Abstract
The purpose of this is to evaluate the effect of supplementation of enteral feed volume with preterm versus term donor human milk (DHM) on short-term physical growth in very low birth weight (VLBW) neonates. In this open-label, variable block-sized, superiority, randomized controlled trial with allocation concealment, VLBW neonates with insufficient volume of mother’s own milk (MOM) were assigned to receive either preterm (n = 48) or term (n = 54) DHM till discharge. Preterm DHM was defined as the breast milk expressed within 28 days of delivery at ≤ 34 weeks of gestation. The primary outcome was days to regain birth weight. Maternal and neonatal demographic variables were comparable in the two study groups. Days to regain birth weight were significantly more in the preterm DHM group, 17.4 (7.7) vs 13.6 (7.2) days, mean difference (95% CI) being 3.74 (0.48–7.0) days, P = 0.02). The proportion of MOM use was 82% in preterm vs 91.1%, P = 0.03 in the term milk group. Duration of skin-to-skin contact was also significantly lower in the preterm vs term milk group, the median (IQR) was 4 (0, 6) vs 4 (2, 6) hours/day, P < 0.01. However, bronchopulmonary dysplasia was higher in the preterm milk group (13% vs. 4%, P = 0.17). The velocity of gain in weight was similar in the two groups from week 1–3 but higher in the term DHM supplementation group during the 4th week.
Conclusion: Supplementing MOM with preterm DHM did not result in a faster regaining of birth weight.
Trial registration: CTRI/2020/02/023569; Date: 17.02.2020.
What is Known: • Human milk content is influenced by various parameters including maternal, neonatal, and methodological. • Preterm DHM should cause rapid short-term weight gain in VLBW neonates as it has higher protein content. | |
What is New: • In the setting of MOM contributing a major fraction of feeding volume, selective supplementation of feeding volume with preterm DHM does not result in rapid short-term weight gain in VLBW neonates. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extrauterine growth retardation (EUGR) develops in approximately 75–97% of the very low birth weight (VLBW) babies during postnatal life [1]. Auxological extrauterine growth retardation is associated with adverse long-term outcomes [2].
The nutritional regimen for VLBW infants should be able to achieve a postnatal growth rate that mimics intrauterine growth. The protein needed of preterm neonates is particularly high and plays a very important role during the postnatal period. Mother’s own milk (MOM) is considered the best nutrition during the first six months of birth [3]. Pasteurized donor human milk (DHM) is the preferred alternative for vulnerable neonates in cases of unavailability or insufficient quantity of MOM [4, 5].
The energy, protein, and fat content of milk are dependent upon: the type of milk as colostrum, mature or transition milk, term, and preterm milk, or human milk fortifiers. Although fortification of human milk in preterm neonates is standard global practice, the World Health Organization guidelines on the feeding of VLBW infants do not support routine multicomponent fortification except in infants who fail to gain weight despite adequate breast milk feeding [6]. Different feeding strategies aim to match growth velocity to the intrauterine foetal growth with an average weight gain of 24–26 g/d in the late third trimester [7]. Milk volume for neonates up to 200 ml/kg/d has been used in different studies and found to be associated with improved weight gain [8]. The use of any exogenous protein is akin to additional cost and gut dysbiosis [9]. Preterm milk is a naturally available human source of protein.
Preterm milk is usually defined as milk donated by women within 28 days of giving birth at less than 34 weeks of gestation, and term milk is donated by women delivered after 34 weeks of gestation or after 28 days of birth if delivered at less than 34 weeks of gestation [10]. Since preterm milk has higher protein content with a maximum mean difference of up to 0.7 g/dL, it should provide extra calories and should have a short-term effect on growth with potential long-term implications [11]. Protein supplementation increases the fat-free mass (FFM) accretion in infants. Weight gain resulting from FFM gain increases brain size and reduces the risk of adverse neurodevelopmental outcomes among preterm infants [12].
Human milk content is influenced by many parameters which are broadly classified as maternal, neonatal, and methodological [13]. Because of differences in the macronutrient composition of preterm and term milk, pooled pasteurized preterm DHM should ideally cause early short-term weight gain in VLBW neonates. Although the protein content will be variable and will differ with the proportion of milk used [13], the evidence on the same is yet to be generated. More so, donor milk is generally collected from mothers of term infants and at a mature stage of lactation when milk production is more than the own baby’s requirement [14]. To the best of our knowledge, this is the first study comparing the effect of preterm and term DHM on the growth of VLBW babies where MOM is insufficient.
Methods
The randomized controlled trial was conducted from July 2020 to March 2021 in a level III neonatal intensive care unit (NICU) at a tertiary care hospital in India.
Study subjects
Potentially eligible neonates were identified from a clean labour room nursery immediately following birth. All consecutive inborn VLBW (birth weight ≤ 1500 g) neonates on enteral feeds having an insufficient volume of MOM were enrolled in the study after obtaining written informed consent from the mother/guardian. Insufficient volume of MOM was defined as MOM volume less than 80% on any day after the beginning of enteral nutrition and later any volume lesser than the requirement of that particular day. Neonates with major congenital malformation, necrotizing enterocolitis stage IIa or higher before enrolment, HIV-positive mothers, birth at gestation age < 27 weeks or birth weight < 800 g, galactosemia or other contraindications to human milk feeding, and maternal infection by severe acute respiratory syndrome by coronavirus 2 (SARS-CoV-2) were excluded from the study.
Randomization and allocation concealment
A researcher not involved in the study recruitment or outcome assessment generated the random number sequence of block sizes 4 or 6 using a web-based random number generator. The random number sequence was stratified by birth weight (≤ 1000 g and > 1000 g). The sequence was kept in serially numbered opaque sealed envelopes. Multiple births were assigned to the same study group. Physicians, researchers, and nurses were aware, whereas parents were unaware of the group allocation. Physicians were not blinded as they were involved in providing intervention. Independent investigator cross-verified outcome of random samples. Milk disbursal bottles were labelled and stored by milk bank staff who were not aware of group allocation and were not involved in any clinical care.
Interventions
Participants were randomly assigned to receive enteral feed volume supplementation with preterm (preterm milk group) or term pasteurized DHM (term milk group) whenever MOM volume was insufficient. A dedicated milk bank staff is used to screen and enrol mothers, maintain both online and offline records, and collect milk according to the term and preterm groups. Colour-coded labels were applied to the collection and disbursal bottles of both the milk groups. Milk collection and disbursal according to groups were ensured daily by the primary investigator. DHM was frozen at − 200 °C after the Holder method of pasteurization (30 min at 62.5 °C) and thawed in lukewarm water at the time of disbursal for use. A daily log of the time of initiation of the first feed, total feed volume per day, and the proportion of MOM and DHM was maintained. Patients were followed up till 40 weeks of postmenstrual age or discharge whichever was earlier. Criteria for discharge included resolution of medical illness, weight ≥ 1450 g with a gain of weight for consecutive 3 days, postmenstrual age ≥ 34 weeks, acceptance of feed by spoon or direct breastfeeding, and ability to maintain normal body temperature outside a radiant warmer or incubator.
Anthropometric data collection
The weight of the baby was taken daily at approximately the same time of the day using the electronic weighing scale with an accuracy of 10 g. Once a week, the accuracy of the weighing scale was checked using a standard weight. Time of initiation and quantity of human milk fortifier, nutritional supplements, feed intolerance episodes, and daily duration of skin-to-skin (STS) contact hours were recorded. The length of the neonate was measured using an infantometer to the nearest 1 mm correction while the occipitofrontal circumference (OFC) was measured using a non-stretchable plastic measuring tape with 0.1 cm accuracy. Anthropometric measurements were done using the methodology prescribed by the Intergrowth-21 study [15].
Feeding protocol and other interventions common to both groups
The eligible neonates were assessed daily for feed initiation. Once neonates were hemodynamically stable with the soft abdomen and audible bowel sounds, feeds were initiated as intermittent boluses at 2-h intervals. The standard protocol of feeding was uniformly followed in both groups. For neonates born at 260 to 286 weeks of gestation, minimal enteral nutrition was started at 10–15 ml/kg/day on day 1, and the enteral feed volume was increased by 20 to 30 ml/kg/day. For neonates born at 290 to 306 weeks of gestation, the feed was started at 20 to 30 ml/kg/day and increased by 30 to 40 ml/kg/day. Neonates born at ≥ 310 weeks of gestation were given all milk feed (80 ml/kg/day total fluid volume) from day 1 of life. Later daily total fluid and milk requirement of every neonate was decided according to day of life, loss or gain of weight every day, and associated comorbidities like patent ductus arteriosus and urine output. Advancement of feeds was done till infants reached a feed volume of 180 to 200 mL/kg/day. Once infants were transitioned from gavage feeds to direct feeds, volume was not controlled but was offered ad libitum. MOM feeding was strongly recommended for all neonates. Every possible effort was made to procure MOM for each neonate. As per the unit policy, all the mothers were counselled to express breast milk within 6 h of delivery and then two to three times a day, by the lactation counsellor, resident doctors, and nursing staff. Human milk fortifier (macronutrient composition in grams in 1 g powder sachet: protein 0.27; total fat, 0.04; carbohydrates, 0.49) was added (1 sachet per 25 ml of expressed breast milk) once the baby reached 100 ml/kg/day of enteral feeds. Neonates who were not on total enteral feeds were initiated on parenteral nutrition to achieve a total protein intake of 3 to 3.5 g/kg/day and calorie intake of 120 to 130 kcal/kg/day. Probiotics, caffeine, and other nutrition supplements were added to both groups as per the standard policy of the unit.
Outcome measures
The primary outcome of interest was days to regain birth weight. The number of days taken to regain birth weight after initial physiological weight loss was noted. It was taken as the first of three successive days when the weight was greater than or equal to the birth weight.
Secondary outcomes included rate of gain in weight, length, and OFC till discharge or 40 weeks postmenstrual age whichever came earlier, time to achieve full feed (day 1 of 120 ml/kg/day if tolerated for 3 consecutive days), the incidence of NEC stage IIa or above, and episodes of feeding intolerance. Feed intolerance was defined as the presence of any of the following signs: significant gastric residuals of > 33% on 2 consecutive occasions or > 50% on a single occasion when the total feed volume was > 8 ml or aspirates of > 4 ml on two occasions if the total feed volume was < 8 ml, increase in abdominal girth by > 2 cm from the previous value, and the presence of brownish/bilious/bloodstained gastric aspirates or vomiting for which feeds had to be withheld for ≥ 12 h [16]. NEC was defined by modified Bell’s staging criteria [17].
The primary outcome, time to regain birth weight, was calculated as mean days in achieving birth weight beginning from day one to the day of birth. The secondary outcome, weight gain velocity, was calculated by the 2-point average weight model. This was calculated by dividing the total difference in weight at 2 points by the number of days and average weight using the following formula [18].
\(\frac{\left[1000\times \left({W}_{n}-{W}_{1}\right)\right]}{\left\{\left({D}_{n}-{D}_{1}\right)\times \left[\frac{\left({W}_{n}-{W}_{1}\right)}{2}\right]\right\}}\) (Wn = Weight at endpoint of primary outcome, W1 = weight on day 1 of regaining of birth weight, Dn = last day of primary outcome, D1 = first day of regaining of birth weight)
Sample size calculation
The sample size was calculated based on a previous study where DHM was used in neonates (birth weight < 1600 g and gestation age 27 to 33 weeks) as a sole diet and reported 7 days lesser time in regaining birth weight with preterm as compared with term milk [19]. Based on the unit’s experience, neonates in the current study would need 20 to 80% DHM as a supplement to MOM on any given day. A difference in duration of regaining birth weight of 3 days was predicted in the index study due to the lesser need for DHM. Taking a difference of 3 (5) days between two groups (18 days preterm and 21 days in term milk), the minimum required sample size with 80% power and a 2-sided significance of 5% was 44 subjects in each group. Considering an attrition rate of 20%, 50 VLBW neonates were enrolled in each group.
The trial was approved by the institutional ethics committee and was prospectively registered in the clinical trials registry of India, (CTRI/2020/02/023569).
Statistical analysis
Continuous variables were expressed as mean and standard deviation if normally distributed and as median and interquartile range when skewed. Categorical variables were expressed as numbers and proportions. Quantitative data with normal distribution were compared using the Student’s t test and those with skewed distribution were analysed using the Mann–Whitney U test. Categorical data were compared using chi-square or Fisher exact test as applicable. A p value of < 0.05 was considered significant. Time to regain birth weight was also analysed using the Kaplan–Meier survival analysis and log-rank test. A linear regression analysis was done to adjust for the variables that could influence the primary outcome and were found to be significantly different in the two study groups in univariate analysis. The regression model included days to achieve birth weight (primary outcome) as the dependent variable and study group (binary variable), birth weight less than 1000 g (binary variable), the proportion of DHM in the total milk intake during the hospital stay (continuous variable), and total STS hours during the hospital stay (continuous variable) as the independent (predictor) variables. The collinearity of the included variables was checked by calculating the variance inflation factors (not found to be significant) and robust estimates were calculated. Analysis was done using the intention to treat principle. Statistical analysis was conducted using SPSS version 23.0.
Results
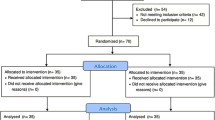
Out of a total of 173 VLBW neonates born over the study period of nine months (July 2020 to March 2021), 102 were enrolled: 54 in preterm and 48 in the term milk group and 71 neonates were excluded as per predefined criteria (Fig. 1). Maternal and neonatal baseline characteristics were comparable in the two groups (Table 1).
Time to regain birth weight, mean (SD) in preterm group 17.4 (7.7) as compared to term 13.6 (7.2) milk group, was longer, 3.74 (0.48 to 7.0); P = 0.03 (Fig. 2). The number of days taken to regain birthweight remained significantly lower in the term DHM group with an adjusted mean difference (95% CI) being 3.6 (− 5 to − 2.2) days even after adjusting for birth weight being less than 1000 g, the proportion of DHM in cumulative feed volume during the hospital stay and the cumulative duration of STS during the hospital stay. The average duration of weight loss was 7.8 (4.4) and 6.7 (5) days (P = 0.28) in the preterm and term milk groups, respectively. The volume of MOM was similar but the proportion of its usage was significantly less in the preterm milk group (82% vs 91.1%, P = 0.03) (Supplementary Fig. 1). Daily DHM requirement was significantly lesser in the term milk group (Supplementary Fig. 2). No difference was found in calculated energy 123.35 (7.32) vs 121 (9.97), P = 0.23, and protein 2.84 (0.48) vs 2.75 (0.39), P = 0.37, intake in both the study groups (Table 2).
Weight gain velocity was significantly less during the fourth week of hospital stay in the preterm milk group (Table 3 and Fig. 3). However, among 7 neonates out of a total of 14, who weighed < 1000 g at birth and survived till discharge, days to regain birth weight in the preterm and term milk group were 16.7 (7.8) vs 28.3 (12.4) days, respectively, mean difference (95% CI) being − 11.5 (− 36 to 13) days, P = 0.2). Time to achieve full feed, the incidence of NEC stage IIa or above, and episodes of feeding intolerance were similar in both the groups. However, bronchopulmonary dysplasia and neonates who died or were discharged against medical advice were more in the preterm milk group (Table 4).
Discussion
In low- and middle-income countries where the higher cost and limited availability of human milk-based fortifiers exists, preterm milk with higher protein content can provide a better form of nutrition for at-risk neonates. This study randomized VLBW neonates to receive either preterm or term DHM when their MOM was not sufficiently available. Time to regain birth weight in the study was significantly more in preterm as compared to the term milk group which was non-consistent with the hypothesis. Firstly, this might be a chance finding. The second cause of faster regaining of birth weight in the term milk group could be the effect of co-interventions like overall lesser DHM supplementation and significantly higher use STS contact duration.
The overall need for DHM was less than 20% in the current study. A dedicated lactation counsellor in the unit helped all the mothers in expressing milk soon after delivery. All the mothers were also advised daily to 3–4 L of water intake, a balanced diet, and sleep by a lactation counsellor. In most neonates, the available MOM volume was less than 20% on the day of initiation of feeds but it soon increased after daily counselling for milk expression, diet, and liquid intake. On subsequent days, the available MOM volume was more than 80–90%. Neonates once enrolled remained in the study irrespective of DHM volume requirement. This brought large variation in DHM and MOM volume in the two groups.
In a previous study where DHM was given as the sole diet, birth weight was regained earlier in the preterm milk supplementation group (11.4 vs 18.8 days). Each aliquot of DHM was also analysed for nutrient content both before and after pasteurization.15 More protein, fat, and energy content of mother’s milk improves weight gain in comparison with DHM where these macronutrients are affected by pasteurization [20]. A reduction of 3.5% in fat, 3.9% in protein, and 2.8% in energy has been reported post pasteurization [21, 22]. Duration of STS (hours/d) in the current study was also significantly higher 4 (0, 6) vs 4 (2, 6), P < 0.01 in the term milk group, and it is a well-described intervention associated with better weight gain. A recent Cochrane review showed that STS was associated with increased weight gain of 4.1 (2.3 to 5.9) g/d [23].
The third cause could be the difference in sickness level of the two groups during the progression of the study. Higher mortality of the sick neonates in the term milk group who at enrolment had a difference in the score of neonatal acute physiology and hence the survival of relatively more stable babies in term milk group in the current study overestimated the result in rapid and better weight gain, although this difference was not statistically significant. Few parameters causing a negative effect on growth such as bronchopulmonary dysplasia were higher in the preterm vs term milk group (13% vs. 4%, P = 0.17).
More than one-third of the study population was with severe intrauterine growth retardation which had a poor genetic potential for postnatal growth [24]. These small for gestation age neonates had relatively advanced gestation age and started taking direct breastfeeds with measured feeds after the acute illness was over. This led to a total quantified feed volume of 120–130 ml/kg/d and protein intake of less than 3 g/kg/d. The study population characteristics (30% infants with shock and sepsis, 12% infants with severe IVH) could also be a barrier to optimal weight gain.
Among the stratified group of neonates weighing less than 1000 g showed a faster regain of the birth weight and better weight, length, and OFC gain velocities till discharge. However, the number of enrolled neonates weighing less than 1000 g was less due to the limitation of the number of deliveries due to the SARS-CoV-2 pandemic, and only seven neonates survived till discharge. Time to regain birth weight remained significantly lesser in the term milk group even after adjusting for birth weight being less than 1000 g, the proportion of DHM in cumulative feed volume during the hospital stay, and the cumulative duration of STS during the hospital stay.
The preterm DHM has higher protein content as compared to the term milk. However, this difference becomes narrower after the first week (from 0.7 to 0.2 g/dL). Hence, the clinical significance of more proteins is likely to be high during the initial week [11]. Gross et al. have also shown a similar trend of protein difference in preterm and term DHM with a difference in protein content up to 0.9 to 1.2 g/dL during the initial 2 weeks. This difference reduces to 0.2 to 0.4 g/dL over the next 2 weeks [19]. There is emerging data that higher protein intake is associated with better head growth and neurodevelopment [3]. Low intakes of proteins during the first week of life have found to be associated with impaired neurocognitive development at 18–22 months of age [25]. Isolated weight gain could be a manifestation of fat mass accumulation which may not reflect brain growth [26]. The study has tried to see the effect of preterm DHM on weight gain; however, head growth could be a better parameter. However, there was no difference in head growth velocity in this study during the first week and throughout the hospital stay. A larger sample size might be required to see the effect of preterm and term DHM on head growth and neurodevelopmental outcome. Growth is a multidimensional and continuous process, and it depends upon several other factors such as body composition, gender, and genetic makeup [27]. Hence, valid estimation requires consideration of these parameters with long-term follow-up too.
Variability in the composition of MOM or DHM obtained from a single donor and multiple donor pool has been observed. High variability in the composition of fat, protein, and energy was found for all types of samples. This variability remained persistent even after standard fortification. Feeding with pooled DHM may result in inadequate or nonuniform growth hence individualized fortification was recommended [20]. Short of biochemical analysis for nutrients in each pooled sample of milk in our study, the actual higher protein content of preterm milk cannot be assured.
Strengths of the study
Every step was meticulously followed from preterm and term milk collection to disbursal to prevent cross-contamination between the groups.
Limitations
Limitations of the study are the non-availability of biochemical analysis of pooled DHM, the absence of long-term neurodevelopmental follow-up, fewer neonates in < 1000 g stratified group, and an open-label design. The overall requirement of DHM was lesser than the assumption while calculating sample size and limits the validity of the results. Further, the findings of this study could be related to important confounders such as MOM intake per day and STS duration, even though such confounding could creep in when a pragmatic approach to study conduct is undertaken.
Conclusion
In a setting where MOM constitutes a large fraction of enteral feeding volume, selective supplementation with preterm donor milk to meet the feed volume requirement did not result in improved physical growth during the hospital stay. Future research with preterm milk as supplemental DHM is warranted in extremely low birth weight neonates who are at higher risk for EUGR. Further, the same may be supplemented with biochemical analysis of macronutrients in pooled or single donor human milk, used as supplemental feed. Differences in human milk composition at different gestation and different time points can also be assessed to correlate the effect on growth.
Abbreviations
- BPD:
-
Bronchopulmonary dysplasia
- DHM:
-
Donor human milk
- EUGR:
-
Extrauterine growth retardation
- FFM:
-
Fat-free mass
- MOM:
-
Mother’s own milk
- NEC:
-
Necrotizing enterocolitis
- NICU:
-
Neonatal intensive care unit
- OFC:
-
Occipitofrontal circumference
- STS:
-
Skin-to-skin
- VLBW:
-
Very low birth weight
References
Dusick AM, Poindexter BB, Ehrenkranz RA, Lemons JA (2003) Growth failure in the preterm infant: can we catch up? Semin Perinatol 27:302–310. https://doi.org/10.1016/s0146-0005(03)00044-2
De Curtis M, Rigo J (2004) Extrauterine growth restriction in very-low-birthweight infants. Acta Paediatr 93:1563–1568. https://doi.org/10.1080/08035250410022198
Agostoni C, Buonocore G, Carnielli VP et al (2010) Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr 50:85–91. https://doi.org/10.1097/MPG.0b013e3181adaee0
Committee ESPGHAN, on Nutrition, Arslanoglu S, Corpeleijn W, et al (2013) Donor human milk for preterm infants: current evidence and research directions. J Pediatr Gastroenterol Nutr 57:535–542. https://doi.org/10.1097/MPG.0b013e3182a3af0a
Committee on Nutrition; Section on Breastfeeding; Committee on Fetus and Newborn, (2017) Donor human milk for the high-risk infant: preparation, safety, and usage options in the United States. Pediatrics 139:e20163440. https://doi.org/10.1542/peds.2016-3440
World Health Organization (WHO) (2011) Guidelines on optimal feeding of low birth-weight infants in low- and middle-income countries [Internet]. Geneva, Switzerland: World Health Organization. http://www.ncbi.nlm.nih.gov/books/NBK298973/PubMed. Accessed 6 Dec 2014
Owen P, Donnet ML, Ogston SA, Christie AD, Howie PW, Patel NB (1996) Standards for ultrasound fetal growth velocity. Br J Obstet Gynaecol 103:60–69. https://doi.org/10.1111/j.1471-0528.1996.tb09516.x
Abiramalatha T, Thomas N, Thanigainathan S (2021) High versus standard volume enteral feeds to promote growth in preterm or low birth weight infants. Cochrane Database Syst Rev 9(3):CD012413. https://doi.org/10.1002/14651858.CD012413.pub3
Chan GM, Lee ML, Rechtman DJ (2007) Effects of a human milk-derived human milk fortifier on the antibacterial actions of human milk. Breastfeed Med 2:205–208. https://doi.org/10.1089/bfm.2007.0015
Faerk J, Skafte L, Petersen S, Peitersen B, Michaelsen KF (2001) Macronutrients in milk from mothers delivering preterm. Adv Exp Med Biol 501:409–413. https://doi.org/10.1007/978-1-4615-1371-1_51
Gidrewicz DA, Fenton TR (2014) A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatr 14:216. Published 2014 Aug 30. https://doi.org/10.1186/1471-2431-14-216
Ramel SE, Gray HE, Christiansen E, Boys C, Georgieff MK, Demerath EW (2016) Greater early gains in fat-free mass, but not fat mass, are associated with improved neurodevelopment at 1 year corrected age for prematurity in very low birth weight preterm infants. J Pediatr 173:108–115. https://doi.org/10.1016/j.jpeds.2016.03.003 (Epub 2016 Apr 4)
Hsu YC, Chen CH, Lin MC, Tsai CR, Liang JT, Wang TM (2014) Changes in preterm breast milk nutrient content in the first month. Pediatr Neonatol 55:449–454. https://doi.org/10.1016/j.pedneo.2014.03.002
Fomon SJ, Ziegler EE, Vázquez HD (1977) Human milk and the small premature infant. Am J Dis Child 131:463–467. https://doi.org/10.1001/archpedi.1977.02120170089018
Villar J, Cheikh Ismail L, Victora CG et al (2014) International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the Intergrowth-21st Project. Lancet 384:857–868. https://doi.org/10.1016/S0140-6736(14)60932-6
Dorling J, Abbott J, Berrington J et al (2019) (2019) Controlled trial of two incremental milk-feeding rates in preterm infants. N Engl J Med 381:1434–1443. https://doi.org/10.1056/NEJMoa1816654
Neu J (1996) Necrotizing enterocolitis: the search for a unifying pathogenic theory leading to prevention. Pediatr Clin North Am 43:409–432. https://doi.org/10.1016/s0031-3955(05)70413-2
Patel AL, Engstrom JL, Meier PP, Jegier BJ, Kimura RE (2009) Calculating postnatal growth velocity in very low birth weight (VLBW) premature infants. J Perinatol 29:618–622. https://doi.org/10.1038/jp.2009.55
Gross SJ (1983) Growth and biochemical response of preterm infants fed human milk or modified infant formula. N Engl J Med 308:237–241. https://doi.org/10.1056/NEJM198302033080501
de Halleux V, Rigo J (2013) Variability in human milk composition: benefit of individualized fortification in very-low-birth-weight infants. Am J Clin Nutr 98:529S-S535. https://doi.org/10.3945/ajcn.112.042689
García-Lara NR, Vieco DE, De la Cruz-Bértolo J, Lora-Pablos D, Velasco NU, Pallás-Alonso CR (2013) Effect of Holder pasteurization and frozen storage on macronutrients and energy content of breast milk. J Pediatr Gastroenterol Nutr 57:377–382. https://doi.org/10.1097/MPG.0b013e31829d4f82
Vieira AA, Soares FV, Pimenta HP, Abranches AD, Moreira ME (2011) Analysis of the influence of pasteurization, freezing/thawing, and offer processes on human milk’s macronutrient concentrations. Early Hum Dev 87:577–580. https://doi.org/10.1016/j.earlhumdev.2011.04.016
Conde-Agudelo A, Diaz-Rossello JL, Belizan JM (2003) Kangaroo mother care to reduce morbidity and mortality in low birth weight infants. Cochrane Database Syst Rev (2):CD002771. https://doi.org/10.1002/14651858.CD002771
Sharma D, Shastri S, Sharma P (2016) Intrauterine growth restriction: antenatal and postnatal aspects. Clinical Medicine Insights Pediatr 10:67–83. https://doi.org/10.4137/CMPed.S40070
Stephens BE, Walden RV, Gargus RA, Tucker R, McKinley L, Mance M, Nye J, Vohr BR (2009) First-week protein and energy intakes are associated with 18-month developmental outcomes in extremely low birth weight infants. Pediatrics 123:1337–1343. https://doi.org/10.1542/peds.2008-0211
Bell KA, Matthews LG, Cherkerzian S, Palmer C, Drouin K, Pepin HL, Ellard D, Inder TE, Ramel SE, Belfort MB (2019) Associations of growth and body composition with brain size in preterm infants. J Pediatr 214:20-26.e2. https://doi.org/10.1016/j.jpeds.2019.06.062
Merrick J (2013) Child health and human development over the lifespan. Front Public Health 19(1):1. https://doi.org/10.3389/fpubh.2013.00001
Author information
Authors and Affiliations
Contributions
Dr. Vimlesh Soni collected data, carried out the initial analyses, and drafted the initial manuscript. Dr. Suksham Jain conceptualized and designed the study, supervised data collection, and reviewed and revised the manuscript. Dr. Deepak Chawla carried out the analyses and reviewed the manuscript for intellectual content. Dr. Supreet Khurana carried out the analyses and interpretation of data and reviewed the manuscript for intellectual content. Dr. Shikha Rani carried out the analyses and interpretation of data and reviewed the manuscript for intellectual content. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by ethics committee of GMCH no. GMCH/IEC/2019/238 Dated 30.12.2019.
Consent to participate
Written informed consent was obtained from parents of all participants.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Gregorio Milani
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Summary Different macronutrient compositions of preterm donor human milk than of term should cause early short-term weight gain in VLBW neonates. The current study was done to assess the same.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Soni, V., Jain, S., Chawla, D. et al. Supplementation of mother’s own milk with term versus preterm donor human milk: a randomized controlled trial. Eur J Pediatr 182, 709–718 (2023). https://doi.org/10.1007/s00431-022-04711-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-022-04711-5