Abstract
Cystic fibrosis (CF) is predominantly a lung disease but is also characterised by impaired skeletal muscularity and a reduction in fat-free mass. We aimed to test the hypothesis that clinical and anthropometric parameters would determine fat-free mass impairment in adolescents with CF. We measured the fat-free mass index (FFMI) using bioelectrical impedance, the lung function using spirometry, the number of shuttles as a measure of exercise tolerance and the reported physical activity in children and young people with CF in a tertiary centre at King’s College Hospital, London, UK. CF-related liver disease was diagnosed by abnormal liver enzymes and/or ultrasonography. We studied 28 children and young people (11 male) with a median (interquartile range (IQR)) age of 15 (13–17) years. They had a median (IQR) FFMI of 13.5 (11.6–15.1) kg/m2. The FFMI significantly correlated with age (rho = 0.568, p = 0.002), number of shuttles (rho = 0.691, p < 0.001) and reported hours of activity per day (rho = 0.426, p = 0.024). The median (IQR) FFMI was significantly higher in male [15.1 (13.1–18.6) kg/m2] compared to female participants [12.7 (11.6–14.1) kg/m2, p = 0.008]. The median (IQR) FFMI was significantly lower in the 10 (36%) participants with liver disease [11.9 (11.5–13.4) kg/m2] compared to the FFMI in the remaining 18 participants without liver disease [14.4 (12.5–15.9) kg/m2, p = 0.027].
Conclusion: Fat-free mass increases with increasing age and growth in adolescents with CF. Physical activity exerts a beneficial effect on fat-free mass, and CF-related liver disease negatively affects fat-free mass in adolescents with CF.
What is Known: • Health behaviours in adolescence influence lifelong health in cystic fibrosis (CF). • A normal body mass index in CF might fail to reveal a low fat-free mass (FFM), and quality of life in CF is strongly associated with a reduced FFM. | |
What is New: • FFM increases with increasing age and growth in adolescents with CF. • Physical activity exerts a beneficial effect, and liver disease negatively affects FFM in adolescents with CF. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cystic fibrosis (CF) is a life-shortening, hereditary disease with patients commonly suffering from frequent lung infections and obstructive pulmonary disease. Nutritional impairment is common in CF and is typically due to a chronic negative energy balance secondary to malabsorption [1]. Monitoring of weight, height and the corresponding body mass index (BMI) are frequently used in clinical practice to support optimal pulmonary function in children and adolescents, with a recommendation to maintain a BMI above the fiftieth percentile [2]. The BMI, however, cannot inform on body composition which is known to be globally affected in CF, including a decreased fat-free mass which corresponds to decreased skeletal muscularity and muscle atrophy [3]. A seemingly normal BMI might fail to reveal a low fat-free mass [4], and a high BMI could mask a significantly reduced muscle mass [5] in the context of overweight or obesity which is becoming increasingly common in the CF population [6].
Peripheral muscle abnormalities go often hand-in-hand with respiratory muscle impairment [7] and are thought to be the combined result of different pathophysiological processes, such as physical inactivity, chronic inflammation, and the frequent use of agents that cause muscle atrophy such as systemic corticosteroids [8].The cystic fibrosis transmembrane conductance regulator is also expressed in human skeletal muscle, leaving open a possibility of an intrinsic primary effect of the disease on the skeletal muscles [9]. Reduced muscle mass is of critical clinical importance in CF as outcomes such as lung function, quality of life and mortality have been more strongly associated with a reduced fat-free mass rather than with a low body mass index [8].
New health behaviours appear during adolescence, which track into adulthood and influence lifelong health and morbidity. Poor adherence to treatment in adolescence is often considered to be a major contributor to the decline in CF lung health [10]. The determinants of fat-free mass have not been previously investigated in adolescents with CF. We hypothesised that clinical and anthropometric parameters would be associated with the fat-free mass in adolescents with CF. Our aim was to determine these parameters.
Methods and materials
Study design
This study was a secondary analysis of a study cohort which aimed to validate results from a bioelectrical impedance device against dual-energy X-ray absorptiometry (DEXA) [11]. The study investigated clinically stable children and young people with CF aged between 12 and 19 years. The study was conducted between June 2016 and January 2017 at the tertiary CF centre at King’s College Hospital National Health System Foundation Trust, London, UK [11]. Consecutive children and young people were enrolled. Any children or young people with a pulmonary exacerbation in the past 2 weeks or acute illness and hospitalisation were excluded from the study. Age, gender and genotype were recorded. The genotype was classified as homozygous for ΔF508, heterozygous for ΔF508 or other mutations. Information on the presence of chronic infection (three or more positive microbiology sputum cultures in the preceding 12 months) [11], CF-related diabetes, CF-related liver disease (abnormal liver enzymes and/or ultrasonography) [12] and pancreatic insufficiency, concurrent use of systemic steroids and 5-year survival was recorded. The participants underwent bioelectrical impedance analysis, pulmonary function and exercise tolerance tests. Ethical approval was granted by the East Midlands—Nottingham II Research Ethics Committee (reference: 16/EM/0174). Participants aged 16 years and older gave written consent, whilst participants younger than 16 years gave written assent, and consent was provided by their parents/guardians.
Fat-free mass and body mass index
The fat-free mass was measured by bioelectrical impedance analysis [13] with the InBody S10 Body Composition Analyser (InBody Ltd, Cerritos, CA, USA). Fat-free mass was estimated by impedance using the four compartment model representing the body in terms of water, protein, fat and mineral components. Measurements were taken using the tetra-polar 8-Point Tactile Electrode system in the sitting position, following 2 h of fasting and micturition within 30 min prior to testing. The fat-free mass was adjusted using a correction equation that was derived following regression with DEXA as gold standard and was specific for this bioelectric impedance device [11]. The fat-free mass index (FFMI) was calculated as fat-free mass/height2 (kg/m2) [14]. Height was measured to the nearest cm by a stadiometer and weight to the nearest 0.1 kg by digital scales. The corresponding BMI z-score and percentile were calculated [15].
Pulmonary function and respiratory muscle testing
Spirometry and body plethysmography were performed on the same day with bioelectrical impedance analysis, according to the American Thoracic Society (ATS) and the European Respiratory Society (ERS) guidelines [16, 17]. The Jaeger MasterScreen PFT/IOS/Body, CareFusion Ltd, Basingstoke, UK, was used to measure pulmonary function. The highest value of forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1) and functional residual capacity (FRC) was recorded and was expressed in z-scores [18]. Maximal inspiratory pressure and maximal expiratory pressure were measured and expressed as percentage predicted [19]. Maximal inspiratory and maximal expiratory pressures were measured using a handheld, manometer according to ATS/ERS guidelines (Micro RPM Respiratory Muscle Analyser, CareFusion, San Diego, CA, USA) [20].
Habitual activity estimation scale
Participants completed the Habitual Activity Estimation Scale on the day of the assessment. The parents and/or members of the research team assisted as required. They reported the percentage of an average day that they were inactive (lying down), somewhat inactive (sitting), somewhat active (walking) and active (activities that make you breathe hard and increase your heart rate). They documented their waking, sleeping and meal times. The research team was thus able to calculate the hours spent on each of these activity levels. The hours of being active and somewhat active were then summed to calculate the time spent being moderately-vigorously active per day.
Modified shuttle walk test
The modified shuttle walk test was used to assess exercise tolerance. Patients walked/run between two cones, on an enclosed flat corridor with externally paced instructions. Initial walking speed was 0.50 m/s and was increased by 0.17 m/s every minute until the participant completed all 15 levels or became too breathless to maintain the required speed or failed to complete the shuttle in the allocated time [21].
Statistical analyses
Continuous data were tested for normality with the Kolmogorov–Smirnov test, were found to be non-normally distributed and were presented as median and interquartile range (IQR). The relationship between the FFMI and pulmonary function, respiratory muscle function and exercise variables were examined using Spearman’s rho bivariate correlation analysis (rho). The FFMI was compared in binary conditions such as sex, chronic infection, CF-related diabetes, pancreatic insufficiency and CF-related liver disease using the chi-square test. To examine the independent association of the fat-free mass with pulmonary function, exercise and clinical parameters, the continuous parameters that exhibited a significant correlation with the FFMI and the binary parameters in which the FFMI was significantly different (p < 0.05) were inserted in a multivariable linear regression model with the FFMI as the outcome variable. Multi-collinearity among the independent variables in the regression analysis was assessed by examination of a correlation matrix for the independent variables. Univariate linear regression analysis was used to graphically depict the relationship of the FFMI with the number of shuttles. Statistical analysis was performed using SPSS software, version 27.0 (SPSS Inc., Chicago, IL, USA).
Results
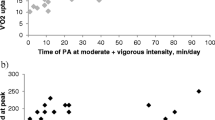
During the study period, 28 children and young people (11 male) were recruited to the study with a median (IQR) age of 15 (12–19) years. One subject that was recruited in the primary study was excluded from this study as fat-free mass could not be measured as part of the original data collection. The demographic and clinical characteristics of the included subjects are presented in Table 1. They had a median (IQR) fat-free mass of 37.7 (30.9–47.4) kg and a FFMI of 13.5 (11.6–15.1) kg/m2. The FFMI significantly correlated with age (rho = 0.568, p = 0.002), number of shuttles (rho = 0.691, p < 0.001, Fig. 1), reported hours of activity per day (rho = 0.426, p = 0.024), FVC z-score (rho = 0.388, p = 0.041), maximum inspiratory (rho = 0.394, p = 0.038) and maximum expiratory (rho = 0.493, p = 0.008) pressure but not with BMI z-score (rho = 0.178, p = 0.364), FEV1 z-score (rho = 0.229, p = 0.240) and FRC z-score (rho = − 0.299, p = 0.156). The median (IQR) FFMI was significantly higher in male [15.1 (13.1–18.6) kg/m2] compared to female participants [12.7 (11.6–14.1) kg/m2, p = 0.008] and significantly lower in participants with liver disease [11.9 (11.5–13.4) kg/m2] compared to participants without liver disease [14.4 (12.5–15.9) kg/m2, p = 0.027, Fig. 1]. The ultrasonographic findings and liver function tests of the subjects with liver disease are presented in Table 2. The median (IQR) FFMI was not different in patients with chronic infection [12.2 (11.5–14.9) kg/m2] compared to patients without chronic infection [13.9 (12.8–15.3) kg/m2, p = 0.151]. The median (IQR) FFMI was not different in participants with CF-related diabetes [12.2 (11.3–14.5) kg/m2] compared to participants without diabetes [14.0 (11.6–15.3) kg/m2, p = 0.352]. The FFMI was not compared in participants with and without pancreatic insufficiency as there was only one subject without pancreatic insufficiency. A summary of the associations of the fat-free mass with the included clinical and anthropometric parameters is graphically presented in Fig. 2.
Weight and age were not included in the multivariable model due to collinearity with height, since the FFMI is a product of the square of height. The hours of activity per week were collinear with the number of shuttles and were thus not included in the model. Following multivariable linear regression analysis, the fat-free mass was independently associated with male sex (adjusted p = 0.028, 95% CI = 0.19 to 3.07), number of shuttles (adjusted p = 0.004, 95% CI = 0.02 to 0.08) and CF-related liver disease (adjusted p = 0.033, 95% CI = − 2.91 to − 0.14) but not with the FVC z-score (adjusted p = 0.773, 95% CI = − 0.63 to 0.48).
Discussion
We have demonstrated that fat-free mass increases with increasing age and growth in adolescents with cystic fibrosis and that the fat-free mass is positively affected by aerobic activity and negatively affected by the development of CF-related liver disease. Our hypothesis that clinical and anthropometric parameters would be associated with the fat-free mass in adolescents with CF was thus accepted.
Our population is clinically comparable to previous studies of body composition in CF. Scully and colleagues studied 38 adolescents and adults with CF at a mean age of 28 years with a mean FEV1 of 73% predicted and reported a mean lean mass of 42.2 kg measured by DEXA [22]. In our study, we measured a younger population at a median age of 15 years and reported a median FEV1 of 77.3% predicted and a FFMI of 37.7 kg. The actual values of the fat-free mass in our subjects are, as expected, lower compared to healthy children and young people of a similar age [22]. In our study, we report a median fat-free mass of 37.7 kg, while population studies in healthy 13–18 year old children have reported mean fat-free mass values of 38.9–41.8 kg [23]. The finding of a more reduced fat-free mass in CF patients with liver disease is a novel finding in the whole CF population and not only in CF adolescents. The precise mechanism underlying how liver disease can induce muscle atrophy has not been completely elucidated. A recent animal study described a potential mechanism where liver fibrosis (induced in mice by bile duct ligation) leads to the upregulation of the atrophy-inducing, tumour necrosis factor α in a culture of human myotubes [24]. It is interesting to note that focal biliary fibrosis is the main pathological process in CF-related liver disease [12]. Furthermore, liver disease is associated with a hypermetabolic state, increased energy requirements and a reduction in caloric intake and absorption. These phenomena might also explain the skeletal muscle atrophy seen with liver disease. Kyrana et al. compared the fat-free mass measured by DEXA in seventeen children with end-stage chronic liver disease (none of them with CF) at a median age of 7.4 years to 14 healthy controls and reported that resting energy expenditure correlated strongly with fat-free mass, but the ratio of the resting energy expenditure to the fat-free mass was not different between patients and controls [25].
The finding of a higher fat-free mass and improved muscularity in adolescents with higher reported physical activity and exercise capacity would be an expected finding as active individuals would have a higher muscle mass. Our study did not include an exercise intervention, so we cannot infer whether the improved muscularity in CF is the result of increased physical activity. Establishing this association would be clinically important as qualitative indices of body composition such as fat-free mass have been linked with better respiratory outcomes and better quality of life [11, 26]. In this sense, the next line of research might be a randomised trial of a structured aerobic activity programme to adolescents with CF and establishing whether this intervention would lead to improvements in their aerobic capacity and whether an improved aerobic state would be associated with improved spirometric and clinical outcomes such as hospitalisation and reported quality of life. A short residential rehabilitation program consisting of supervised respiratory and nutritional treatment and daily physical activity for 3 weeks was associated with a significant increase in FFM in 34 patients with CF between 6 and 40 years old [27].
In our study, we report a lack of association between fat-free mass and the presence of chronic infection. Dufresne et al., similarly, did not find that inflammation could predict fat-free mass and quadriceps muscle strength in adults with CF [28]. Some other studies, however, have suggested that CF might be characterised by a generalised systemic myopathy that probably is the result of a spill-over of circulating pro-inflammatory cytokines [29]. In a group of 122 CF patients with a median age of 13 years, impaired peripheral muscularity was reported in the children and young people with chronic Pseudomonas aeruginosa infection compared to the CF subjects with less severe or no infection [30]. Van de Weert-van Leeuwen et al. also studied 149 adolescents with CF and reported that the response to exercise was decreased in a state of increased inflammation, possibly via a catabolic effect of circulating cytokines on the peripheral muscles [31].These conflicting results might be partially explained by population differences and the complexity of controlling for all potential contributors in a multifactorial disease such as CF.
Although our study population included adolescents with abnormal indices of airway obstruction (FEV1) and hyperinflation (FRC), these indices were not related to the fat-free mass. This implies that in our population, the mechanism of fat-free mass depletion might not involve airflow limitation and might be predominantly related to muscle disuse and deconditioning. This finding is in agreement with some previous studies that have reported that although inhaled bronchodilators can improve lung function indices such as FEV1, they have no effect on exercise tolerance [32].
Although only one participant in our study was receiving systemic corticosteroids at the time of study, corticosteroids are sometimes used in shorter or longer courses to treat inflammation in CF lung disease. The negative impact of these agents on peripheral muscle function has been well reported in numerous patient groups [33]. The term steroid myopathy refers to a myopathy with a symmetric distribution involving the proximal extremity muscles, associated with long-term muscle atrophy, notably with very minimal or no associated pain [34]. The possible cumulative effects of these agents on fat-free mass should also be considered in the clinical context.
Our study has strengths and some limitations. To our knowledge, we were the first to investigate the determinants of fat-free mass in stable adolescents with cystic fibrosis, and we highlighted the potential beneficial effect of exercise on maintaining skeletal muscularity and overall health in cystic fibrosis. The association of liver disease with impaired fat-free mass is also a novel finding in the adolescent CF population, which has not been previously reported. We should acknowledge as a limitation our limited population size, but our study focused only on adolescents and was conducted in a single CF centre, and thus, uniformity of care was guaranteed. We also conducted a comprehensive assessment of lung function, respiratory muscle function, body composition, and exercise capacity, and we were able to derive meaningful conclusions on the interactions of these parameters. Some previous papers have described that bioelectrical impedance measurements might underestimate the real fat-free mass in CF, suggesting that this method has limited applicability in the assessment of body composition in individual patients with CF [35]. In our study, however, we adjusted our fat-free mass measurements by correcting with an equation that was specific to our device and was derived by gold standard measurements made with dual energy X-ray absorptiometry. Future studies could include the evaluation of inflammatory indices such as tumour necrosis factor α and interleukin 6 to further elucidate the role of inflammation in CF myopathy.
In conclusion, we have demonstrated that in adolescents with CF, physical activity exerts a beneficial effect on fat-free mass, while, contrarily, CF-related liver disease negatively affects fat-free mass.
Data Availability
Data is available from the corresponding author upon reasonable request.
Abbreviations
- ATS:
-
American Thoracic Society
- BMI:
-
Body mass index
- CF:
-
Cystic fibrosis
- ERS:
-
European Respiratory Society
- FEV1 :
-
Forced expiratory volume in 1 s
- FFMI :
-
Fat-free mass index
- FRC:
-
Functional residual capacity
- IQR:
-
Interquartile range
References
Brownell JN, Bashaw H, Stallings VA (2019) Growth and nutrition in cystic fibrosis. Semin Respir Crit Care Med 40:775–791
Smyth AR, Bell SC, Bojcin S, Bryon M, Duff A, Flume P et al (2014) European cystic fibrosis society standards of care: best practice guidelines. J Cyst Fibros 13(Suppl 1):S23-42
Calella P, Valerio G, Brodlie M, Donini LM, Siervo M (2018) Cystic fibrosis, body composition, and health outcomes: a systematic review. Nutrition 55–56:131–139
Ionescu AA, Evans WD, Pettit RJ, Nixon LS, Stone MD, Shale DJ (2003) Hidden depletion of fat-free mass and bone mineral density in adults with cystic fibrosis. Chest 124:2220–2228
Ritchie H, Nahikian-Nelms M, Roberts K, Gemma S, Shaikhkhalil A (2021) The prevalence of aberrations in body composition in pediatric cystic fibrosis patients and relationships with pulmonary function, bone mineral density, and hospitalizations. J Cyst Fibros 20:837–842
Harindhanavudhi T, Wang Q, Dunitz J, Moran A, Moheet A (2020) Prevalence and factors associated with overweight and obesity in adults with cystic fibrosis: a single-center analysis. J Cyst Fibros 19:139–145
Dassios T (2015) Determinants of respiratory pump function in patients with cystic fibrosis. Paediatr Respir Rev 16:75–79
Gruet M, Troosters T, Verges S (2017) Peripheral muscle abnormalities in cystic fibrosis: etiology, clinical implications and response to therapeutic interventions. J Cyst Fibros 16:538–552
Lamhonwah AM, Bear CE, Huan LJ, Kim Chiaw P, Ackerley CA, Tein I (2010) Cystic fibrosis transmembrane conductance regulator in human muscle: dysfunction causes abnormal metabolic recovery in exercise. Ann Neurol 67:802–808
Knudsen KB, Pressler T, Mortensen LH, Jarden M, Skov M, Quittner AL et al (2016) Associations between adherence, depressive symptoms and health-related quality of life in young adults with cystic fibrosis. Springerplus 5:1216
Papalexopoulou N, Dassios TG, Lunt A, Bartlett F, Perrin F, Bossley CJ et al (2018) Nutritional status and pulmonary outcome in children and young people with cystic fibrosis. Respir Med 142:60–65
Colombo C (2007) Liver disease in cystic fibrosis. Curr Opin Pulm Med 13:529–536
Calella P, Valerio G, Brodlie M, Taylor J, Donini LM, Siervo M (2019) Tools and methods used for the assessment of body composition in patients with cystic fibrosis: a systematic review. Nutr Clin Pract Official Publ Am Soc Parent Enteral Nutr 34:701–714
Schutz Y, Kyle UU, Pichard C (2002) Fat-free mass index and fat mass index percentiles in Caucasians aged 18–98 y. Int J Obes Relat Metab Disord 26:953–960
Martinez-Millana A, Hulst JM, Boon M, Witters P, Fernandez-Llatas C, Asseiceira I et al (2018) Optimisation of children z-score calculation based on new statistical techniques. PLoS ONE 13:e0208362
Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R et al (2005) General considerations for lung function testing. Eur Respir J 26:153–161
Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F et al (2005) Standardisation of the measurement of lung volumes. Eur Respir J 26:511–522
Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH et al (2012) Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 40:1324–1343
Wilson SH, Cooke NT, Edwards RH, Spiro SG (1984) Predicted normal values for maximal respiratory pressures in Caucasian adults and children. Thorax 39:535–538
Wells JC, Williams JE, Chomtho S, Darch T, Grijalva-Eternod C, Kennedy K et al (2012) Body-composition reference data for simple and reference techniques and a 4-component model: a new UK reference child. Am J Clin Nutr 96:1316–1326
Bradley J, Howard J, Wallace E, Elborn S (1999) Validity of a modified shuttle test in adult cystic fibrosis. Thorax 54:437–439
Scully KJ, Jay LT, Freedman S, Sawicki GS, Uluer A, Finkelstein JS et al (2022) The relationship between body composition, dietary intake, physical activity, and pulmonary status in adolescents and adults with cystic fibrosis. Nutrients 14
Shypailo RJ, Wong WW (2020) Fat and fat-free mass index references in children and young adults: assessments along racial and ethnic lines. Am J Clin Nutr 112:566–575
Kurosawa T, Goto M, Kaji N, Aikiyo S, Mihara T, Ikemoto-Uezumi M et al (2021) Liver fibrosis-induced muscle atrophy is mediated by elevated levels of circulating TNFalpha. Cell Death Dis 12:11
Kyrana E, Williams JE, Wells JC, Dhawan A (2019) Resting energy expenditure of children with end-stage chronic liver disease before and after liver transplantation. J Pediatr Gastroenterol Nutr 69:102–107
Charatsi AM, Dusser P, Freund R, Maruani G, Rossin H, Boulier A et al (2016) Bioelectrical impedance in young patients with cystic fibrosis: validation of a specific equation and clinical relevance. J Cyst Fibros 15:825–833
Van Biervliet S, Declercq D, Dereeper S, Vermeulen D, Wurth B, De Guschtenaere A (2021) The effect of an intensive residential rehabilitation program on body composition in patients with cystic fibrosis. Eur J Pediatr 180:1981–1985
Dufresne V, Knoop C, Van Muylem A, Malfroot A, Lamotte M, Opdekamp C et al (2009) Effect of systemic inflammation on inspiratory and limb muscle strength and bulk in cystic fibrosis. Am J Respir Crit Care Med 180:153–158
Divangahi M, Matecki S, Dudley RW, Tuck SA, Bao W, Radzioch D et al (2004) Preferential diaphragmatic weakness during sustained Pseudomonas aeruginosa lung infection. Am J Respir Crit Care Med 169:679–686
Dassios TG, Katelari A, Doudounakis S, Dimitriou G (2014) Chronic Pseudomonas aeruginosa infection and respiratory muscle impairment in cystic fibrosis. Respir Care 59:363–370
van de Weert-van Leeuwen PB, Slieker MG, Hulzebos HJ, Kruitwagen CL, van der Ent CK, Arets HG (2012) Chronic infection and inflammation affect exercise capacity in cystic fibrosis. Eur Respir J 39:893–898
Dodd JD, Barry SC, Daly LE, Gallagher CG (2005) Inhaled beta-agonists improve lung function but not maximal exercise capacity in cystic fibrosis. J Cyst Fibros 4:101–105
Pereira RM (2011) Freire de Carvalho. J Glucocorticoid-induced myopathy Joint Bone Spine 78:41–44
Surmachevska N, Tiwari V (2022) Corticosteroid induced myopathy. StatPearls. Treasure Island (FL)
King S, Wilson J, Kotsimbos T, Bailey M, Nyulasi I (2005) Body composition assessment in adults with cystic fibrosis: comparison of dual-energy X-ray absorptiometry with skinfolds and bioelectrical impedance analysis. Nutrition 21:1087–1094
Funding
Niovi Papalexopoulou received funding from the Isaac Schapera Research Trust. The research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at the Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The funding sources had no involvement in the conduct of the research or the preparation of the article.
Author information
Authors and Affiliations
Contributions
TD and AG designed the study; MRM and NP collected the data; TD, MRM, and AG undertook the analysis. All authors were involved in drafting or revising the manuscript for important intellectual content. They all gave consent to submission and publication of the work and able to publicly discuss and defend the manuscript’s content.
Corresponding author
Ethics declarations
Ethics approval
Ethical approval was granted by the East Midlands—Nottingham II Research Ethics Committee (reference: 16/EM/0174).
Consent to participate
Participants aged 16 years and older gave written consent, whilst participants younger than 16 years gave written assent, and consent was provided by their parents/guardians.
Consent for publication
Participants aged 16 years and older gave written consent, whilst participants younger than 16 years gave written assent, and consent was provided by their parents/guardians.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Peter de Winter
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dassios, T., Mitakidou, M.R., Dhawan, A. et al. Physical activity and liver disease affect the fat-free mass in adolescents with cystic fibrosis. Eur J Pediatr 182, 769–775 (2023). https://doi.org/10.1007/s00431-022-04752-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-022-04752-w