka R; DAN05 Consortium (2022) Cambridge hybrid closed-loop algorithm in children and adolescents with type 1 diabetes: a multicentre 6-month randomised controlled trial. Lancet Digit Health 4(4):e245–e255. https://doi.org/10.1016/S2589-7500(22)00020-6
Tauschmann M, Thabit H, Bally L, Allen JM, Hartnell S, Wilinska ME, Ruan Y, Sibayan J, Kollman C, Cheng P, Beck RW, Acerini CL, Evans ML, Dunger DB, Elleri D, Campbell F, Bergenstal RM, Criego A, Shah VN, Leelarathna L, Hovorka R; APCam11 Consortium (2018) Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet 392(10155):1321–1329. https://doi.org/10.1016/S0140-6736(18)31947-0. Erratum. In: Lancet 2018;392(10155):1310
Ware J, Allen JM, Boughton CK, Wilinska ME, Hartnell S, Thankamony A, de Beaufort C, Schierloh U, Fröhlich-Reiterer E, Mader JK, Kapellen TM, Rami-Merhar B, Tauschmann M, Nagl K, Hofer SE, Campbell FM, Yong J, Hood KK, Lawton J, Roze S, Sibayan J, Bocchino LE, Kollman C, Hovorka R; KidsAP Consortium (2022) Randomized trial of closed-loop control in very young children with type 1 diabetes. N Engl J Med 386(3):209–219. https://doi.org/10.1056/NEJMoa2111673
Brown SA, Forlenza GP, Bode BW, Pinsker JE, Levy CJ, Criego AB, Hansen DW, Hirsch IB, Carlson AL, Bergenstal RM, Sherr JL, Mehta SN, Laffel LM, Shah VN, Bhargava A, Weinstock RS, MacLeish SA, DeSalvo DJ, Jones TC, Aleppo G, Buckingham BA, Ly TT; Omnipod 5 Research Group (2021) Multicenter trial of a tubeless, on-body automated insulin delivery system with customizable glycemic targets in pediatric and adult participants with type 1 diabetes. Diabetes Care 44(7):1630–1640. https://doi.org/10.2337/dc21-0172
Messer LH, Berget C, Vigers T, Pyle L, Geno C, Wadwa RP, Driscoll KA, Forlenza GP (2020) Real world hybrid closed-loop discontinuation: Predictors and perceptions of youth discontinuing the 670G system in the first 6 months. Pediatr Diabetes 21(2):319–327. https://doi.org/10.1111/pedi.12971
Lal RA, Basina M, Maahs DM, Hood K, Buckingham B, Wilson DM (2019) One year clinical experience of the first commercial hybrid closed-loop system. Diabetes Care 42(12):2190–2196. https://doi.org/10.2337/dc19-0855
Roze S, Buompensiere MI, Ozdemir Z, de Portu S, Cohen O (2021) Cost-effectiveness of a novel hybrid closed-loop system compared with continuous subcutaneous insulin infusion in people with type 1 diabetes in the UK. J Med Econ 24(1):883–890. https://doi.org/10.1080/13696998.2021.193970
Author information
Authors and Affiliations
Contributions
Chiara Mameli: desing and analysis; Giulia Maria Smylie: data collection, analysis and writing manuscript; Alessio Galati: data collection, analysis and writing manuscript; Biagio Rapone: analysis; Roque Cardona-Hernandes: writing manuscript; Gianvincenzo Zuccotti: conduct and writing manuscript; Maurizio Delvecchio: design, conduct and writing manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Peter de Winter.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mameli, C., Smylie, G.M., Galati, A. et al. Safety, metabolic and psychological outcomes of Medtronic MiniMed 670G in children, adolescents and young adults: a systematic review. Eur J Pediatr 182, 1949–1963 (2023). https://doi.org/10.1007/s00431-023-04833-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-023-04833-4