Abstract
NIV-NAVA mode for respiratory support in preterm infants is not well-studied. This study aimed to describe the diaphragmatic function, diaphragmatic excursion (DE), and thickness fraction (DTF), in preterm infants < 30 weeks’ gestation supported by NIV-NAVA compared to NIPPV using bedside ultrasonography. In this consecutive prospective study, DE, diaphragmatic thickness at end of expiration (DTexp), end of inspiration (DTins), and DTF were assessed using bedside ultrasound. Lung aeration evaluation using lung ultrasound score (LUS) was performed for the two groups. Diaphragmatic measurements and LUS were compared for the 2 groups (NIV-NAVA group versus NIPPV group). Statistical analyses were conducted using the SPSS software version 22. Out of 70 infants evaluated, 40 were enrolled. Twenty infants were on NIV-NAVA and 20 infants on NIPPV with a mean [SD] study age of 25.7 [0.9] weeks and 25.1 [1.4] weeks respectively (p = 0.15). Baseline characteristics and respiratory parameters at the time of the scan showed no significant difference between groups. DE was significantly higher in NIV-NAVA with a mean SD of 4.7 (1.5) mm versus 3.5 (0.9) mm in NIPPV, p = 0.007. Additionally, the mean (SD) of DTF for the NIV-NAVA group was 81.6 (30) % vs 78.2 (27) % for the NIPPV group [p = 0.71]. Both groups showed relatively high LUS but no significant difference between groups [12.8 (2.6) vs 12.6 (2.6), p = 0.8].
Conclusion: Preterm infants managed with NIV-NAVA showed significantly higher DE compared to those managed on NIPPV. This study raises the hypothesis that NIV-NAVA could potentially improve diaphragmatic function due to its synchronization with patients’ own breathing. Longitudinal studies to assess diaphragmatic function over time are needed.
Trial registry: Clinicaltrials.gov (NCT05079412). Date of registration September 30, 2021.
What is Known: • NIV-NAVA utilizes diaphragmatic electrical activity to provide synchronized breathing support. • Evidence for the effect of NIV-NAVA on diaphragmatic thickness fraction (DTF) and excursion (DE) is limited. | |
What is New: • Ultrasonographic assessment of diaphragmatic function (DTF and DE) is feasible. • In preterm infants, DE was significantly higher in infants supported with NIV-NAVA compared to those supported with NIPPV. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past few decades, avoidance of invasive mechanical ventilation (IMV) and promoting of the use of non-invasive ventilation (NIV) in infants born prematurely has been accepted as a standard of care to reduce lung injury and subsequent development of bronchopulmonary dysplasia (BPD) [1]. Although nasal continuous positive airway pressure (nCPAP) is the most frequently used NIV mode for infants born before 32 weeks gestation, NIPPV was found to decrease the incidence of reintubation within 2–7 days of life compared to nCPAP [2]. Furthermore, synchronized NIPPV is considered the most effective NIV in preventing extubation failure in preterm neonates with respiratory distress syndrome [3]. The advantages of NIPPV over nCPAP include the ability to deliver higher mean airway pressure (MAP) and carbon dioxide (CO2) clearance [4, 5].
Recently, neurally adjusted ventilatory assist (NAVA) for invasive and non-invasive ventilation (NIV-NAVA) has emerged as a new respiratory support mode for preterm infants with respiratory insufficiency. Typically, NAVA mode (invasive and non-invasive) uses the electrical activity (Edi) of the diaphragm to trigger, set the amount of pressure, and cycle off the ventilator which in turn reduces asynchrony during NIV [6, 7]. In comparison to NIPPV, several studies have reported that NIV-NAVA is associated with a higher success rate of preventing reintubation [8,9,10], alongside fewer episodes of bradycardia and apnea of prematurity per day [11]. However, data regarding the effect of NIV-NAVA compared to NIPPV on diaphragmatic function and dimensions in preterm infants remains unknown.
Lung ultrasound (LU) has been increasingly used in neonates as a non-invasive and radiation-free imaging modality to assess lung aeration and diaphragm function. Moreover, there is a growing interest among researchers in using ultrasound to monitor the evolution of diaphragmatic contractility and dimensions during IMV, for clinical and research purposes [12,13,14,15]. Several parameters have been investigated to evaluate diaphragmatic functions, such as diaphragmatic excursion (DE) and diaphragmatic thickness and its fraction (DTF). While DE is known as the distance in which diaphragm can move during the respiratory cycle, DTF is a ratio between diaphragm thickness during inspiration and expiration [16, 17]. Although data about interpreting those parameters in preterm infants are still emerging, high DE was found to be a good indicator for prediction of extubation success in preterm infants < 32 weeks [18]. Likewise, DTF was found to be greater in preterm infants with BPD indicating increased diaphragmatic function to compensate for or the underlying parenchymal disease in this population [19]. Despite NIV-NAVA utilizes diaphragmatic electrical activity to provide synchronized breathing support, evidence for the effect of NAVA on DTF and DE is scarce.
We hypothesized that infants on NIV-NAVA will have better diaphragmatic function (DE and DTF) compared to infants on NIPPV. Thus, in this study, our primary objective was to characterize the DE, diaphragmatic thickness at end of expiration (DTexp) and end of inspiration (DTins), and DTF in preterm infants (< 30 weeks’ gestation) who were managed on NIV-NAVA compared to those managed on NIPPV using bedside ultrasonography. The secondary objective was to evaluate lung aeration using lung ultrasound score (LUS) in infants on NIV-NAVA compared to those on NIPPV.
Methods
Study design
We conducted a consecutive prospective study between March 2020 and November 2021 on eligible infants who were admitted to the neonatal intensive care unit (NICU) at Mount Sinai Hospital, Toronto, Canada. Local research ethics approval was obtained (Mount Sinai Hospital REB (19–0324-E), and consecutive patients were enrolled after informed written consent was obtained from parents or guardians. This study was registered with the US Library of Medicine clinical trials registry, www.clinicaltrials.gov (NCT: 05,079,412). Registration has been delayed because of concomitant COVID pandemic announcement that led to withhold all interventional studies include this study for almost a year. Reporting of this study followed the Strengthening Reporting of Observational Studies in Epidemiology (STROBE) Statement [20].
Study participants
Consecutive infants born at < 30 weeks’ gestation who were receiving NIV-NAVA or NIPPV at 2–4 weeks after birth and for at least 24 h at the time of recruitment were included. Infants who had congenital or chromosomal abnormalities, neuromuscular disease, known lung malformation or diaphragm dysfunction, or whose parents declined to consent were excluded.
Respiratory management for preterm infants < 30 weeks gestational age
Respiratory management of preterm neonates < 30 weeks’ gestation at our unit was by unit guidelines developed as part of “Bronchopulmonary Dysplasia Prevention bundle.” The primary mode of non-invasive ventilatory support for infants born at < 24 weeks’ gestation post-extubation is nasal intermittent positive pressure ventilation (NIPPV). Infants born at ≥ 24 weeks’ gestation and spontaneously breathing are initiated on nCPAP (6–10 cmH2O). If infants experience bradycardia and/or apnea while on nCPAP, they will be transitioned to NIPPV. FiO2 is titrated to maintain SpO2 within the target range (91–95%). Given the limited number of NAVA ventilators available in our unit during the study period, NIV-NAVA was used as a rescue NIV respiratory mode for cases failing NIPPV (FiO2 > 0.4–0.5 to maintain oxygen saturation target, and pCO2 > 65 mmHg with a pH < 7.20) to prevent reintubation. Another indication for the use of NIV-NAVA was the presence of significant abdominal distension that can potentially lead to compromised ventilation.
Study procedure
All eligible infants were consecutively approached for the parent’s consent. We evaluated diaphragmatic thickness (DT) and DE for all enrolled infants using bedside ultrasound (US). We used a high-resolution L20–5 MHz linear probe for the measurement of DT and a C10–3 MHz curvilinear probe for assessing DE (Z. One—Mindray, Inc.). All US assessments were undertaken using the standard technique while infants were in the supine position. Scans were performed either before or 1 h after feeding to avoid any concerns of a full stomach on diaphragmatic assessment. Infants were on continuous cardiorespiratory monitoring for apnea, bradycardia (heart rate < 80 beats per minute), and desaturation (oxygen saturation < 85%) events during the scan. Sonographic assessment of the diaphragm was completed by personnel trained in point-of-care neonatal US with a minimum of 6 months of experience in lung and diaphragm ultrasounds. To minimize errors in measurements and improve our measurement accuracy, we standardized the technique for obtaining diaphragmatic views and how to measure diaphragm thickness and excursion among all investigators prior to study enrollment.
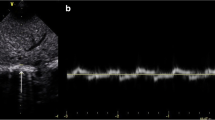
Using a recently published standardized technique [12, 19, 21,22,23,24,25], the right hemidiaphragm was assessed with the infant in a relaxed state facilitated by modified swaddling and occasionally pacifier administered by a second person. For DE measurements, the probe was placed subcostal between the anterior axillary and midclavicular line with a curvilinear probe using a standardized protocol for image acquisition. DE was measured as the difference in the position of the outer line of the diaphragm in M-mode at peak inspiration and expiration (Fig. 1a). DT was measured at the zone of apposition where thickening and shortening of the diaphragm could be appropriately evaluated. DT was obtained by placing the linear transducer at the 9th or 10th intercostal space near the mid-axillary line while the transducer was perpendicular to the chest wall [23, 24]. By B-mode, the diaphragm was identified as the hypo-echoic area (muscular layer) bordered by two echogenic lines of the diaphragmatic pleura (upper line) and peritoneum (lower line). Using M-mode tracing, the end of expiratory and inspiratory DT was calculated by determining the maximum perpendicular distance between the pleural and peritoneal layers, measuring only the distance of the hypoechoic area (Fig. 1b). To assess the efficiency of the diaphragm as a pressure generator, the DTF was determined [25]. DTF was calculated as the change in DTexp DTins using the following formula:
a, b Measurement of diaphragmatic excursion and thickness. a Measurement of diaphragmatic excursion using M-mode from a study patient, marked by the length of the orange arrows pointing up. b Measurement of diaphragmatic thickness at the end of inspiration and expiration using M-mode from a study patient, marked by the vertical distance (orange bidirectional arrows) between the 2 layers of diaphragm
For each diaphragm parameter (DTexp, DTins, DTF, and DE), the average from at least three respiratory cycles was reported to reduce the risk of over or underestimating the diaphragmatic measurements [26]. Standard neonatal LU views, three chest areas on each side (upper anterior, lower anterior and lateral) were obtained, and LUS using grading score (ranges from 0–18 points) were determined as previously described [27, 28].
At the end of the study, all scans for the two groups were anonymized. All scans collected during the study were kept confidential and securely locked in computerized files saved on hospital drive which is password protected. Assessment of diaphragm function (DE and DTF) was performed by one of the investigators who was blind to study groups. Similarly, LUS was evaluated by another study investigator who was unaware of the study groups or results of diaphragmatic measurements of the study patients. To assess the inter-observer correlation, 25% of the anonymized study scans (n = 10 cases of DT and 10 cases of DE) were randomly assigned and revaluated. Measurements were compared, and the intra-class correlation coefficient (ICC) was calculated.
Outcomes and clinical data
The primary outcome was the sonographic evaluation of DE, DTexp, DTins, and DTF for infants born at < 30 weeks’ gestation after receiving NIV-NAVA or NIPPV for ≥ 24 h. Secondary outcome was LUS for the two groups. Demographic and respiratory data at the time of the scan was collected. Typically, respiratory parameters are documented by respiratory therapist or bedside nurse every hour in the patient electronic medical record. The average from three consecutive measured PIP and MAP prior to LUS scan was reported to minimize risk of bias.
Sample size
A convenience sample was planned over one year (the study period) since no previous data on DE or DTF in preterm infants on NIV-NAVA was available. Due to COVID-19 pandemic, study recruitment was extended until November 2021.
Statistical analysis
Statistical analysis was performed using commercially available software (SPSS for Windows Inc. Version 22. Chicago, Illinois). The Kolmogorov–Smirnov test was performed to examine the distribution of data. Independent Student t test was used to compare continuous parametric variables to determine the differences between groups; Mann–Whitney U test was used for continuous non-parametric variables; chi-square test (χ2) or Fisher exact test was used for categorical variables when appropriate. Clinical data and ultrasonography measurements were presented as mean and standard deviation or median and interquartile range for continuous variables or frequency and percentages for categorical variables. The ICC was determined for variables with continuous measurements (mixed factorial design). We calculated the ICC using 30% of the images, which were evaluated by another individual blinded to the groups. p values less than 0.05 were considered significant.
Results
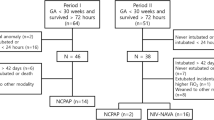
We evaluated 70 infants born at < 30 weeks’ gestation in a primary screen, of whom 46 cases met eligibility and consented to participate. Out of the 46 consented patients, 40 infants completed the study protocol (20 infants in each group). Six infants (4 cases in the NIPPV group and 2 infants from NIV-NAVA group) were not scanned due to timing issues and were excluded (Fig. 2). Baseline characteristics and respiratory status prior to the LU scanning of the two groups were summarized in Tables 1 and 2. There was no significant difference between the NIV-NAVA group compared to the NIPPV group regarding birth gestation (GA) or postmenstrual age (PMA) at the time of the scan. DE was significantly higher in NIV-NAVA [mean SD 4.7 (1.5)] versus [3.5 (0.9)] in NIPPV, p = 0.007 (Table 3). DTF was higher in NIV-NAVA compared to NIPPV group [mean (SD) 81.6 (30) vs 78.2 (27), p = 0.71). There was no significant difference regarding DTexp and DTins between groups. Both groups showed relatively high LUS but no significant difference [mean (SD) 12.8 (2.6) versus 12.6 (2.6) p = 0.8] (Fig. 3). Duration of IMV and incidence of BPD did not show significant difference between NIV-NAVA and NIPPV group [mean (SD) 5.35 (6.2) days versus 3.35 (4.4) days p = 0.24] and [80% versus 70% p = 0.85], respectively.
Lung ultrasound patterns and scoring: Lung ultrasound findings were categorized into 4 patterns 1 A-lines pattern (score = 0) indicates presence of A-lines artifact, pleural line sliding, and less than 3 B-lines; 2 Nonconfluent B-lines pattern (interstitial syndrome, score = 1), indicates more than 3 B-lines, and pleural line sliding; 3 Confluent B-lines pattern (white lung pattern, score = 2) indicates confluent B-lines, and pleural line sliding; 4 consolidation pattern (subpleural consolidation > 5 mm, score = 3) with absent A-lines, confluent B lines
We found a strong inter-observer agreement for the measurement of DT and DE (ICC 0.86 [95% confidence interval (CI) 0.84–0.87] and 0.93 [95% CI 0.91–0.94] respectively). The ultrasonographic exams were generally well tolerated, and none of the scans were aborted because of bradycardia or desaturation during the ultrasonographic exams.
Discussion
Our study is the first to report that DE was significantly higher in preterm infants on NIV-NAVA compared to DE in infants supported by NIPPV. The significantly higher DE in preterm infants supported with NIV-NAVA can be explained by the fact that this mode of ventilation has a better patient-ventilator synchronization which will eventually augment diaphragmatic contractility. Several studies have suggested that better DE indicates improved diaphragmatic function and can potentially predict successful weaning from mechanical ventilation [29, 30]. Our results aligned with Hadda et al., who randomized adult patients to either invasive NAVA or conventional ventilators. Authors of this study found that invasive NAVA was associated with a higher DE, in comparison to patients supported by conventional ventilators [31].
Alam et al. [32] reported that successful extubation was significantly correlated with DE with an area under the curve (AUC) of 0.83 (p < 0.001) and sensitivity 77.8% and specificity 84.6%. These results were consistent with several other studies that showed promising findings for using NIV-NAVA to facilitate extubation in preterm infants [10, 33, 34]. During our study period, NIV-NAVA was used as rescue mode for infants who failed NIPPV. Currently, attending physicians in our unit are recommending the use of NIV-NAVA as the primary NIV mode post extubation to improve rate of successful extubation. Interestingly, NAVA has been useful in preterm neonate with postsurgical unilateral diaphragmatic paralysis and cases of congenital diaphragmatic hernia with encouraging results [35,36,37].
Furthermore, Rehan et al. found that a higher level of PEEP in preterm infants supported by nCPAP causes significant reduction in DE suggesting impaired diaphragmatic functions with high PEEP [38]. While the PEEP used in NIV-NAVA and NIPPV in our study were relatively high in both groups, infants on NIV-NAVA had less reduced DE compared to DE in infants receiving similar PEEP on NIPPV.
Additionally, our study did not show a significant difference between the 2 groups regarding the diaphragmatic thickness (DTins and DTexp) or DTF. There is no available data regarding the effect of NIV-NAVA on diaphragmatic dimensions in preterm infants to compare with our findings. However, in a recent study conducted in our unit by Yeung et al., authors reported higher DTF in preterm infants who were diagnosed with BPD [19]. Despite up to 80% of our study population had been diagnosed with BPD at 36 weeks PMA, our results did not show similar results as Yeung et al. This could be explained by the fact that our study was done during the earlier neonatal period before BPD established. Another potential reason of not finding difference in DTF between groups was due to the small sample size of our study. In contrast, our results were aligned with Hadda et al., who evaluated the DTF in adults’ patients admitted to ICU with acute respiratory failure, to evaluate the effect of invasive NAVA on the diaphragmatic function. In this study, investigators found no significant difference in DT and DTF between invasive NAVA and conventional ventilator [31].
Furthermore, we evaluated lung aeration using LUS in both groups. Although we found no significant difference between NIV-NAVA and NIPPV regarding the LUS, we noted that LUS were significantly high in both groups when compared with our cut-off score (> 10 points) for early prediction of BPD in similar preterm population [27]. The high LUS in both groups is likely because our study population were born at a mean GA of 25 weeks’ gestation with very immature lung. Several studies that evaluated lung aeration using LUS in the first 2 weeks of postnatal age in preterm infants found similar high LUS that accurately predict the diagnosis of BPD at 36 weeks PMA [39,40,41].
We acknowledge our study limitations. First, we had a small sample size that could be attributed to interrupted/low recruitment rate due to the COVID-19 pandemic. Second, the study design was based on consecutive recruitment of all eligible patients but lacked randomization. Thirdly, we did not do a serial ultrasonographic assessment to evaluate the changes of diaphragmatic dimensions and functions over time while infants were supported by these two types of NIV. Another limitation is that infants in the NIV-NAVA group were scanned after short duration post transitioning from NIPPV and the risk of “carryover effect” cannot be ruled out completely. Finally, diaphragm ultrasound is operator dependent; therefore, some variations in the measurements are not uncommon. However, our study results have shown high interobserver reliability which validate, to some degree, the study findings.
Conclusion
In infants born at < 30 weeks’ gestation, NIV-NAVA was associated with significantly higher DE compared to NIPPV reflecting improvement in the diaphragmatic functions. There were no significant differences regarding other measurement such as DTexp, DTins, DTF, and LUS. Further studies, with a larger sample size and serial assessment of the diaphragm are needed to draw a firm conclusion.
Data availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
Abbreviations
- DE:
-
Diaphragm excursion
- DTexp :
-
Diaphragm thickness at end of expiration
- DTF:
-
Diaphragm thickness fraction
- DTins :
-
Diaphragm thickness at end of inspiration
- ICC:
-
Intraclass correlation coefficient
- IMV:
-
Invasive mechanical ventilation
- LU:
-
Lung ultrasound
- LUS:
-
Lung ultrasound score
- NAVA:
-
Neurally adjusted ventilatory assist
- nCPAP:
-
Nasal continuous positive airway pressure
- NICU:
-
Neonatal intensive care unit
- NIPPV:
-
Nasal intermittent positive pressure ventilation
- NIV:
-
Non-invasive ventilation
- RCT:
-
Randomized control trial
- SD:
-
Standard deviation
References
Stein H, Beck J, Dunn M (2016) Non-invasive ventilation with neurally adjusted ventilatory assist in newborns. Semin Fetal Neonatal Med 21:154–161
Lemyre B, Davis PG, De Paoli AG, Kirpalani H (2017) Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation. Cochrane Database Syst Rev 2:Cd003212
Ramaswamy VV, More K, Roehr CC, Bandiya P, Nangia S (2020) Efficacy of noninvasive respiratory support modes for primary respiratory support in preterm neonates with respiratory distress syndrome: systematic review and network meta-analysis. Pediatr Pulmonol 55:2940–2963
Mukerji A, Belik J (2015) Neonatal nasal intermittent positive pressure ventilation efficacy and lung pressure transmission. J Perinatol 35:716–719
Latremouille S, Bhuller M, Shalish W, Sant’Anna G (2021) Cardiorespiratory effects of NIV-NAVA, NIPPV, and NCPAP shortly after extubation in extremely preterm infants: A randomized crossover trial. Pediatr Pulmonol 56:3273–3282
Firestone KS, Beck J, Stein H (2016) Neurally adjusted ventilatory assist for noninvasive support in neonates. Clin Perinatol 43:707–724
Treussart C, Decobert F, Tauzin M, Bourgoin L, Danan C, Dassieu G, Carteaux G, Mekontso-Dessap A, Louis B, Durrmeyer X (2022) Patient-ventilator synchrony in extremely premature neonates during non-invasive neurally adjusted ventilatory assist or synchronized intermittent positive airway pressure: a randomized crossover pilot trial. Neonatology 119:386–393
Makker K, Cortez J, Jha K, Shah S, Nandula P, Lowrie D, Smotherman C, Gautam S, Hudak ML (2020) Comparison of extubation success using noninvasive positive pressure ventilation (NIPPV) versus noninvasive neurally adjusted ventilatory assist (NI-NAVA). J Perinatol 40:1202–1210
Yonehara K, Ogawa R, Kamei Y, Oda A, Kokubo M, Hiroma T, Nakamura T (2018) Non-invasive neurally adjusted ventilatory assist versus nasal intermittent positive-pressure ventilation in preterm infants born before 30 weeks’ gestation. Pediatr Int 60:957–961
Shin SH, Shin SH, Kim SH, Song IG, Jung YH, Kim EK, Kim HS (2022) Noninvasive neurally adjusted ventilation in postextubation stabilization of preterm infants: a randomized controlled study. J Pediatr 247:53-59.e51
Tabacaru CR, Moores RR Jr, Khoury J, Rozycki HJ (2019) NAVA-synchronized compared to nonsynchronized noninvasive ventilation for apnea, bradycardia, and desaturation events in VLBW infants. Pediatr Pulmonol 54:1742–1746
Zhao XQ, Tang ZJ, Xia B (2021) Clinical application of ultrasound in paediatric diaphragmatic dysfunction. Pediatr Dimens 6:1–6
Kharasch SJ, Dumas H, O’Brien J, Shokoohi H, Al Saud AA, Liteplo A, Schleifer J, Kharasch V (2021) Detecting ventilator-induced diaphragmatic dysfunction using point-of-care ultrasound in children with long-term mechanical ventilation. J Ultrasound Med 40:845–852
Wait JL, Johnson RL (1997) Patterns of shortening and thickening of the human diaphragm. J Appl Physiol 83:1123–1132
Boon AJ, Harper CJ, Ghahfarokhi LS, Strommen JA, Watson JC, Sorenson EJ (2013) Two-dimensional ultrasound imaging of the diaphragm: quantitative values in normal subjects. Muscle Nerve 47:884–889
Ferrari G, De Filippi G, Elia F, Panero F, Volpicelli G, Aprà F (2014) Diaphragm ultrasound as a new index of discontinuation from mechanical ventilation. Crit Ultrasound J 6:8
Kim WY, Suh HJ, Hong SB, Koh Y, Lim CM (2011) Diaphragm dysfunction assessed by ultrasonography: influence on weaning from mechanical ventilation. Crit Care Med 39:2627–2630
Bahgat E, El-Halaby H, Abdelrahman A, Nasef N, Abdel-Hady H (2021) Sonographic evaluation of diaphragmatic thickness and excursion as a predictor for successful extubation in mechanically ventilated preterm infants. Eur J Pediatr 180:899–908
Yeung T, Mohsen N, Ghanem M, Ibrahim J, Shah J, Kajal D, Shah PS, Mohamed A (2022) Diaphragmatic thickness and excursion in preterm infants with bronchopulmonary dysplasia compared with term or near term infants: a prospective observational study. Chest
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370:1453–1457
El-Halaby H, Abdel-Hady H, Alsawah G, Abdelrahman A, El-Tahan H (2016) Sonographic evaluation of diaphragmatic excursion and thickness in healthy infants and children. J Ultrasound Med 35:167–175
El-Mogy M, El-Halaby H, Attia G, Abdel-Hady H (2018) Comparative study of the effects of continuous positive airway pressure and nasal high-flow therapy on diaphragmatic dimensions in preterm infants. Am J Perinatol 35:448–454
Grosu HB, Lee YI, Lee J, Eden E, Eikermann M, Rose KM (2012) Diaphragm muscle thinning in patients who are mechanically ventilated. Chest 142:1455–1460
Cohn D, Benditt JO, Eveloff S, McCool FD (1997) Diaphragm thickening during inspiration. J Appl Physiol 83:291–296
Goligher EC, Laghi F, Detsky ME, Farias P, Murray A, Brace D, Brochard LJ, Bolz SS, Rubenfeld GD, Kavanagh BP, Ferguson ND (2015) Measuring diaphragm thickness with ultrasound in mechanically ventilated patients: feasibility, reproducibility and validity. Intensive Care Med 41:642–649
Umbrello M, Formenti P, Longhi D, Galimberti A, Piva I, Pezzi A, Mistraletti G, Marini JJ, Iapichino G (2015) Diaphragm ultrasound as indicator of respiratory effort in critically ill patients undergoing assisted mechanical ventilation: a pilot clinical study. Crit Care 19:161
Mohamed A, Mohsen N, Diambomba Y, Lashin A, Louis D, Elsayed Y, Shah PS (2021) Lung ultrasound for prediction of bronchopulmonary dysplasia in extreme preterm neonates: a prospective diagnostic cohort study. J Pediatr 238:187-192.e182
Brat R, Yousef N, Klifa R, Reynaud S, Shankar Aguilera S, De Luca D (2015) Lung ultrasonography score to evaluate oxygenation and surfactant need in neonates treated with continuous positive airway pressure. JAMA Pediatr 169:e151797
Hayat A, Khan A, Khalil A, Asghar A (2017) Diaphragmatic excursion: does it predict successful weaning from mechanical ventilation? J Coll Physicians Surg Pak 27:743–746
Zambon M, Greco M, Bocchino S, Cabrini L, Beccaria PF, Zangrillo A (2017) Assessment of diaphragmatic dysfunction in the critically ill patient with ultrasound: a systematic review. Intensive Care Med 43:29–38
Hadda V, Pahuja S, Mittal S, Madan K, Khan MA, Mohan A, Guleria R (2022) Effects of neurally adjusted ventilation assist (NAVA) and conventional modes of mechanical ventilation on diaphragm functions: a randomized controlled trial. Heart Lung 53:36–41
Alam MJ, Roy S, Iktidar MA, Padma FK, Nipun KI, Chowdhury S, Nath RK, Rashid HO (2022) Diaphragm ultrasound as a better predictor of successful extubation from mechanical ventilation than rapid shallow breathing index. Acute Crit Care 37:94–100
Lee BK, Shin SH, Jung YH, Kim E-K, Kim H-S (2019) Comparison of NIV-NAVA and NCPAP in facilitating extubation for very preterm infants. BMC Pediatr 298
Yagui AC, Gonçalves PA, Murakami SH, Santos AZ, Zacharias RSB, Rebello CM (2021) Is noninvasive neurally adjusted ventilatory assistance (NIV-NAVA) an alternative to NCPAP in preventing extubation failure in preterm infants? J Matern Fetal Neonatal Med 34:3756–3760
Soreze Y, Motte E, Dell’Orto V, Yousef N, De Luca D (2018) Use of neurally adjusted ventilator assist in postsurgical hemidiaphragmatic paralysis. Arch Dis Child Fetal Neonatal Ed 103:F86-f87
Gentili A, Masciopinto F, Mondardini MC, Ansaloni S, Reggiani ML, Baroncini S (2013) Neurally adjusted ventilatory assist in weaning of neonates affected by congenital diaphragmatic hernia. J Matern Fetal Neonatal Med 26:598–602
Roosens S, Derriks F, Cools F (2016) Case report: Non-invasive neurally adjusted ventilatory assist in a newborn with unilateral diaphragmatic paralysis. Pediatr Pulmonol 51:E37-e39
Rehan VK, Laipraset J, Wallach M, McCool FD (1999) Effects of CPAP on diaphragm dimensions in the neonate. Pediatr Res 45:317–317
Alonso-Ojembarrena A, Lubián-López SP (2019) Lung ultrasound score as early predictor of bronchopulmonary dysplasia in very low birth weight infants. Pediatr Pulmonol 54:1404–1409
Loi B, Vigo G, Baraldi E, Raimondi F, Carnielli VP, Mosca F, De Luca D (2021) Lung ultrasound to monitor extremely preterm infants and predict bronchopulmonary dysplasia. A multicenter longitudinal cohort study. Am J Respir Crit Care Med 203:1398–1409
Oulego-Erroz I, Alonso-Quintela P, Terroba-Seara S, Jiménez-González A, Rodríguez-Blanco S (2021) Early assessment of lung aeration using an ultrasound score as a biomarker of developing bronchopulmonary dysplasia: a prospective observational study. J Perinatol 41:62–68
Acknowledgements
We would also like to thank the families, nurses, and respiratory therapists in Mount Sinai Hospital who helped us complete our study.
Guarantor
AM, who takes responsibility for the content of the manuscript, including the data and analysis (Original Research).
Author information
Authors and Affiliations
Contributions
ME and LT has equal contribution to the study design, data collection, and manuscript writing. AM had full access to all of the data and takes responsibility for the content of this manuscript, including study design, data, and data analysis. The study design was conducted by LT, ME, JI, NN, and AM; data collection was performed by JI, ME, and AM. Data analysis was performed by NN and AM. The manuscript was prepared by ME and LT then edited by JI, NN, and AM. All authors of this study approved the final draft of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Local research ethics approval was obtained (Mount Sinai Hospital REB (19–0324-E), Toronto, ON, Canada.
Consent to participate
Written informed consent was obtained from parents or guardians.
Consent for publication
All the authors have seen the final version of the manuscript and gave their full consent for the publication.
Conflicts of interest
The authors declare no competing interests.
Additional information
Communicated by Daniele De Luca
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abstract publication/presentation
Portions of this paper were presented at the Pediatric Academic Society (PAS) meeting in Denver, USA, in May 2022 a as poster presentation as well as at the 4th Neonatal Research Day- Toronto, Canada, on April 2022 as an oral presentation.
Rights and permissions
About this article
Cite this article
Elkhouli, M., Tamir-Hostovsky, L., Ibrahim, J. et al. Ultrasonographic assessment of diaphragmatic function in preterm infants on non-invasive neurally adjusted ventilatory assist (NIV-NAVA) compared to nasal intermittent positive-pressure ventilation (NIPPV): a prospective observational study. Eur J Pediatr 182, 731–739 (2023). https://doi.org/10.1007/s00431-022-04738-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-022-04738-8