Abstract
We aimed to describe characteristics and management of children with comorbidities attending European emergency departments (EDs) with fever. MOFICHE (Management and Outcome of Fever in children in Europe) is a prospective multicentre study (12 European EDs, 8 countries). Febrile children with comorbidities were compared to those without in terms of patient characteristics, markers of disease severity, management, and diagnosis. Comorbidity was defined as a chronic underlying condition that is expected to last > 1 year. We performed multivariable logistic regression analysis, displaying adjusted odds ratios (aOR), adjusting for patient characteristics. We included 38,110 patients, of whom 5906 (16%) had comorbidities. Most common comorbidities were pulmonary, neurologic, or prematurity. Patients with comorbidities more often were ill appearing (20 versus 16%, p < 0.001), had an ED-Paediatric Early Warning Score of > 15 (22 versus 12%, p < 0.001), or a C-reactive protein > 60 mg/l (aOR 1.4 (95%CI 1.3–1.6)). They more often required life-saving interventions (aOR 2.7, 95% CI 2.2–3.3), were treated with intravenous antibiotics (aOR 2.3, 95%CI 2.1–2.5), and were admitted to the ward (aOR 2.2, 95%CI 2.1–2.4) or paediatric intensive care unit (PICU) (aOR 5.5, 95% CI 3.8–7.9). They were more often diagnosed with serious bacterial infections (aOR 1.8, 95%CI 1.7–2.0), including sepsis/meningitis (aOR 4.6, 95%CI 3.2–6.7). Children most at risk for sepsis/meningitis were children with malignancy/immunodeficiency (aOR 14.5, 8.5–24.8), while children with psychomotor delay/neurological disease were most at risk for life-saving interventions (aOR 5.3, 4.1–6.9) or PICU admission (aOR 9.7, 6.1–15.5).
Conclusions: Our data show how children with comorbidities are a population at risk, as they more often are diagnosed with bacterial infections and more often require PICU admission and life-saving interventions.
What is Known: • While children with comorbidity constitute a large part of ED frequent flyers, they are often excluded from studies. | |
What is New: • Children with comorbidities in general are more ill upon presentation than children without comorbidities. • Children with comorbidities form a heterogeneous group; specific subgroups have an increased risk for invasive bacterial infections, while others have an increased risk of invasive interventions such as PICU admission, regardless of the cause of the fever. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is an important health paradox regarding children with comorbidities attending the emergency department (ED). On one hand, these children form an increasing group [1]; constitute a large part of the so-called “ED frequent flyers” [2, 3]; have an increased risk for invasive bacterial infections (sepsis/meningitis) as well as serious bacterial infections, such as urinary tract infections, pneumonia, or bone and joint infections [4,5,6,7]; and use a larger amount of ED and hospital resources [8, 9], yet on the other hand are very often excluded from studies [10,11,12,13]. When research does include children with comorbidities, it usually focuses on specific types of comorbidities, such as febrile neutropenia [14]. The most compelling evidence that shows how children with comorbidities form a very fragile population comes from a recent key paper, showing half of the infection-related deaths in the UK occur in children with comorbidities [15].
However, no details were provided regarding type of comorbidities or presenting problems. Our aim was to address this knowledge gap, by assessing presenting signs and symptoms, clinical management, and cause of infection of febrile children with comorbidities attending the ED in a large European cohort.
Materials and methods
Study design
This study is part of the MOFICHE (Management and Outcome of Febrile children in Europe) study, which is embedded in the PERFORM (Personalized Risk assessment in Febrile illness to Optimise Real-life Management across the European Union) study. The MOFICHE study is an observational multicentre study that evaluates the clinical management and outcome of febrile children in Europe using routinely collected data [16].
Ethics statement
The study was approved by the ethical committees of all the participating hospitals, and no informed consent was needed for this study: Austria (Ethikkommission Medizinische Universitat Graz, ID:28-518ex15/16), Germany (Ethikkommission Bei Der LMU München, ID:699–16), Greece (Ethics committee, ID:9683/18.07.2016), Latvia (Centrala medicinas etikas komiteja, ID:14.07.201.6.No. Il16-07–14), Slovenia (Republic of Slovenia National Medical Ethics Committee, ID:0120–483/2016–3), Spain (Comité Autonómico de Ética de la Investigación de Galicia, ID:2016/331), The Netherlands (Commissie Mensgebonden onderzoek, ID:NL58103.091.16), UK (Ethics Committee, ID:16/LO/1684, IRAS application no. 209035, Confidentiality advisory group reference: 16/CAG/0136).
In all the participating UK settings, an additional opt-out mechanism was in place.
Study population and setting
Twelve EDs from eight different European countries (Austria, Germany, Greece, Latvia, the Netherlands (n = 3), Spain, Slovenia, and the UK (n = 3)) participated in the study. Participating hospitals were either tertiary university hospitals or large teaching hospitals (Appendix 1). Data were collected for at least 1 year, between January 2017 and April 2018. Inclusion criteria were children aged 0 months to 18 years presenting with fever to the ED (temperature ≥ 38.0 °C) or a history of fever in the previous 72 h.
Data collection
Data were obtained from patient records and entered into an electronic case report form. Data included general patient characteristics (age, sex, comorbidity, previous medical care, time of arrival, referral (self, primary care physician, Emergency Medical Services, or other), triage urgency, vital signs, and presence of “red traffic light” alarming signs for identifying risk of serious illness (National Institute for Health and Care Excellence (NICE) guideline on fever [17]. These alarming signs include level of consciousness, ill-appearance, increased work of breathing, age < 3 months, non-blanching rash, meningeal signs, status epilepticus, and focal neurological signs.
Data collection ranged from a 1 week per month sample to all visits, depending on the number of ED visits per hospital (Appendix 1).
Definitions
The presence of comorbidity and type of comorbidity (e.g., organ system involved) were taken directly from the patient’s chart. Comorbidity was pre-defined as a chronic underlying condition that is expected to last at least one year. During data analysis, comorbidity was classified as either simple or complex. Complex comorbidity was defined as comorbidity that affects two or more body system, malignancy, or a progressive condition [18,19,20]. Children with malignancy and immunodeficiency were analysed together, as were children with neurologic disease and psychomotor delay.
Vital signs were classified as abnormal according to APLS reference ranges.
ED-Paediatric Early Warning Score (ED-PEWS) were calculated based on the PEWS specifically developed and validated for the ED by Zachariasse et al. (age, vital signs, capillary refill time, level of consciousness, and work of breathing combined into an ED- PEWS score) [21].
Triage categories were grouped together into low urgency (non-urgent, standard) and high urgency (urgent, very urgent, and immediate).
A previous ED visit was defined as a visit to either the same or a different ED in the previous 5 days. Duration of fever was defined as the duration of fever upon presentation at the ED.
Immediate life-saving interventions (ILSI) were categorized into the following categories: airway and breathing support, electrical therapy, emergency procedures, hemodynamic support, and emergency medications (Appendix 2) [22, 23].
Focus of infection was categorized as upper respiratory tract infection (otitis media, tonsillitis/pharyngitis, other), lower respiratory tract infection (LRTI), gastro-intestinal tract and surgical abdomen, urinary tract, skin, musculoskeletal, sepsis, meningitis/central nervous system, flulike illness, childhood exanthemas, inflammatory syndromes, undifferentiated fever, or other [16].
The consortium developed a consensus-based flowchart [16, 24] combining all available clinical data and test results. A more detailed description was published by Hagedoorn et al. [16] This flowchart was used to classify the presumed cause of infection for each patient visit (Appendix 3), depending on clinical signs, C-reactive protein (CRP), and microbiological tests (bacterial cultures, viral or bacterial PCR).
Serious bacterial infections were defined as “definite/probable bacterial” with a focus of infection from the gastro-intestinal tract, LRTI, urinary tract, bone and joints, central nervous system, or sepsis [22]. Invasive bacterial infection (sepsis/meningitis) was defined as a focus from the central nervous system or sepsis and “definite/probable bacterial” from the consensus-based flowchart [25].
Data quality and missing data
The use of a digital training module, which included the clarification of the National Institute for Health and Care Excellence alarming signs, allowed optimization and standardization of the clinical assessment and data collection processes. Universal guidance on entering the standardized data was issued prior to commencement of the study period. Furthermore, monthly teleconferences and biannual meetings were organized and quarterly reports of data quality were discussed with all partners.
Missing determinants were handled by using multiple imputation. Imputation was performed by using the MICE package in R, version 3.4. SPSS version 25 was used for the analysis of the data. This imputation process resulted in twenty databases on which statistical analysis was performed and pooled for a final result.
Data analysis
We performed descriptive analyses for general patient characteristics, vital signs, ED-PEWS and presence of National Institute for Health and Care Excellence alarming signs, clinical management (diagnostic tests, intravenous antibiotics, oxygen therapy, immediate life-saving interventions), disposition (discharge, hospital admission, Paediatric Intensive Care Unit (PICU) admission), and diagnosis (focus of infection, viral or bacterial disease). Characteristics of children with and without comorbidities were compared using chi-squared-tests and Mann–Whitney U tests. Results were deemed significant with a p-value < 0.05.
We analysed differences in management, disposition, and presumed cause of infection for children with and without comorbidities by multivariable logistic regression adjusted for ED of presentation and general patient characteristics (sex, duration of fever, previous medical care, time of arrival, and mode of referral).
Results
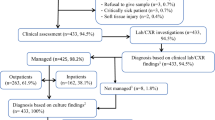
After excluding 370 (1.0%) patients with missing data regarding comorbidities, 38,110 patients were left for analysis. In total, 5906 patients had comorbidities (16%, range between EDs 5.3 and 62%) of whom 1678 (28%, range 8.6–60%) were classified as complex comorbidities (Table 2). The most common types of comorbidities were pulmonary, neurologic/psychomotor delay, prematurity (gestational age < 37 weeks), urology/nephrology, cardiac, and malignancy/immunodeficiency (Table 1). Details regarding missing variables are provided in Table 2.
Patient characteristics
Patients with comorbidities were older (median age 3.7 years versus 2.6 years, p < 0.001) and more often were male (59% versus 54%, p < 0.001) than children without comorbidities.
Patients with comorbidities more often presented with a fever duration of less than 24 h (44% versus 35%, p < 0.001), more often were referred by a specialist (22% versus 8.3% (p < 0.001), and more often had a high triage urgency (53% versus 32%, p < 0.001), abnormal vital signs, or an ED-PEWS of 15 or higher (22% versus 12%, p < 0.001).
All differences were more pronounced in children with complex comorbidities (Table 2).
Furthermore, they more often were described as ill appearing (range according to comorbidity: 18–25%), had increased work of breathing (range 6.2–31%), or presented with neurological signs or symptoms (range 3.8–18%, Table 2; Appendix 4).
Clinical management
In children with comorbidities, diagnostic tests such as general blood tests (aOR 2.0, 95 CI 1.9–2.2), CRP (aOR 2.0, 95% CI 1.8–2.1), blood cultures (aOR 3.0, 95% CI 2.7–3.3), and imaging (aOR 1.6, 95% CI 1.5–1.7) were performed more often after correcting for general patient characteristics. Furthermore, test results, such as CRP, and blood cultures (aOR 2.3, 95% CI 1.6–3.3) were more often abnormal (Table 3).
Regarding therapy, children with comorbidities more often required immediate life-saving interventions (aOR 2.7, 95 CI 2.2–3.3) and oxygen (aOR 4.9, 4.2–5.7) and were treated more frequently with intravenous antibiotics (aOR 2.3, 95% CI 2.1–2.5; Table 3). Children with comorbidities were admitted more often to the general ward (aOR 2.2, 95% CI 2.1–2.4) as well as the PICU (aOR 5.5, 95% 3.8–7.9; Table 3).
Children with a history of malignancy/immunodeficiency (aOR 5.8, 95% CI 4.8–7.0) or neurologic problems/psychomotor delay (aOR 2.9, 95% CI 2.5–3.3) were most often admitted, while children with a history of neurologic/psychomotor delay (aOR 9.7, 95% CI 6.1–15.5), children with a history of pulmonary disease (aOR 8.8, 95% CI 5.2–14.8), or children with a history of prematurity (8.1, 95% CI 4.4–14.7) were most often admitted to the PICU. Children with a history of neurologic disease/psychomotor delay (aOR 5.3, 95% CI 4.1–6.9), pulmonary disease (aOR 3.0, 95% CI 2.1–4.3), or cardiac disease (aOR 2.7, 95% CI 1.7–4.5) most often required immediate life-saving interventions (Appendix 5).
Focus and presumed cause of infection
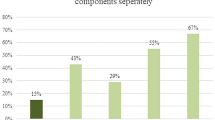
Most common types of infections in almost all subgroups of comorbidity were upper respiratory tract infections, lower respiratory tract infections, and undifferentiated fever (Appendix 6). Children with comorbidities were more often diagnosed with lower respiratory tract (20.4 versus 13.6, p < 0.001; Fig. 1), undifferentiated fever (10.8 versus 7.3, p < 0.001), and sepsis/meningitis (1.6 versus 0.6%, p < 0.001), while presentations for upper respiratory tract infections (41 versus 55%, p < 0.001) were less common.
Focus of infection in children with and without comorbidity. Data shown as percentages within the groups of children with and without comorbidity. LRTI = lower respiratory tract infection; gastro-intestinal = gastro-intestinal and surgical abdomen; UTI = urinary tract infection, exanthems = exanthems and flulike illness; musculoskeletal = soft-tissue, skin and musculoskeletal infection. URTI (not shown in graphic) = upper respiratory tract infection: without comorbidity 54.5%, with comorbidity 41.2%
After correcting for general patient characteristics, patients with comorbidities more often were categorized as having a serious bacterial infection (aOR 1.8, 95% CI 1.7–2.0) and invasive bacterial infections (sepsis/meningitis) in particular (aOR 4.6, 95% CI 3.2–6.7). Children most at risk for sepsis/meningitis were children with a history of malignancy/immunodeficiency (aOR 14.5, 95% CI 8.5–24.8), neurologic disease/psychomotor delay (aOR 4.6, 95% CI 2.6–8.1), or prematurity (aOR 4.5, 95% CI 2.2–9.2; Appendix 5). These results were similar when only including children with culture-proven sepsis/meningitis (Appendix 7). In our study population, Escherichia coli, Staphylococcus aureus, coagulase-negative Staphylococci, and Streptococcus pneumoniae were the most common pathogens found in children with comorbidities, while Neisseria meningitidis, group B Streptococcus, Streptococcus pneumoniae, and Escherichia coli were the most common pathogens in febrile children without comorbidities.
Discussion
Main findings
Children with comorbidities, with 16%, form a substantial part of the paediatric ED population. Our data show that children with comorbidities in general are more ill upon presentation than children without comorbidities, as they more often have abnormal vital signs, and a high ED-Paediatric Early Warning Score. In contrast with this, in our study, children with comorbidity presented with a shorter duration of fever, possibly due to awareness of a higher risk for serious illness. They more often present to the ED with common diseases such as lower respiratory tract infections as well as invasive bacterial infections such as sepsis/meningitis. While they are managed differently, with higher rates of resource use such as blood tests, admission, intravenous antibiotics, and rates of serious interventions (immediate life-saving interventions, PICU admission), this seems adequate as they are diagnosed with serious bacterial infections more often. Secondly, our data show that children with comorbidities form a heterogeneous group, with different types of comorbidities requiring a distinct pattern of management. As expected, children with malignancy/immunodeficiency more often had serious bacterial infections and were treated with intravenous antibiotics, and children with psychomotor delay/neurological disease most often required immediate life-saving interventions and PICU admission.
Findings in relation to previous literature
Studies on the prevalence of chronic comorbidities in the paediatric population are scarce and many articles base their numbers on US studies that took place more than a decade ago [26,27,28]. Using these numbers might underestimate the current prevalence and health care burden of children with comorbidities [1].
Furthermore, comparing studies is not straightforward due to the fact that studies differ in study methods (e.g. self-reported comorbidity versus hospital data versus national registries), definitions used and whether mental health is included in the definition [19, 20, 28,29,30,31].
These differences might explain the large variation that is found in the prevalence of these children in Europe, which varies between 10 and 40% [29, 31, 32].
However, there is convincing data that regardless of the definition used, children with comorbidity have higher health care utilization and an increased risk of in-hospital mortality [20, 28, 33,34,35].
For example, a recent European study showed that depending on the setting, between 10 and 38% of all ED visits were comprised by children with comorbidities [33].
Furthermore, several studies showed that between 40 and 75% of cases of childhood mortality were comprised by children with comorbidities [28, 34, 35].
Our study is in line with previous studies that found increased resource use in children with comorbidities and an increased risk of serious bacterial infections [4, 9, 14, 36]. With our study, we aimed to provide a more detailed overview of these children and identify children at risk for serious illness.
Implications for clinical practice and research
Our data demonstrate how children with comorbidities form a fragile patient population. Clinicians should be aware of the increased risk for serious bacterial infections and PICU admission when evaluating febrile children with comorbidities at the ED.
Given their increasing numbers on one hand and increased risk for serious bacterial infections on the other hand, it is imperative that they are not being left out of studies or guidelines. Our study provides insight on which specific subgroups are specifically at risk for serious bacterial infections and interventions such as immediate life-saving interventions or PICU admission. Children most at risk for sepsis/meningitis were children with a history of malignancy/immunodeficiency, prematurity or neurologic disease/psychomotor delay. These data could be used to maintain a lower threshold for diagnostic tests, and start antibiotic therapy based on the combination of clinical assessment and diagnostic test results, children with a history of neurologic/psychomotor delay, pulmonary disease, cardiac disease, or prematurity most often required PICU admission or immediate life-saving interventions. Further research should identify which subgroups of children are most at risk for serious illness and provide detailed information on the disease course. This information should ideally be used to improve early recognition and interventions in order to improve the outcome of these children.
As most, though not all [37], studies predicting serious bacterial infections in febrile children have excluded children with comorbidities, future studies should focus on validating existing clinical prediction rules for this population, and if necessary, develop guidelines and prediction rules specifically targeted to this population. Our data show that, although overall, children with comorbidity have an increased risk for serious bacterial infections; this is not true for all subgroups of comorbidity. This data can be used to target antibiotic therapy.
Lastly, future research would benefit from the use of a uniform classification of children with comorbidities that can be used to provide an overview of the prevalence and resource use in these children at all levels of care. Furthermore, using a uniform classification can facilitate the comparison of different studies.
Strengths and limitations
To our knowledge, our study is the first to include a large multicenter cohort of febrile children with different types of comorbidities and includes detailed information on presenting signs and symptoms, management, diagnostic test results, and cause of infection.
Data were collected year-round and included different EDs with different rates of children with comorbidities, which largely increases the generalizability of the results [16, 38].
Furthermore, we have included a large number of children with serious and invasive bacterial infections, which was determined by a uniformly applied and validated flowchart [16].
Using routinely collected data has its limitations. However, to ensure data quality and completeness, all study sites were extensively trained regarding the accurate documentation of patient characteristics and quality checks were performed regularly. Missing data were limited, and its effects were further reduced by using multiple imputation for missing values [39].
A second limitation is that in some settings, children that are likely to be admitted, for example due to a high risk for serious bacterial infections, such as children with febrile neutropenia, are sometimes seen at the ward directly and bypass the ED [40]. Furthermore, data were only collected at the ED and not at primary care facilities. Therefore, the patients included in our study might not represent the complete group of febrile children with comorbidity. Furthermore, although comorbidity was grouped by body system, these groups could still be heterogeneous. However, heterogeneity was reduced by further analysing children by level of complexity.
Lastly, although this study provided detailed information by children with comorbidity by body system, we did not study resource use and risk of serious illness for specific diagnoses.
Conclusion
Our data show that children with comorbidity form important part of the paediatric ED population. Febrile children with comorbidities in general are more ill with a shorter duration of symptoms, more frequently have abnormal test results, more often require admission and PICU admission and life-saving interventions, and more often are diagnosed with serious and invasive bacterial infections.
Data sharing statement
Individual participant data that underlie the results reported in this article, including a data dictionary, will be made available after de-identification to researchers who provide a methodologically sound proposal. Proposals should be directed to d.borensztajn@erasmusmc.nl. To gain access, data requestors will need to sign a data access agreement.
Abbreviations
- aOR:
-
Adjusted odds ratio
- CRP:
-
C-reactive protein
- ED:
-
Emergency department
- MOFICHE:
-
Management and Outcome of Fever in children in Europe
- NICE:
-
National Institute for Health and Care Excellence
- ILSI:
-
Immediate life-saving interventions
- PERFORM:
-
Personalized Risk assessment in Febrile illness to Optimize Real-life Management across the European Union
- PEWS:
-
Paediatric Early Warning Score
- PICU:
-
Paediatric Intensive Care Unit
References
Carvalho KM, De Carvalho MSN, Grando RL, De Menezes LA (2021) Children with complex chronic conditions: an evaluation from the standpoint of academic publications. Int J Contemp Pediatr 8:594
Greenfield G, Okoli O, Quezada-Yamamoto H et al (2021) Characteristics of frequently attending children in hospital emergency departments: a systematic review. BMJ Open 11:e051409
Vrijlandt SEW, Nieboer D, Zachariasse JM, Oostenbrink R (2022) Characteristics of pediatric emergency department frequent visitors and their risk of a return visit: A large observational study using electronic health record data. PLoS ONE 17:e0262432
Trautner BW, Caviness AC, Gerlacher GR, Demmler G, Macias CG (2006) Prospective evaluation of the risk of serious bacterial infection in children who present to the emergency department with hyperpyrexia (temperature of 106 degrees F or higher). Pediatrics 118:34–40
Irwin AD, Drew RJ, Marshall P et al (2015) Etiology of childhood bacteremia and timely antibiotics administration in the emergency department. Pediatrics 135:635–642
Irwin AD, Grant A, Williams R et al (2017) Predicting risk of serious bacterial infections in febrile children in the emergency department. Pediatrics 140:e20162853
Chang SSY, Lim AZ, Ong GY et al (2021) Predictors of serious bacterial infections using serum biomarkers in an infant population aged 0 to 90 days: a prospective cohort study. BMJ Paediatr Open 5:e000861
Russell CJ, Simon TD (2014) Care of children with medical complexity in the hospital setting. Pediatr Ann 43:e157–e162
O’Mahony L, O’Mahony DS, Simon TD, Neff J, Klein EJ, Quan L (2013) Medical complexity and pediatric emergency department and inpatient utilization. Pediatrics 131:e559–e565
Van den Bruel A, Haj-Hassan T, Thompson M, Buntinx F, Mant D (2010) European RNORSII. Diagnostic value of clinical features at presentation to identify serious infection in children in developed countries: a systematic review. Lancet 375:834–845
Nijman RG, Vergouwe Y, Thompson M et al (2013) Clinical prediction model to aid emergency doctors managing febrile children at risk of serious bacterial infections: diagnostic study. BMJ 346:f1706
Trippella G, Galli L, De Martino M, Lisi C, Chiappini E (2017) Procalcitonin performance in detecting serious and invasive bacterial infections in children with fever without apparent source: a systematic review and meta-analysis. Expert Rev Anti Infect Ther 15:1041–1057
Mintegi S, García S, Martín MJ et al (2020) Clinical Prediction Rule for Distinguishing Bacterial From Aseptic Meningitis. Pediatrics 146:e20201126
Arif T, Phillips RS (2019) Updated systematic review and meta-analysis of the predictive value of serum biomarkers in the assessment and management of fever during neutropenia in children with cancer. Pediatr Blood Cancer 66:e27887
Ferreras-Antolín L, Oligbu G, Okike IO, Ladhani S (2020) Infection is associated with one in five childhood deaths in England and Wales: analysis of national death registrations data, 2013–15. Arch Dis Child 105:857–863
Hagedoorn NN, Borensztajn DM, Nijman R et al (2020) Variation in antibiotic prescription rates in febrile children presenting to emergency departments across Europe (MOFICHE): A multicentre observational study. PLoS Med 17:e1003208
NICE Guidelines. Available from: https://www.nice.org.uk/guidance. Accessed 05 September 2022
Simon TD, Cawthon ML, Stanford S et al (2014) Pediatric medical complexity algorithm: a new method to stratify children by medical complexity. Pediatrics 133:e1647–e1654
McCulloch H, Breneol S, Stewart SA et al (2022) Identifying children with medical complexity in administrative datasets in a Canadian context: study protocol. BMJ Open 12:e057843
Leyenaar JK, Schaefer AP, Freyleue SD et al (2022) Prevalence of Children With Medical Complexity and Associations With Health Care Utilization and In-Hospital Mortality. JAMA Pediatr 176:e220687
Zachariasse JM, Nieboer D, Maconochie IK et al (2020) Development and validation of a Paediatric Early Warning Score for use in the emergency department: a multicentre study. Lancet Child Adolesc Health 4:583–591
Hagedoorn NN, Zachariasse JM, Borensztajn D et al (2021) Shock Index in the early assessment of febrile children at the emergency department: a prospective multicentre study. Arch Dis Child 2020archdischild
Borensztajn DM, Hagedoorn NN, Carrol ED et al (2021) A NICE combination for predicting hospitalisation at the Emergency Department: a European multicentre observational study of febrile children. Lancet Reg Health Eur 8:100173
Nijman RG, Oostenbrink R, Moll HA et al (2021) A novel framework for phenotyping children with suspected or confirmed infection for future biomarker studies. Front Pediatr 9:688272
Borensztajn D, Hagedoorn NN, Carrol E et al (2022) Characteristics and management of adolescents attending the ED with fever: a prospective multicentre study. BMJ Open 12:e053451
Perrin JM, Bloom SR, Gortmaker SL (2007) The increase of childhood chronic conditions in the United States. JAMA 297:2755–2759
Perrin JM, Anderson LE, Van Cleave J (2014) The rise in chronic conditions among infants, children, and youth can be met with continued health system innovations. Health Aff (Millwood) 33:2099–2105
Wijlaars LP, Gilbert R, Hardelid P (2016) Chronic conditions in children and young people: learning from administrative data. Arch Dis Child 101:881–885
Andersen AD, Michelsen P (2018) S. 5.N. Round table: improving primary health care for children and adolescents: what are the future steps. Eur J Public Health 28
Bai G, Herten MH, Landgraf JM, Korfage IJ, Raat H (2017) Childhood chronic conditions and health-related quality of life: findings from a large population-based study. PLoS ONE 12:e0178539
Barrio Cortes J, Suárez Fernández C, Bandeira de Oliveira M et al (2020) Chronic diseases in the paediatric population: comorbidities and use of primary care services. An Pediatr (Engl Ed) 93:183–193
Mazur A, Dembinski L, Schrier L, Hadjipanayis A, Michaud PA (2017) European Academy of Paediatric consensus statement on successful transition from paediatric to adult care for adolescents with chronic conditions. Acta Paediatr 106:1354–1357
Zachariasse JM, Espina PR, Borensztajn DM et al (2021) Improving triage for children with comorbidity using the ED-PEWS: an observational study. Arch Dis Child 2021archdischild
Armocida B, Monasta L, Sawyer S et al (2022) Burden of non-communicable diseases among adolescents aged 10–24 years in the EU, 1990–2019: a systematic analysis of the Global Burden of Diseases Study 2019. Lancet Child Adolesc Health 6:367–383
Hardelid P, Dattani N, Gilbert R, Programme BOTRCOPACH, Child DOWG (2014) Estimating the prevalence of chronic conditions in children who die in England, Scotland and Wales: a data linkage cohort study. BMJ Open 4:e005331
Wells R, Le Doare K, Sharland M, Heath P, Ladhani SN, CABIN N (2015) Targeted empiric antibiotic therapy for children with non-oncological comorbidities and community-onset invasive bacterial infections. J Infect 71:294–301
Hagedoorn NN, Borensztajn D, Nijman RG et al (2021) Development and validation of a prediction model for invasive bacterial infections in febrile children at European Emergency Departments: MOFICHE, a prospective observational study. Arch Dis Child 106:641–647
Borensztajn D, Yeung S, Hagedoorn NN et al (2019) Diversity in the emergency care for febrile children in Europe: a questionnaire study. BMJ Paediatr Open 3:e000456
Vergouwe Y, Royston P, Moons KG, Altman DG (2010) Development and validation of a prediction model with missing predictor data: a practical approach. J Clin Epidemiol 63:205–214
Hoffner B, Rubin KM (2019) Meeting the challenge of immune-related adverse events with optimized telephone triage and dedicated oncology acute care. J Adv Pract Oncol 10:9–20
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 668303 and No. 848196. The research was supported by the National Institute for Health Research Biomedical Research Centre based at Imperial College (JH, ML) and at Newcastle Hospitals NHS Foundation Trust and Newcastle University (EL, ME), grant number not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed to the design and data collection of the study. DB and NH verified and analysed the data. DB, NH, and HM interpreted the data. DB, NH, and HM drafted the manuscript. All authors critically evaluated and revised the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. This publication is the work of the authors who will serve as guarantors for the contents of this paper.
Corresponding author
Ethics declarations
Disclaimer
The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
Conflict of interest
DB, UB, EC, ME, MF, NH, BK, FMT, HM, EL, ML, MP, IRC, MT, CV, DZ, and WZ report grants from the European Union Horizon 2020 research and innovation programme during the study conduct. MP reports a grant from Pfizer and financial support from Pfizer and Sanofi outside the submitted work. MF reports a grant from CSL Behring outside the submitted work. RN reports a grant from the National Institute for Health Research during the study conduct. ME reports financial support from the National Institute for Health Research Biomedical Research Centre based at Newcastle Hospitals NHS Foundation Trust and Newcastle University during the study conduct. MT is a member of the Advisory Board of MSD and Pfizer, a member of the National Committee on Immunization Practices, and a member of the national Scientific Advisory Group for the management of the pandemic. All other authors declare no competing interests.
Additional information
Communicated by Peter de Winter
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Borensztajn, D.M., Hagedoorn, N.N., Carrol, E.D. et al. Febrile children with comorbidities at the emergency department — a multicentre observational study. Eur J Pediatr 181, 3491–3500 (2022). https://doi.org/10.1007/s00431-022-04552-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-022-04552-2