Abstract
In patients with congenital heart disease (CHD), reduced exercise capacity can be a predictor for late complications and may be used to guide interventions. Yet, the interpretation of exercise capacity is challenged by changes in body composition during growth. Our aim was to create an overview of disease-specific exercise capacity in children with CHD. We performed a multicentre retrospective study of exercise capacity of CHD patients, aged 6–18 years, tested between January 2001 and October 2018. Sex-specific distribution graphs were made using the LMS method and height to relate to body size. We included all CHD with N > 50, including severe defects (e.g., univentricular heart, tetralogy of Fallot) and “simple” lesions as ventricular septum defect and atrial septum defect. We included 1383 tests of 1208 individual patients for analysis. The peak oxygen uptake (VO2peak, 37.3 ml/min/kg (25th–75th percentile 31.3–43.8)) varied between specific defects; patients with univentricular hearts had lower VO2peak compared with other CHD. All groups had lower VO2peak compared to healthy Dutch children. Males had higher VO2peak, Wpeak and O2pulsepeak than females. Sex- and disease-specific distribution graphs for VO2peak, Wpeak and O2pulsepeak showed increase in variation with increase in height.
Conclusion: Disease-specific distribution graphs for exercise capacity in children with CHD from a large multicentre cohort demonstrated varying degrees of reduced VO2peak and Wpeak. The distribution graphs can be used in the structured follow-up of patients with CHD to predict outcome and identify patients at risk.
What is Known: • Children with congenital heart disease (COnHD) are at risk to develop heart failure, arrhytmia’s and other complications. Exercise capacity may be an important predictor for outcome in children with ConHD. In children, the interpretation of exercise capacity poses an additional challenge related to physical changes during growth. | |
What is New: • In this report of a multi-center cohort >1300 childrewn with ConHD, we related the changes in exercise capacity to length. We demonstrated that exercise capacity was reduced as compared with healthy children and we observed variation between disease groups. Patients with a univentricular circulation (Fontan) had worse exercise capacity. We constructed disease specific charts of development of exercise capacity throughout childhood, accessible via a web-site. These graphs may help practitioner to guide children with ConHD. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The last decades have seen major improvements in treatment of patients with congenital heart disease (CHD). The concomitant increase in prevalence of patients surviving with a CHD leads to a new population at risk for major long-term adverse events [1]. Many children treated for CHD have some degree of structural abnormality remaining after surgical correction, which can predispose to develop complications such as arrhythmias or heart failure [2, 3]. These complications may lead to impaired exercise tolerance, re-intervention, rehospitalisation, or death [4, 5].
A major quest in the follow-up of children with CHD is to predict who will develop these complications and when to intervene. In adults, exercise capacity is a good predictor of mortality in Fontan patients [6] and can be used as a surrogate of morbidity [7]. Exercise capacity could also be used in the paediatric population with CHD as it can be serially assessed with a cardiopulmonary exercise test (CPET) [8]. There is a wide variety in types of CHD with potentially different effects on baseline exercise capacity, e.g., univentricular heart, tetralogy of Fallot, transposition of the great arteries, or ventricular septal defect (VSD). The use of exercise capacity in the growing cohort of patients with CHD has been restricted by limited numbers in this wide variety of specific lesions. Recent guidelines state that exercise capacity should be related to disease specific reference values, yet to the best of our knowledge, there is only limited data available for peak oxygen consumption for children with CHD [9, 10].
In children, the interpretation of CPET poses an additional challenge related to physical changes during growth [11]. In general, exercise capacity increases with growth to reach a maximum in early adulthood [12,13,14,15]. To avert confounding, there is a need to relate CPET parameters with metrics of growth. An ideal metric is easy to attain and allows comparison of data throughout growth.
Our aim was to provide an overview for exercise capacity for children with CHD. For that purpose, we first tested available metrics related to growth (age, length, weight and BSA) and next analysed CPETs obtained during routine follow-up visits at the outpatient clinic.
Materials and methods
This study is a retrospective multicentre cohort study of children treated for CHD in four university hospitals, (i) Erasmus Medical Centre — Sophia Children’s Hospital, (ii) University Medical Centre Utrecht — Wilhelmina Children’s Hospital, (iii) Leiden University Medical Centre — Willem-Alexander Children’s Hospital and (iv) Radboud University Medical Centre — Amalia Children’s Hospital. Institutional review boards from all centres approved retrospective data collection.
Study population
We included children who had performed a CPET between January 2001 and October 2018. These CPETs were conducted in either regular clinical follow-up or in studies performed in one of the centres. We included all patients between the ages of 6 and 18 with a structural heart defect who were able to perform a CPET. We excluded children who underwent and intervention after the CPET (defined as admission to the hospital within 3 months after the exercise test) as these children may represent a group with clinical deterioration. We excluded children with a respiratory exchange ratio (RER) at peak exercise of less than 1.00 as an index of maximal effort. Patients could be included more than once if multiple CPETs were available.
Clinical information on patients was gathered either from the national database KinCor [16] or from the patient information systems from the local hospitals. The CHD diagnosis was classified using the classification system of KinCor, which is based on the ICD classification. Patients with multiple defects were categorized in the most “severe” group; the hierarchy of severity was based on the ICD classification. A detailed description of disease classification is given in the supplement.
We choose a minimum group size of 50 to analyse a group separately. If a group was < n = 50, it was combined with a comparable group if possible. Some diagnostic groups were deemed too small to provide reliable overview values, i.e. congenital corrected transposition of the great arteries (n = 6), atrioventricular septal defect (n = 30), pulmonary atresia with VSD classified in the group of tetralogy of Fallot (n = 12), tricuspid valve abnormalities (n = 49) and mitral valve abnormalities (n = 29). The latter two were combined to atrioventricular (AV) valve abnormality; the others were excluded. All children with transposition of the great arteries were operated using an arterial switch operation, and all children with an ASD and VSD were operated.
Exercise tests
All exercise tests were performed on a cycle ergometer in the upright position, while breath-by-breath gas analyses were done. Cycle ergometry is the most used form of exercise testing in Europe. In all centres, the Godfrey protocol was used, which consists of a 3-min warm-up period followed by a progressive increase in workload depending on the height of the child: 10 W/min for children less than 120 cm in height, 15 W/min for children from 120 to 150 cm and 20 W/min for children over 150 cm until failure. During this increase in workload, continuous electrocardiography and ventilation measurements are taken [17]. Exact test equipment is given in the online supplement. All tests were overseen by a medical qualified person to conduct these tests.
VO2peak was defined as the highest VO2 measured over an average of 30 s. Wpeak was defined as the highest average value for the last 30 s. Peak heart rate was defined as the highest heart rate achieved averaged over a total of 10 s, and O2pulse was defined as VO2peak/heart rate for the last 30 s. For predicted VO2peak,Wpeak and peak heart rate (HRpeak) data from 214 healthy Dutch children between 8 and 18 years old were used [12, 18]. VE/VCO2 slope was calculated until peak exercise.
Statistics
Quality control and standardization methods
We analysed the best metric to standardize the reference values. We included the following metrics: age, height, weight and body surface area (BSA) in our analyses and stratified the analyses for sex. We calculated BSA using the Haycock formula. We tested differences between models using VO2peak (ml/min) and peak workload (Wpeak) as outcome parameters. Differences between models were tested using a log likelihood test.
Baseline characteristics and LMS curves
We tested all variables for normal distribution visually with histograms, q-q plots and using the Shapiro-Wilcox test. Variables which are normally distributed are shown as mean ± standard deviation (SD) and parameters which are not normally distributed are displayed as median and 25th–75th percentile. We tested all CPET outcome parameters with height using Spearman’s correlation. Those with a significant correlation are shown as graphs, and those without are shown as mean ± standard deviation or median and 25th–75th percentile. Differences between groups are tested with ANOVA and post hoc analysis with the Tukey test. To construct the reference value graphs, we used the lambda mu sigma (LMS) method described by Cole et al. [19]. All analyses (except for constructing the LMS charts) were done in R version 3.4.4, and LMS Chartmaker Light by Cole et al. was used to construct LMS models [20].
Results
Patient characteristics
We included 1383 tests of 1208 individual children for analysis (Suppl. Figure 1) with ASD, VSD, atrioventricular (AV) valve abnormality, pulmonary stenosis, aortic stenosis, aortic coarctation, transposition of the great arteries, tetralogy of Fallot and univentricular hearts. Patients could be included more than once in this cohort. Patient characteristics are shown in the supplement (Suppl. Table 1). Of the patients, 57.5% were male, the median age was 13.3 years (25–75% range 7.2–18.0), the median weight was 48.0 kg (25–75% range 37.3–58.6), and the median height was 160.3 cm (25–75% range 149.0–170.0).
Standardization method
To obtain an easy and reliable metric, we assessed the R2 for the parameters VO2peak and Wpeak using the metrics: age, height, weight and BSA, stratified for sex (Suppl. Figure 1). Of these metrics, height, weight and BSA performed significantly better than age. This pattern was similar for both sexes (male and female) and both outcome parameters (VO2peak and Wpeak). There were small but statistically significant differences: height performed better in explaining variance in Wpeak whereas weight and BSA performed slightly better in explaining variance in VO2peak. We decided to use height from here on because BSA is more complicated to compose, and many different formulas are used [21]. Also, height is known to be more consistent in the population over time. For example, the height of Dutch children has not changed over the last 20 years whereas there has been a substantial rise in obesity rates complicating the use of weight [22].
Exercise parameters
There was a large and significant variation in exercise capacity between disease groups (Tables 1 and 2). Patients with a univentricular heart performed worse than all other patient groups, except for AV valve abnormalities in VO2peak and Wpeak (Suppl. Table 2–10 shows ANOVA with corresponding Tukey tests). VE/VCO2 was elevated in patients with univentricular hearts as compared with all other diagnostic groups (Table 1; Fig. 2, supplementary Fig. 3–8).
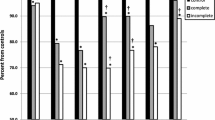
All patient groups had lower VO2peak, Wpeak and HRpeak as percentage of predicted based upon an age-matched healthy control group; again, patients with a univentricular heart performed worse compared to all other patient groups except for AV valve abnormalities (Fig. 1) [12]. VO2peak, as predicted for their age, ranged from 86% (25–75% range 70%–99%) in patients with aortic stenosis to 63% (25–75% range 53–73%) in patients with univentricular hearts. Wpeak as percentage of predicted ranged between 88 (25–75% range 75–98%) for patients with ASDs and 63% (25–75% range 53–72%) in patients with univentricular hearts. The lowest median HRpeak was 92% (25–75% range 85–97%) of predicted in patients with univentricular hearts (p < 0.001). All percentages of predicted are displayed graphically in Fig. 1.
Histograms of PeakVO2, peak workload and peak heart rate as percentage of predicted in the different disease groups. The number above the histogram represents the median. The red dotted line represents 100%. On the right side the number of patients per group, median VO2/kg, median age of the group and distribution of sex is given
To relate our results to body size, we constructed disease-specific graphs and values using height as metric.
Figure 2 shows the distribution graphs for VO2peak, Wpeak and O2pulsepeak for males and females separately in patients with univentricular hearts (Fig. 2a), tetralogy of Fallot (Fig. 2b) and transposition of the great arteries (Fig. 2c). All distribution graphs can be found and downloaded at “https://pedcardio.shinyapps.io/Racer2/”. Reference plots for all disease groups are in the supplement (Suppl. Figure 3–8) The parameters HRpeak, VO2peak/kg, VO2peak as % of predicted, Wpeak as % of predicted, HRpeak as % of predicted and the last 30 s 2 were not significantly associated with height and therefore shown as a median with 25th and 75th percentile in the table as follows. For all parameters, males had higher values compared with females. Also, the variance of the data (displayed by the centile curves) increased with height.
a Sex-specific distribution graphs for VO2peak, Wpeak and O2pulsepeak and median HRpeak, VO2peak/kg, VO2 as% of predicted, Wpeak as % of predicted, HR as % of predicted and VE/VCO2 slope in patients with Univentricular hearts related to height (in cm). Dark blue/dark red areas represents 80 centiles, lighter shade represents 95 centiles. Table at the bottom shows median and 25-75 quantiles. b Sex-specific distribution graphs for VO2peak, Wpeak and O2pulsepeak and median HRpeak, VO2peak/kg, VO2 as% of predicted, Wpeak as % of predicted, HR as % of predicted and VE/VCO2 slope in patients with tetralogy of Fallot related to height (in cm). Dark blue/dark red areas represents 80 centiles, lighter shade represents 95 centiles. Table at the bottom shows median and 25-75 quantiles. c Sex-specific distribution graphs for VO2peak, Wpeak and O2pulsepeak and median HRpeak, VO2peak/kg, VO2 as% of predicted, Wpeak as % of predicted, HR as % of predicted and VE/VCO2 slope in patients with transposition of the great arteries related to height (in cm). Dark blue/dark red areas represents 80 centiles, lighter shade represents 95 centiles. Table at the bottom shows median and 25-75 quantiles
In patients with univentricular hearts, the VO2peak/kg decreased with age (0.97 ± 0.23 ml/kg/min per year), and in patients with biventricular circulations, there was no association between age and VO2peak/kg.
Lastly, we analysed the effect of left ventricular dominance vs right ventricular dominance in patients with univentricular circulations. Patients with right ventricular dominance had lower VO2peak as % of predicted (67.8 [57.9–75.1] vs. 60.3 [52.6–68.3], p = 0.015) and Wpeak as % of predicted (65.5 [57.2–74.2] vs. 62.1 [52.2–68.4], p = 0.025). No differences in HR as % of predicted were observed.
Discussion
In this study, we present the exercise capacity in a large cohort of children with CHD. In healthy children, exercise capacity increases with the increase in body size during childhood. To construct graphs of the observed distribution in exercise capacity in children with CHD, we related the exercise parameters to height. Exercise capacity was reduced in all CHD groups as compared to the normal population, and we observed a large variation between different groups of CHD. Therefore, we constructed disease-specific distribution graphs. These graphs and values can be used to guide clinicians during the serial follow-up of patients with CHD, e.g. in transition to adult services, and can be used as an indication for abnormal changes in CPET results and emphasize the need for identification of the cause, such as cardiac or other disease or inadequate changes in physical activity. There is a need for large datasets of exercise capacity to identify patients at risk for adverse outcome. Recent studies suggest that lack of normal development of exercise capacity during children can predict worse outcome [23]. As we have come increasingly aware of the potential of preventive strategies to postpone long-term complications in CHD, it is important to analyse exercise capacity of patients with CHD during childhood growth. Previous studies of exercise capacity in adults with CHD, covering a large age range of patients treated in a different era, although valuable, did not account for the change in body size and growth during childhood [24].
In comparison with previously published data, we observed similar VO2peak/kg and lower VO2peak as percentage of predicted in most patient groups [10, 25,26,27,28]. These differences may be due to the use of a different reference group. Several studies [10, 25, 27] used the data published in 1984 by Wasserman and Cooper, in which 107 American children were studied [15]. We used a recent Dutch cohort of 214 children described in 2011 [12, 13]. It should be noted that in patients with a univentricular circulation, in general, European cohorts describe a higher VO2/kg than cohorts from USA and Japan (~ 32 ml/kg/min vs. ~ 27 ml/kg/min) [25,26,27]. In the cohort described by Paridon et al., only 166 out of 411 patients had been able to complete a maximal exercise test. There are large differences in cohort size, ranging from 25 to 311 patients with a univentricular circulation. These differences may also explain different results when comparing patients with left ventricular dominance with those with right ventricular dominance. In this cohort, exercise capacity was lower in patients with right morphology dominant univentricular hearts. Smaller size cohorts of univentricular hearts have found different results, but on average, most large size cohorts showed similar differences [29].
In adults with a univentricular circulation, a decrease in VO2peak as percentage of predicted of > 3% per year has been shown to be a predictor for death or cardiac surgery [30]. In the present study, we describe a similar decrease in VO2 peak/kg in patients with a univentricular circulation, and recently, Janousek et al. described a similar decrease in a group of patients ranging from 10 to 30 years of age [31]. Follow-up studies in this cohort are necessary to determine the effects of attrition on outcome. It should be noted that VO2 peak (in ml/min) increases with height during childhood in the normal population but also in children with CHD. The use of VO2peak in ml/min plotted versus height until adulthood and versus age thereafter would facilitate comparison as well as transition into adult care services. Thus, the use of disease specific and growth specific exercise capacity graphs can identify deviations from expected development of VO2peak. Predictive value of exercise capacity for cardiac-related hospitalization has been demonstrated in one study of children with tetralogy of Fallot [8] and in several studies in patients > 18 year of age. Further research is needed to identify personalized risk scores for adverse outcome using development of exercise capacity in addition to other clinical parameters.
In this study, all patients with CHD had a decreased exercise capacity, even patients with lesions that are deemed less severe such as an ASD and VSD. Previous studies in adults also indicated that despite excellent survival, exercise capacity in “simple lesions” was not normal [24, 32]. There is no obvious explanation for these findings, e.g. there was no limitation to increase peak heart rate. For patients with a VSD, a dysfunctional septal architecture has been suggested to be involved [33]. For patients with an ASD, timing of closure and hence duration of increased RV preload may affect the ability for remodelling [34]. The results of several studies to exercise capacity in”simple” CHD lesions favour a more rigorous follow up of “simple lesions” during childhood and beyond.
There is a discussion in paediatrics which metric to use to standardize exercise capacity during growth. A systematic review in 2015, describing six different metrics to standardize exercise capacity in paediatrics (age, height, weight, BSA, lean body mass and pubertal stage), did not provide a conclusion which metric best corrects for differences in body size [35]. Most studies used age to standardize CPET in childhood, as in adults [35, 36]. Yet, in adults, VO2peak and Wpeak decrease with age, whereas body composition is relatively stable [24, 37]. In contrast, in children, VO2peak and Wpeak increase with age and the main factors seem to be the development and growth of muscle mass, lung capacity, and cardiac output [12]. The findings of our study would suggest the use of height, weight or BSA rather than age to standardize exercise capacity in children with CHD. Our observations are in line with a previous study assessing reference values for the 6-min walk test [38]. In comparison with body weight, height is relatively unaffected by obesity. Thus, to reduce variation, we choose height over weight and BSA as metric to standardize CPET.
The strength of our study is the number of children with CHD included. This allowed us to construct disease-specific distribution graphs. Another strength is that we included all CPETs conducted and therefore have a good representation for day-to-day clinical CPETs. Yet, this study design also comes with some limitations; particularly, the inclusion of all CPETs may also lead to selection bias. Our cohort included a range of different CHD, with an overrepresentation of the more severe conditions such as tetralogy of Fallot and univentricular hearts [39]. In these patients in general, more rigorous follow-up surveillance is performed. To achieve adequately sized groups, we had to combine heterogenic groups such as mitral and tricuspid valve abnormalities, although these conditions may have very different physiologies. Furthermore, we were not able to add clinical data such as echocardiograms and MRIs to our study. Also, we did not select or correct for medication use of our patients, specifically beta blockers which may limit peak exercise performance [40]. In adult cohorts, the percentage of patients using beta blockers is ~ 15% [41]. In our study, peak heart rate was > 95% in most groups, with exception for the patients with univentricular hearts. In patients with univentricular heart, a reduced peak heart rate has been described previously and is not related to beta blocker use, rather to abnormal physiology [42]. We justify this approach, since excluding these patients may have yielded another selection bias. Also, we did not correct for any other diseases, socioeconomic or life-style factors including sport habits. Our data are largely in agreement with data from the literature; hence, these effects probably are either small or bias to every study. The positive result of this approach is that our cohort is representative of a present-day cohort of children of CHD. Lastly, the lack of ethnic diversity in our cohort may reduce the generalizability. Our data applies to children from Caucasian background predominantly. In order to circumvent this problem of variance between ethnic groups, we used height to relate CPET outcomes to body size. This is important since the Dutch are amongst the tallest of human populations in the world. Expressing the CPET results per height makes our value set usable in other Caucasian population outside of the Netherlands.
In conclusion, we constructed disease-specific distribution graphs for exercise capacity in children with CHD during growth and development using a relatively large national multi-centre cohort. Children with CHD had reduced exercise capacity varying with specific disease, thereby justifying disease-specific reference values. These values can be used in the structured follow-up of children with CHD, in transition to adult services, and can be used to better identify lack of physical activity.
Abbreviations
- ASD:
-
Atrial septum defect
- BSA:
-
Body surface area
- CHD:
-
Congenital heart diseases
- CPET:
-
Cardio pulmonary exercise test
- RER:
-
Respiratory exchange ratio
- SD:
-
Standard deviation
- VSD:
-
Ventricular septum defect
References
Marelli AJ, Ionescu-Ittu R, Mackie AS, Guo L, Dendukuri N, Kaouache M (2014) Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation 130(9):749–756
Norozi K, Wessel A, Alpers V, Arnhold JO, Geyer S, Zoege M et al (2006) Incidence and risk distribution of heart failure in adolescents and adults with congenital heart disease after cardiac surgery. Am J Cardiol 97(8):1238–1243
Arnaert S, De Meester P, Troost E, Droogne W, Van Aelst L, Van Cleemput J et al (2021) Heart failure related to adult congenital heart disease: prevalence, outcome and risk factors. ESC Heart Failure 8(4):2940–2950
van der Ven JPG, van den Bosch E, Bogers A, Helbing WA (2018) State of the art of the Fontan strategy for treatment of univentricular heart disease. F1000Research 7
van der Ven JPG, van den Bosch E, Bogers A, Helbing WA (2019) Current outcomes and treatment of tetralogy of Fallot. F1000Research 8
Diller GP, Giardini A, Dimopoulos K, Gargiulo G, Muller J, Derrick G et al (2010) Predictors of morbidity and mortality in contemporary Fontan patients: results from a multicenter study including cardiopulmonary exercise testing in 321 patients. Eur Heart J 31(24):3073–3083
Baumgartner H, Bonhoeffer P, De Groot NM, de Haan F, Deanfield JE, Galie N et al (2010) ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 31(23):2915–2957
Tsai YJ, Li MH, Tsai WJ, Tuan SH, Liao TY, Lin KL (2016) Oxygen uptake efficiency slope and peak oxygen consumption predict prognosis in children with tetralogy of Fallot. Eur J Prev Cardiol 23(10):1045–1050
Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM et al (2019) 2018 AHA/ACC guideline for the management of adults with congenital heart disease: executive summary: a reporT of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 73(12):1494–1563
Amedro P, Gavotto A, Guillaumont S, Bertet H, Vincenti M, De La Villeon G et al (2018) Cardiopulmonary fitness in children with congenital heart diseases versus healthy children. Heart 104(12):1026–1036
Cooper DM, Weiler-Ravell D (1984) Gas exchange response to exercise in children. Am Rev Respir Dis 129(2 Pt 2):S47–S48
Bongers BC, van Brussel M, Hulzebos EHJ, Takken T (2014) Pediatric norms for cardiopulmonary exercise testing: in relation to sex and age
Harkel ADJT, Takken T, Van Osch-Gevers M, Helbing WA (2011) Normal values for cardiopulmonary exercise testing in children. Eur J Cardiovasc Prev Rehabil 18(1):48–54
Lai N, Fiutem JJ, Pfaff N, Salvadego D, Strainic J (2021) Relating cardiorespiratory responses to work rate during incremental ramp exercise on treadmill in children and adolescents: sex and age differences. Eur J Appl Physiol 121(10):2731–2741
Cooper DM, Weiler-Ravell D, Whipp BJ, Wasserman K (1984) Growth-related changes in oxygen uptake and heart rate during progressive exercise in children. Pediatr Res 18(9):845–851
Silva LM, Kuipers IM, van den Heuvel F, Mendes R, Berger RM, van Beynum IM et al (2016) KinCor, a national registry for paediatric patients with congenital and other types of heart disease in the Netherlands: aims, design and interim results. Neth Heart J 24(11):628–639
Godfrey S (1974) Exercise testing in children: applications in health and disease: Saunders Limited
Bongers BC, Hulzebos EH, Van Brussel M, Takken T (2012) Pediatric norms for cardiopulmonary exercise testing. Uitgeverij BOXPress, s’-Hertogenbosch 2(3):30–34
Cole TJ (1990) The LMS method for constructing normalized growth standards. Eur J Clin Nutr 44(1):45–60
Team RC (2015) R: a language and environment for statistical computing
Redlarski G, Palkowski A, Krawczuk M (2016) Body surface area formulae: an alarming ambiguity. Sci Rep 6:27966
Schonbeck Y, Talma H, van Dommelen P, Bakker B, Buitendijk SE, HiraSing RA et al (2013) The world’s tallest nation has stopped growing taller: the height of Dutch children from 1955 to 2009. Pediatr Res 73(3):371–377
Ohuchi H, Negishi J, Miike H, Toyoshima Y, Morimoto H, Fukuyama M et al (2019) Positive pediatric exercise capacity trajectory predicts better adult Fontan physiology rationale for early establishment of exercise habits. Int J Cardiol 274:80–87
Kempny A, Dimopoulos K, Uebing A, Moceri P, Swan L, Gatzoulis MA et al (2012) Reference values for exercise limitations among adults with congenital heart disease. Relation to activities of daily life single centre experience and review of published data. Europ Heart J 33(11):1386–1396
Paridon SM, Mitchell PD, Colan SD, Williams RV, Blaufox A, Li JS et al (2008) A cross-sectional study of exercise performance during the first 2 decades of life after the Fontan operation. J Am Coll Cardiol 52(2):99–107
Ohuchi H, Negishi J, Noritake K, Hayama Y, Sakaguchi H, Miyazaki A et al (2015) Prognostic value of exercise variables in 335 patients after the Fontan operation: a 23-year single-center experience of cardiopulmonary exercise testing. Congenit Heart Dis 10(2):105–116
Hock J, Hacker AL, Reiner B, Oberhoffer R, Hager A, Ewert P et al (2019) Functional outcome in contemporary children and young adults with tetralogy of Fallot after repair. Arch Dis Child 104(2):129–133
Gavotto A, Huguet H, Picot MC, Guillaumont S, Matecki S, Amedro P (1985) The Ve/Vco2 slope: a useful tool to evaluate the physiological status of children with congenital heart disease. J Appl Physiol 2020;129(5):1102–1110
Bossers SSM, Helbing WA, Duppen N, Kuipers IM, Schokking M, Hazekamp MG et al (2014) Exercise capacity in children after total cavopulmonary connection: lateral tunnel versus extracardiac conduit technique. J Thorac Cardiovasc Surg 148(4):1490–1497
Egbe AC, Driscoll DJ, Khan AR, Said SS, Akintoye E, Berganza FM et al (2017) Cardiopulmonary exercise test in adults with prior Fontan operation: the prognostic value of serial testing. Int J Cardiol 235:6–10
Illinger V, Materna O, Slaby K, Jicinska D, Kovanda J, Koubsky K et al (2022) Exercise capacity after total cavopulmonary anastomosis: a longitudinal paediatric and adult study. ESC Heart Fail 9(1):337–344
Maagaard M, Eckerstrom F, Boutrup N, Hjortdal VE (2020) Functional capacity past age 40 in patients with congenital ventricular septal defects. J Am Heart Assoc 9(19):e015956
Buckberg G, Hoffman JI (2014) Right ventricular architecture responsible for mechanical performance: unifying role of ventricular septum. J Thorac Cardiovasc Surg 148(6):3166–3171 e1–4
Gomes RS, Skroblin P, Munster AB, Tomlins H, Langley SR, Zampetaki A et al (2016) “Young at heart”: Regenerative potential linked to immature cardiac phenotypes. J Mol Cell Cardiol 92:105–108
Blais S, Berbari J, Counil FP, Dallaire F (2015) A systematic review of reference values in pediatric cardiopulmonary exercise testing. Pediatr Cardiol 36(8):1553–1564
Wasserman K, Hansen JE, Sue DY, Whipp BJ, Froelicher VF (1987) Principles of exercise testing and interpretation. J Cardiopulm Rehabil Prev 7(4):189
Koch B, Schaper C, Ittermann T, Spielhagen T, Dorr M, Volzke H et al (2009) Reference values for cardiopulmonary exercise testing in healthy volunteers: the SHIP study. Eur Respir J 33(2):389–397
Li AM, Yin J, Au JT, So HK, Tsang T, Wong E et al (2007) Standard reference for the six-minute-walk test in healthy children aged 7 to 16 years. Am J Respir Crit Care Med 176(2):174–180
Moons P, Sluysmans T, De Wolf D, Massin M, Suys B, Benatar A et al (2009) Congenital heart disease in 111 225 births in Belgium: birth prevalence, treatment and survival in the 21st century. Acta Paediatr 98(3):472–477
Ladage D, Schwinger RH, Brixius K (2013) Cardio-selective beta-blocker: pharmacological evidence and their influence on exercise capacity. Cardiovasc Ther 31(2):76–83
Baggen VJ, van den Bosch AE, Eindhoven JA, Schut AW, Cuypers JA, Witsenburg M et al (2017) Prognostic value of N-terminal Pro-B-type natriuretic peptide, troponin-T, and growth-differentiation factor 15 in adult congenital heart disease. Circulation 135(3):264–279
Claessen G, La Gerche A, Van De Bruaene A, Claeys M, Willems R, Dymarkowski S et al (2019) Heart rate reserve in Fontan patients: chronotropic incompetence or hemodynamic limitation? J Am Heart Assoc 8(9):e012008
Acknowledgements
B. Bannink for helping with accessing raw data.
Funding
The RACER study was made possible by financial support from the “Stichting Hartekind”. WvG was supported by a grant from Dutch Heart Foundation and Stichting Hartekind (01–002-2019-B002).
Author information
Authors and Affiliations
Contributions
WG, WH, TT and BB contributed to the design of the study; all the authors contributed to the acquisition, analysis and interpretation of the data. WG, WH and BB drafted the manuscript, and all the authors critically revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This is an observational study. The Research Ethics Committee of the i) Erasmus Medical Centre—Sophia Children’s Hospital, ii) University Medical Centre Utrecht—Wilhelmina Children’s Hospital, iii) Leiden University Medical Centre—Willem-Alexander Children’s Hospital, and iv) Radboud University Medical Centre—Amalia Children’s Hospital have confirmed that no ethical approval is required.
Consent to participate
Because of the retrospective nature of this study on existing data no informed consent was obtained.
Consent for publication
Because of the retrospective nature of this study on existing data no informed consent was obtained.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Peter de Winter
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van Genuchten, W.J., Helbing, W.A., Ten Harkel, A.D.J. et al. Exercise capacity in a cohort of children with congenital heart disease. Eur J Pediatr 182, 295–306 (2023). https://doi.org/10.1007/s00431-022-04648-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-022-04648-9