Abstract
Reliably assessing the early neurodevelopmental outcomes in infants with neonatal encephalopathy (NE) is of utmost importance to advise parents and implement early and personalized interventions. We aimed to evaluate the accuracy of neuroimaging modalities, including functional magnetic resonance imaging (fMRI) in predicting neurodevelopmental outcomes in NE. Eighteen newborns with NE due to presumed perinatal asphyxia (PA) were included in the study, 16 of whom underwent therapeutic hypothermia. Structural magnetic resonance imaging (MRI), and fMRI during passive visual, auditory, and sensorimotor stimulation were acquired between the 10th and 14th day of age. Clinical follow-up protocol included visual and auditory evoked potentials and a detailed neurodevelopmental evaluation at 12 and 18 months of age. Infants were divided according to sensory and neurodevelopmental outcome: severe, moderate disability, or normal. Structural MRI findings were the best predictor of severe disability with an AUC close to 1.0. There were no good predictors to discriminate between moderate disability versus normal outcome. Nevertheless, structural MRI measures showed a significant correlation with the scores of neurodevelopmental assessments. During sensorimotor stimulation, the fMRI signal in the right hemisphere had an AUC of 0.9 to predict absence of cerebral palsy (CP). fMRI measures during auditory and visual stimulation did not predict sensorineural hearing loss or cerebral visual impairment.
Conclusion: In addition to structural MRI, fMRI with sensorimotor stimulation may open the gate to improve the knowledge of neurodevelopmental/motor prognosis if proven in a larger cohort of newborns with NE.
What is Known: |
• Establishing an early, accurate neurodevelopmental prognosis in neonatal encephalopathy remains challenging. |
• Although structural MRI has a central role in neonatal encephalopathy, advanced MRI modalities are gradually being explored to optimize neurodevelopmental outcome knowledge. |
What is New: |
• Newborns who later developed cerebral palsy had a trend towards lower fMRI measures in the right sensorimotor area during sensorimotor stimulation. |
• These preliminary fMRI results may improve future early delineation of motor prognosis in neonatal encephalopathy. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neonatal encephalopathy (NE) succeeding perinatal asphyxia (PA) contributes considerably to poor neurodevelopmental outcomes [1]. Possible long-term neurodevelopmental sequelae, among survivors, include cerebral palsy (CP), intellectual disabilities, epilepsy, vision, and hearing impairments. Despite early therapeutic hypothermia (TH), current results in high-income countries reveal that the CP rate has remained unchanged around 20% [1; 2], while hearing and visual impairments occur less frequently [2]. It is well recognized that neonates with severe NE at birth have a higher probability of death or disabling neurological and cognitive deficits. However, the prognosis of newborns with mild and moderate NE is more variable, with milder motor deficits and a broader spectrum of cognitive impairments, making prediction more difficult [1, 3]. Therefore, establishing an accurate early neurodevelopmental prognosis in newborns with brain injury, especially in those who will develop a moderate disability, remains a challenging task in the neonatal intensive care unit. Although structural magnetic resonance imaging (MRI) is a robust predictor of neurodevelopmental outcome in newborns with NE due to presumed PA, irrespective of TH [4], it has recently been demonstrated that spectroscopy may have a better accuracy, but requires expertise and harmonization [5]. Hence, currently structural MRI remains the most widely used technique, as it allows to characterize the degree and pattern of brain lesions. However, it is often insufficient to predict long-term disabilities such as fine motor, social, behavioral, sensorial, and cognitive deficits, in particular in mild to moderate cases of NE [6]. Thus, new methods to assess brain function early to optimize prognostic information are lacking [7]. Functional magnetic resonance imaging (fMRI), better established in adults that also requires expertise, could potentially be considered the technique of choice for functional assessment of the newborn brain [7]. We hypothesize that fMRI might identify early brain function changes in neonates with NE related to neurodevelopmental impairments before clinical evidence of pathological signs. If this potential holds true, it could be of extreme importance, given that neuroplasticity and brain adaptability are well documented as most modifiable in neonates [8]. Identifying, as early as possible, functionally affected systems offer considerable likelihood for optimizing brain outcomes by implementing intensive and targeted psychoeducational and therapeutic interventions. The main objective of this study was to evaluate the accuracy of neuroimaging modalities, including fMRI, to predict severe or moderate disability at 18 months of age in newborns with NE due to presumed PA. Additionally, we intend to specifically relate blood-oxygen-level-dependent (BOLD) signal changes, measured with fMRI, during visual, auditory, and sensorimotor stimulation, with detailed sensory and neurodevelopmental function at 12 and 18 months of age, respectively.
Materials and methods
Participants
An observational, exploratory study with prospective data collection was conducted. All procedures have been carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. Informed written consent was obtained from parents of all participants after a full verbal and written explanation of the study. The study was approved by the Ethics Committee of the Faculdade de Medicina da Universidade de Coimbra, Portugal (Reference CE-029–2014). All newborns admitted to the pediatric intensive care unit (PICU) of a tertiary pediatric hospital from Centro Hospitalar e Universitário de Coimbra, Portugal, born at or after 36 gestational weeks, with NE due to presumed PA, defined according to the American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy, 2014 criteria [9], were recruited between April 2016 and March 2017. Exclusion criteria were presence of major congenital abnormalities and inherited inborn errors of metabolism or stroke. Neonatal data collection was retrieved from the PICU electronic clinical database and comprised parents and neonatal characteristics, assessment, evolution, and treatment. Socio-economic status of parents was categorized according to the International Standard Classification of Education (ISCED) and the Portuguese version for European Deprivation Index (EDI-PT) [10]. The NE was characterized with the Modified Sarnat and Thompson scores, applied on admission [11, 12]. The amplitude-integrated electroencephalography (aEEG) worst background pattern at admission and 48 to 72 h of age was classified according to Hellstrom-Westas, 2006 [13].
MRI data acquisition
MRI data were acquired at a mean age of 12 ± 3 days of age, using a 3 T scanner (Siemens AG, Healthcare, Erlangen, Germany), with a 20-channel head coil. Foam cushions and sedation with intravenous midazolam or propofol were used to minimize head motion. The MRI protocol included a 3D T1-weighted MPRAGE (0.83-mm isotropic voxel, 160 slices, TR/TE = 2300/3.5 ms), a 3D T2-weighted SPACE (0.83-mm isotropic voxel, 160 slices, TR/TE = 3200/443 ms), diffusion-weighted imaging (DWI), and three fMRI T2*-weighted sequences sensitive to BOLD (blood-level-oxygen-dependent) contrast (TR/TE = 2080/31 ms, voxel size = 2 × 2 × 2 mm3, 29 axial slices (whole-brain coverage), FOV = 256 × 256 mm2, FA = 90°). Continuous monitoring of the newborns inside the scanner was provided by an intensive care pediatrician and nurse.
Structural MRI grading system of brain injury
Brain lesions characteristic of NE due to presumed PA were graded by two expert neuroradiologists using T1, T2, and DWI sequences, according to a recently reported scoring system [14].
Functional stimulation paradigm
Newborns were submitted to passive visual [7], auditory [15], and sensorimotor stimulation [16], delivered in an optimized block design paradigm [17] in separate runs, described in detail in Online Resource 1.
fMRI data preprocessing
Data were processed using BrainVoyager version 21.2 (Brain Innovation, Maastricht, The Netherlands), and the FMRIB Software Library (FSL) version 4.1.8. fMRI volumes were corrected for motion using rigid transformations and motion parameters were included in data analysis [18]. Motion outliers were accounted through scrubbing of pairs of volumes with > 0.5 mm of translation or 0.5° of rotation between them. We applied slice-scanning time correction, linear trends removal, and temporal high-pass filtering (5 cycles per run). We then performed anatomical-functional registration and finally applied slight spatial smoothing (full-width half-maximum kernel with 3 mm) to functional data.
fMRI subject-level data analysis
A whole-brain voxel-wise general linear model (GLM) was used to estimate the BOLD response to visual, auditory, or sensorimotor stimulation, using a two-gamma hemodynamic response function adapted for term infants [19]. While using a less conservative threshold of p < 0.05, as previously suggested for newborn fMRI studies [7], to compensate for multiple comparisons, only BOLD signal changes in voxels located in primary visual, auditory, or sensorimotor areas identified by visual inspection were considered, and regions of interest (ROIs) were defined as the set of activated voxels within these local anatomical sensitive search spaces.
Sensory assessment at 12 months
The formal auditory and visual assessment included brainstem auditory and visual evoked potentials, described in Online Resource 2 and 3.
Neurodevelopmental assessment at 18 months
Neurodevelopmental and clinical assessment was performed at a mean age of 19 ± 3 months by an experienced neurodevelopment team (neurodevelopmental pediatrician and a psychologist). Besides a detailed classical clinical (including growth evaluation) and neurological examination, these children were submitted to the following assessment tools, currently used in the neurodevelopment unity: the Griffiths Mental Development Scales (GMDS) [20], the Vineland Adaptive Behavior Scales (VABS) [21], and the Modified Checklist for Autism in Toddlers (M-CHAT) [22] for autism spectrum disorders (ASD) screening. Children with a positive M-CHAT, and according to clinical judgment, performed a direct structured proband instrument observation, the Autism Diagnostic Observation Schedule (ADOS), to confirm ASD suspected diagnosis [23]. CP was diagnosed using the clinical criteria from the European Network of Cerebral Palsy [24]. The Gross Motor Function Classification System (GMFCS) for CP classification was also applied. The neurological examination performed was standardized according to the Hammersmith Infant Neurological Examination (HINE) [25]. Epilepsy was diagnosed using the International League Against Epilepsy clinical definition [26]. Microcephaly was defined as head circumference more than two standard deviations (SD) below the mean for age and sex (i.e., less than the 3rd percentile) in the WHO growth charts.
Outcomes at 18 months
Neurodevelopmental outcomes at 18 months of age were classified into three categories:
-
Severe disability, defined if one of the following: (1) death; (2) global developmental quotient (DQ) in GMDS or VABS (total score, adaptive behavior composite - ABC), of 70 or below; (3) CP with a GMFCS 3–5; (4) cerebral visual impairment; (5) sensorineural hearing loss requiring amplification; (6) epilepsy (requiring anticonvulsant therapy at time of assessment); (7) confirmed ASD diagnosis.
-
Moderate disability, defined if one of the following: (1) GMDS (global DQ) or VABS (total score, ABC) between 70 and 84; (2) CP with GMF 1–2.
-
Normal, if GMDS (global DQ) or VABS (total score, ABC) above 84 and no clinical neurological, or neurodevelopmental sequelae at the time of assessment.
Statistical analysis
The IBM-SPSS® software version 27 was used. A level of statistical significance of 5% was considered. A univariate analysis was made, in which central tendency and dispersion measures were calculated for quantitative variables, and absolute and relative frequencies were determined for qualitative variables. Quantitative variables were first checked for normality using the Kolmogorov–Smirnov tests. Receiver operating characteristic (ROC) curves were performed to evaluate the accuracy of neuroimaging biomarkers to predict outcome. The power of the predictors to discriminate was quantified by the area under the ROC curve (AUC). Independent Student’s t test or Mann–Whitney U test were performed as appropriate to compare quantitative variables. Additionally, Spearman correlations were used to evaluate the relationship between quantitative variables without normal distribution.
Results
During the study period, 20 newborns were eligible, of whom two were excluded: one died on the fifth day of age due to an adverse prognosis and redirecting of care and, and the other, had an inherited inborn error of metabolism. The demographic and clinical characteristics of the 18 newborns and their parents are described in Table 1. Parameters regarding PICU assessment, evolution, and treatment are summarized in Table 2. Sixteen newborns were submitted to TH. Regarding structural MRI, the median of the total score was 2 (IQR 1 to 6.75), the median of deep grey matter subscore was 0 (IQR 0 to 1.5), and the median of white matter/cortex subscore was 0 (IQR 0 to 5.25). No lesions in the cerebellum were observed. Three newborns had a total score of 0, four newborns had a total score of 1, scored in the additional subscore due to subdural hemorrhages, and in four newborns the total score ranged from 15 to 26, all of whom had lesions in the thalamus/basal ganglia, posterior limb of the internal capsule, or perirolandic cortex. Regarding fMRI, the summary measures of brain responses during visual, auditory, and sensorimotor stimulation from each newborn can be observed in Online Resources 4. No differences were observed in fMRI BOLD responses in newborns sedated with midazolam or propofol (Online Resources 5). Data from clinical and neurodevelopmental evaluation are summarized in Table 3 and in Online Resources 6. The median of HINE at 18 months of age was 78 (IQR 70 to 78) and five had a suboptimal score (< 74), four of whom had the highest punctuation in MRI deep gray matter subscore (6 to 15). Concerning children with positive score for ASD screening, M-CHAT (n = 5), four had CP or severe global psychomotor delay (GMDS global DQ or VABS total score < 70), and in one child ADOS was conducted, which ruled out ASD.
In Table 4, the accuracy of neuroimaging modalities to predict moderate and/or severe disability at 18 months can be acknowledged after application of ROC curves. Overall, the fMRI measurements were not good predictors of outcome. Nevertheless, the AUC of BOLD signal (% signal change) in the right hemisphere during sensorimotor stimulation was 0.9 (95% CI: 0.694–1.0; p = 0.086) to predict absence of CP (Online Resources 7). The mean of BOLD (% signal change) in the right hemisphere during sensorimotor stimulation was − 0.1275 and 0.7819 in patients with and without CP, respectively (t = 1.6; p = 0.141; Cohen’s d effect size = 1.239).
The significant relationships between the scores GMDS (total or subscales) and the structural MRI score can be observed in Fig. 1. Additionally, a positive Spearman correlation was found between the right brain hemisphere fMRI BOLD signal during sensorimotor stimulation and the GMDS locomotor subscale (Rho = 0.784; p = 0.003; 95% CI 0.413 to 0.956) or the HINE (Rho = 0.652; p = 0.022; 95% CI 0.262 to 0.890) at 18 months.
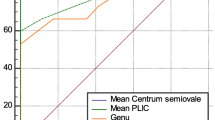
Correlation between GMDS and structural MRI score. Spearman correlation between the global developmental quotient (a), sub-quotient of the locomotor subscale (b), sub-quotient of the personal-social subscale (c) from GMDS and the total score of structural MRI. Global developmental quotient and sub-quotients are standardized to a mean of 100 and a standard deviation of 15. The higher the value, the higher mental development. The total score of structural MRI ranges from 0 to 55 points. The total score has a predictive value for outcome at 2 years and school age. The higher the score, the worse the prognosis. CI, confidence intervals; GMDS, Griffiths Mental Development Scales; MRI, magnetic resonance imaging
Discussion
In our study, the best early predictor of severe disability at 18 months of age, with excellent performance (AUC near 1.0), was structural MRI, measured with the application of the Weeke score for classification of HIE brain lesions, despite performed near the time of DWI pseudonormalization. Its accuracy in predicting jointly moderate and severe neurodevelopmental disabilities relative to normal outcomes was lower, however reaching an AUC of 0.806. The structural MRI score also showed a high negative correlation (Rho Spearman from − 0.713 to − 0.891) with global DQ of GMDS and the sub-quotients from its locomotor and personal-social domains (Fig. 1). Although these results should be interpreted with caution, given the characteristics and small sample size, it is similar to that reported by Weeke et al. in one of the studied cohorts (AUC 0.988 for gray matter subscore) [14]. Different structural MRI scores to classify brain NE lesions due to presumed PA were proved to be highly predictive of outcomes at 24 months [27]. Some of those grading systems are based on categorizing defined brain injury patterns, so they are easy to apply. However, it is challenging to classify brain lesions in infants when they are not adequately included in the standard NE patterns. Accordingly, a current study exposes the limitations of the qualitative interpretation of structural MRI patterns [28]. The recently developed Weeke score [14] we used assesses all relevant brain areas separately and applies an item-based system; it can improve the detection of slight injury in mild NE [29] and has better accuracy and comparable inter-rater reliability relative to other item-based scoring systems [27].
Although we were unable to identify biomarkers that distinguish moderate neurodevelopmental disability from normal outcomes at 18 months of age, which is challenging, we observed a negative correlation between structural MRI total score and the results of neurodevelopmental assessment instruments that can improve the knowledge of the wide prognosis range in newborns with NE.
One of our main and innovative goals was to determine if fMRI can improve the capacity of structural MRI to predict neurodevelopmental outcomes, including potential damage to motor, visual, or auditory pathways. It is well recognized that structural MRI is a good predictor of severe CP, namely if severe basal ganglia/thalamus lesions or a clear abnormal posterior limb of the internal capsule signal are observed [30]. Nevertheless, it may not be as accurate in mild cases of CP [31]. We observed that fMRI measures (mean of BOLD % signal change) in the right brain hemisphere during sensorimotor stimulation were good predictors of CP absence. Children with CP had a trend for lower mean values of BOLD signal when compared to others, although without reaching statistical significance, probably due to the small sample size. Additionally, a positive significant correlation was established between those fMRI measures and the HINE or locomotor subscale of GMDS at 18 months of age. If replicated in larger-scale studies, these results may improve the ability to predict CP in newborns with NE before clinical evidence and promote early targeted educational interventions. We hypothesized that the negative responses observed in the right sensorimotor area of the newborns who later develop CP might be related to oxygen consumption associated with possible synaptogenesis due to brain plasticity and motor reorganization [8, 32]. These mechanisms might also explain why this relationship was only observed in the right hemisphere, in addition to the eventual maturational asymmetry, cortical hemisphere preference, or influence of the stimulus type.
Although the literature on fMRI in newborns with brain injury is still scarce, these findings are supported by a study performed on newborns with perinatal brain lesions, including NE due to presumed PA, which concluded that the CP group had reduced functional connectivity from the right supplementary motor areas when compared with the non-CP group [33]. However, they did not find any difference between the groups in brain activation during a motor task like the one used in our study.
We could not observe differences in standard brain responses during visual and auditory stimulation in newborns with cerebral visual impairment and sensorineural hearing loss when compared with others. These results can be explained due to the inconsistency of reports observed in previous fMRI studies in newborns, the difficulty of normalizing functional activity data or eventually being influenced by sedation used to avoid head motion. It is well recognized that neonatal fMRI studies observed adult-like positive BOLD responses, but also temporally delayed peak BOLD responses or smaller and negative responses [7, 19, 34, 35]. Possible explanations for this variability are the maturity level of neurovascular coupling and of autoregulation mechanisms and different cerebral oxygen metabolic rates in newborns [36]. Despite our efforts to analyze fMRI data with an age-appropriate hemodynamic response function, the best way to detect BOLD signals using task-based fMRI in newborns is not yet fully understood [19]. Furthermore, when using fMRI in newborns with brain injury, specific methodological aspects become more significant and should be considered [37]. Another contributing factor for the lack of power of fMRI analysis may be the predominant involvement of the white matter and thalamus, more than the cortex itself, in the hypoxic-ischemic perinatal insult, highly correlated with neurodevelopmental impairment, especially with motor and visual function [38,39,40]. Thus, normal primary visual cortex or normal optic radiations do not rule out the possibility of an abnormal visual function [40]. Conversely, reduced activation in the occipital cortex and functional connectivity in fMRI during visual stimulation was found in infants with perinatal brain injury [41].
The limitations of this study include a small sample of newborns with NE and a small number of those with CP, cerebral visual impairment, and sensorineural hearing loss, restricting the inference and generalization of results. The maturity level of the local neurovascular coupling underlying the BOLD signal can be potentially compromised in this population of high-risk newborns, precluding a good model of the hemodynamic response. Using a bilateral task for sensorimotor stimulation, instead of an alternating unilateral one, prevents us from thoroughly assessing lateralization and eventually achieving a better comprehension of motor injury and its reorganization. Additionally, the effects of the sedation used to avoid head motion on brain function cannot be entirely excluded.
However, this study has a number of strengths: use of a structured item-based system for classifying brain injury due to presumed PA; a comprehensive and detailed assessment of the neurodevelopmental outcome measures and infant’s needs, optimized with the use of complementary instruments, including VABS, and the multidisciplinary approach comprising otorhinolaryngology and ophthalmology evaluation; inclusion of the challenging fMRI application as a potential tool to improve motor injury prediction in the future.
Conclusion
This exploratory and challenging outcome study of newborns with NE due to presumed PA strengthens structural MRI’s relevance to predict neurodevelopmental impairment. The still unmet possibility of fMRI providing a wealth of new information about the integration of functional cerebral activity in NE can be achieved through better defining normal inter-individual variability and tailored hemodynamic response function. Nevertheless, our fMRI results during sensorimotor stimulation open the door to refining future motor function characterization, allowing accurate prognostic information and early specific and precise therapeutic rehabilitation interventions.
Abbreviations
- ABC:
-
Adaptive Behavior Composite score
- ADOS:
-
Autism Diagnostic Observation Schedule
- aEEG:
-
Amplitude-integrated electroencephalography
- ASD:
-
Autism spectrum disorder
- AUC:
-
Area under the curve
- BOLD:
-
Blood-oxygen-level-dependent
- CI:
-
Confidence interval
- CP:
-
Cerebral palsy
- DQ:
-
Developmental quotient
- DWI:
-
Diffusion-weighted imaging
- EDI-PT:
-
Portuguese version for European Deprivation Index
- fMRI:
-
Functional magnetic resonance imaging
- GLM:
-
General linear model
- GMDS:
-
Griffiths Mental Development Scales
- GMFCS:
-
Gross Motor Function Classification System
- HIE:
-
Hypoxic-ischemic encephalopathy
- HINE:
-
Hammersmith Infant Neurological Examination
- IQR:
-
Interquartile range
- ISCED:
-
International Standard Classification of Education
- M-CHAT:
-
Modified Checklist for Autism in Toddlers
- MRI:
-
Magnetic resonance imaging
- NE:
-
Neonatal encephalopathy
- PA:
-
Perinatal asphyxia
- PICU:
-
Pediatric intensive care unit
- ROC:
-
Receiver operating characteristics
- ROI:
-
Region of interest
- SD:
-
Standard deviation
- TH:
-
Therapeutic hypothermia
- VABS:
-
Vineland Adaptive Behavior Scales
References
Pappas A, Korzeniewski SJ (2016) Long-term cognitive outcomes of birth asphyxia and the contribution of identified perinatal asphyxia to cerebral palsy. Clin Perinatol 43:559–572
Shankaran S, Laptook AR, Pappas A, McDonald SA, Das A, Tyson JE, Poindexter BB et al (2017) Effect of depth and duration of cooling on death or disability at age 18 months among neonates with hypoxic-ischemic encephalopathy: a randomized clinical trial. JAMA 318:57–67
Schreglmann M, Ground A, Vollmer B, Johnson MJ (2020) Systematic review: long-term cognitive and behavioural outcomes of neonatal hypoxic-ischaemic encephalopathy in children without cerebral palsy. Acta Paediatr 109:20–30
Sanchez Fernandez I, Morales-Quezada JL, Law S, Kim P (2017) Prognostic value of brain magnetic resonance imaging in neonatal hypoxic-ischemic encephalopathy: a meta-analysis. J Child Neurol 32:1065–1073
Lally PJ, Montaldo P, Oliveira V, Soe A, Swamy R, Bassett P, Mendoza J et al (2019) Magnetic resonance spectroscopy assessment of brain injury after moderate hypothermia in neonatal encephalopathy: a prospective multicentre cohort study. Lancet Neurol 18:35–45
Walsh BH, Inder TE (2018) MRI as a biomarker for mild neonatal encephalopathy. Early Hum Dev 120:75–79
Seghier ML, Lazeyras F, Huppi PS (2006) Functional MRI of the newborn. Semin Fetal Neonatal Med 11:479–488
Staudt M (2010) Brain plasticity following early life brain injury: insights from neuroimaging. Semin Perinatol 34:87–92
(2014) Executive summary: neonatal encephalopathy and neurologic outcome, second edition. Report of the American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy. Obstet Gynecol 123:896–901
Ribeiro AI, Launay L, Guillaume E, Launoy G, Barros H (2018) The Portuguese version of the European Deprivation Index: development and association with all-cause mortality. PLoS ONE 13:e0208320
Shalak LF, Laptook AR, Velaphi SC, Perlman JM (2003) Amplitude-integrated electroencephalography coupled with an early neurologic examination enhances prediction of term infants at risk for persistent encephalopathy. Pediatrics 111:351–357
Thompson CM, Puterman AS, Linley LL, Hann FM, van der Elst CW, Molteno CD, Malan AF (1997) The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr 86:757–761
Hellstrom-Westas L, Rosen I (2006) Continuous brain-function monitoring: state of the art in clinical practice. Semin Fetal Neonatal Med 11:503–511
Weeke LC, Groenendaal F, Mudigonda K, Blennow M, Lequin MH, Meiners LC, van Haastert IC, Benders MJ, Hallberg B, de Vries LS (2018) A novel magnetic resonance imaging score predicts neurodevelopmental outcome after perinatal asphyxia and therapeutic hypothermia. J Pediatr 192(33–40):e32
Anderson AW, Marois R, Colson ER, Peterson BS, Duncan CC, Ehrenkranz RA, Schneider KC, Gore JC, Ment LR (2001) Neonatal auditory activation detected by functional magnetic resonance imaging. Magn Reson Imaging 19:1–5
Heep A, Scheef L, Jankowski J, Born M, Zimmermann N, Sival D, Bos A, Gieseke J, Bartmann P, Schild H, Boecker H (2009) Functional magnetic resonance imaging of the sensorimotor system in preterm infants. Pediatrics 123:294–300
Cusack R, Wild C, Linke AC, Arichi T, Lee DS, Han VK (2015) Optimizing stimulation and analysis protocols for neonatal fMRI. PLoS ONE 10:e0120202
Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R (1996) Movement-related effects in fMRI time-series. Magn Reson Med 35:346–355
Arichi T, Fagiolo G, Varela M, Melendez-Calderon A, Allievi A, Merchant N, Tusor N, Counsell SJ, Burdet E, Beckmann CF, Edwards AD (2012) Development of BOLD signal hemodynamic responses in the human brain. Neuroimage 63:663–673
(1984) The abilities of young children. University of London Press, London
Sparrow SBD, Cicchetti D (1984) Vineland adaptative behaviour scales: interview edition, survey form. American Guidance Service, Circle Pines, MN
Robins DL, Fein D, Barton ML, Green JA (2001) The Modified Checklist for Autism in Toddlers: an initial study investigating the early detection of autism and pervasive developmental disorders. J Autism Dev Disord 31:131–144
Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, Schopler E (1989) Autism diagnostic observation schedule: a standardized observation of communicative and social behavior. J Autism Dev Disord 19:185–212
Surveillance of Cerebral Palsy in E, (2000) Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Surveillance of Cerebral Palsy in Europe (SCPE). Dev Med Child Neurol 42:816–824
Haataja L, Mercuri E, Regev R, Cowan F, Rutherford M, Dubowitz V, Dubowitz L (1999) Optimality score for the neurologic examination of the infant at 12 and 18 months of age. J Pediatr 135:153–161
Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, Engel J Jr, Forsgren L, French JA, Glynn M, Hesdorffer DC, Lee BI, Mathern GW, Moshe SL, Perucca E, Scheffer IE, Tomson T, Watanabe M, Wiebe S (2014) ILAE official report: a practical clinical definition of epilepsy. Epilepsia 55:475–482
Langeslag JF, Groenendaal F, Roosendaal SD, de Vries LS, Onland W, Leeflang MMG, Groot PFC, van Kaam AH, de Haan TR, PharmaCool Study G (2022) Outcome prediction and inter-rater comparison of four brain magnetic resonance imaging scoring systems of infants with perinatal asphyxia and therapeutic hypothermia. Neonatology 119:311–319
Laptook AR, Shankaran S, Barnes P, Rollins N, Do BT, Parikh NA, Hamrick S et al (2021) Limitations of conventional magnetic resonance imaging as a predictor of death or disability following neonatal hypoxic-ischemic encephalopathy in the late hypothermia trial. J Pediatr 230(106–111):e106
Machie M, Weeke L, de Vries LS, Rollins N, Brown L, Chalak L (2021) MRI score ability to detect abnormalities in mild hypoxic-ischemic encephalopathy. Pediatr Neurol 116:32–38
Martinez-Biarge M, Diez-Sebastian J, Kapellou O, Gindner D, Allsop JM, Rutherford MA, Cowan FM (2011) Predicting motor outcome and death in term hypoxic-ischemic encephalopathy. Neurology 76:2055–2061
Thoresen M, Jary S, Walloe L, Karlsson M, Martinez-Biarge M, Chakkarapani E, Cowan FM (2021) MRI combined with early clinical variables are excellent outcome predictors for newborn infants undergoing therapeutic hypothermia after perinatal asphyxia. EClinicalMedicine 36:100885
Batschelett M, Gibbs S, Holder CM, Holcombe B, Wheless JW, Narayana S (2022) Plasticity in the developing brain: neurophysiological basis for lesion-induced motor reorganization. Brain Commun 4:fcab300
Merhar SL, Gozdas E, Tkach JA, Parikh NA, Kline-Fath BM, He L, Yuan W, Altaye M, Leach JL, Holland SK (2020) Neonatal functional and structural connectivity are associated with cerebral palsy at two years of age. Am J Perinatol 37:137–145
Lee W, Donner EJ, Nossin-Manor R, Whyte HE, Sled JG, Taylor MJ (2012) Visual functional magnetic resonance imaging of preterm infants. Dev Med Child Neurol 54:724–729
Allievi AG, Arichi T, Tusor N, Kimpton J, Arulkumaran S, Counsell SJ, Edwards AD, Burdet E (2016) Maturation of sensori-motor functional responses in the preterm brain. Cereb Cortex 26:402–413
Kozberg M, Hillman E (2016) Neurovascular coupling and energy metabolism in the developing brain. Prog Brain Res 225:213–242
Seghier ML, Huppi PS (2010) The role of functional magnetic resonance imaging in the study of brain development, injury, and recovery in the newborn. Semin Perinatol 34:79–86
Dibble M, O’Dea MI, Hurley T, Byrne A, Colleran G, Molloy EJ, Bokde ALW (2020) Diffusion tensor imaging in neonatal encephalopathy: a systematic review. Arch Dis Child Fetal Neonatal Ed 105:480–488
Martinez-Biarge M, Bregant T, Wusthoff CJ, Chew AT, Diez-Sebastian J, Rutherford MA, Cowan FM (2012) White matter and cortical injury in hypoxic-ischemic encephalopathy: antecedent factors and 2-year outcome. J Pediatr 161:799–807
Mercuri E, Anker S, Guzzetta A, Barnett AL, Haataja L, Rutherford M, Cowan F, Dubowitz L, Braddick O, Atkinson J (2004) Visual function at school age in children with neonatal encephalopathy and low Apgar scores. Arch Dis Child Fetal Neonatal Ed 89:F258-262
Merhar SL, Gozdas E, Tkach JA, Harpster KL, Schwartz TL, Yuan W, Kline-Fath BM, Leach JL, Altaye M, Holland SK (2016) Functional and structural connectivity of the visual system in infants with perinatal brain injury. Pediatr Res 80:43–48
Acknowledgements
We are very grateful to the participants and their families for their involvement in this study. We would like to thank the staff of the pediatric intensive care unit (PICU) of Centro Hospitalar e Universitário de Coimbra. We are also very grateful to Sónia Afonso and Tânia Lopes for the help with the MRI setup and scanning, to Ana Rita Santos and Rita Fonseca for assistance with brainstem auditory evoked potentials, to Liliana Cortez for support with visual evoked potentials, and Elsa Baptista for contribution in neurodevelopmental assessment.
Funding
Open access funding provided by FCT|FCCN (b-on). This work was supported by the FUNDAÇÃO PARA A CIÊNCIA E TECNOLOGIA, LISBOA/COMPETE 2020, Portugal (grant number PTDC/DTP-PIC/6032/2014/POCI-01–0145-FEDER-016781).
Author information
Authors and Affiliations
Contributions
C. R. P. conceptualized and designed the study, acquired, and analyzed the data, interpreted the findings, drafted the manuscript, and obtained funding for the project; J. V. D. acquired and analyzed the data, interpreted the findings, and drafted the manuscript; C. M. acquired and analyzed the data and interpreted the findings; I. N. V., C. P., J. E., and D. J. P. acquired and analyzed the data, interpreted the findings, and revised the manuscript; B. R. C. analyzed the data, interpreted the findings, and revised the manuscript; M. C. B. and G. O. conceptualized the study, obtained funding for the project, supervised the project, and revised the manuscript. All authors approved the final version to be published.
Corresponding author
Ethics declarations
Ethics approval
This study was performed according to the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. Approval was granted by the Ethics Committee of the Faculdade de Medicina da Universidade de Coimbra, Portugal (Reference CE-029–2014).
Consent to participate
Informed written consent was obtained from parents of all participants after a full verbal and written explanation of the study.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Daniele De Luca
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pinto, C.R., Duarte, J.V., Marques, C. et al. The role of early functional neuroimaging in predicting neurodevelopmental outcomes in neonatal encephalopathy. Eur J Pediatr 182, 1191–1200 (2023). https://doi.org/10.1007/s00431-022-04778-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-022-04778-0