Abstract
Fresh frozen plasma (FFP) is largely misused in the neonatal setting. The aim of the study is to evaluate the impact of a Thromboelastography (TEG)-based Quality Improvement (QI) project on perioperative FFP use and neonatal outcomes. Retrospective pre-post implementation study in a level-III NICU including all neonates undergoing major non-cardiac surgery before (01–12/2017) and after (01–12/2019) the intervention. In 2018, the intervention included the following: (1) Training on TEG, (2) Implementation of TEG, and (3) Algorithm for TEG-directed FFP administration in surgical neonates. We compared pre- vs post-intervention patient characteristics, hemostasis, and clinical management. Linear and logistic regression models were used to evaluate the impact of the project on main outcomes. We analyzed 139 neonates (pre-intervention: 72/post-intervention: 67) with a mean (± SD) gestational age (GA) 34.9 (± 5) weeks and birthweight 2265 (± 980) grams which were exposed to 184 surgical procedures (pre-intervention: 91/post-intervention: 93). Baseline characteristics were similar between periods. In 2019, prothrombin time (PT) was longer (14.3 vs 13.2 s; p < 0.05) and fibrinogen was lower (229 vs 265 mg/dl; p < 0.05), if compared to 2017. In 2019, the intraoperative exposure to FFP decreased (31% vs 60%, p < 0.001), while the pre-operative FFP use did not change. The reduction of intraoperative FFP did not impact on mortality and morbidity. Intraoperative FFP use was lower in the post-intervention even after controlling for GA, American Society of Anesthesiologists score, PT, and fibrinogen (Odds ratio: 0.167; 95% CI: 0.070, 0.371).
Conclusion: The TEG-based QI project for the management of FFP during neonatal surgery reduced intraoperative FFP exposure.
What is Known: • PT and aPTT are poor predictors of bleeding risk in acquired neonatal coagulopathy, leading to likely unnecessary fresh frozen plasma (FFP) transfusion in the Neonatal Intensive Care Setting. • As neonatal hemostasis is a delicate balance between the concomitant reduction of pro- and anti-coagulants drivers, thromboelastography (TEG) is a promising alternative for coagulation monitoring. | |
What is New: • The implementation of TEG, training, and shared protocols contributed to reduced intraoperative FFP use, which was not associated with increased mortality or bleeding events. • These findings inform future research showing that there is clinical equipoise to allow for larger studies to confirm the use of TEG in NICUs and to identify TEG cut-offs for transfusion practice. |
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Critically ill neonates are frequently exposed to blood products. After red blood cells, fresh frozen plasma (FFP) transfusion is the most frequent intervention, being around 10% of all admissions to the Neonatal Intensive Care Units (NICUs) [1, 2]. This rate increases among extremely premature infants at risk of hemorrhagic events [1].
Current neonatal transfusion guidelines consider the use of FFP for (1) active bleeding with associated coagulopathy defined by abnormal coagulation tests [Prothrombin Time (PT); Activated Partial Thromboplastin Time (aPPT) and fibrinogen] and (2) increased risk of bleeding associated with coagulopathy [2].
However, in clinical practice, it has been shown that about 60% of FFP transfusions in neonatology are not evidence-based. The main reasons for non-compliance to guidelines were represented by the use of FFP as volume expander, bleeding without coagulopathy, and septic newborns without bleeding or coagulopathy [3].
Furthermore, prolonged PT and aPTT are not predictors of increased bleeding risk in neonatal acquired coagulopathy, as these tests are mainly responsive to procoagulant factors. Indeed, even at the lowest gestational ages, the neonatal hemostatic balance is maintained by the concomitant reduction of both pro-and anti-coagulant drivers when clotting status is defined by global tests such as thromboelastography (TEG) and thrombin generation procedure [4,5,6]. TEG is a viscoelastic evaluation of the clot, from its formation to its lysis; it considers both cellular and plasmatic components of hemostasis, thus allowing a qualitative and dynamic analysis of the in vivo process [7, 8], with an acceptable clinical reproducibility in neonates [9]. We have defined TEG reference intervals for the very low birth weight (VLBW) and term infants at birth and over the first month of life [5]. TEG has been part of the hemostatic diagnostic workup in our NICU since 2018. Furthermore, we planned to implement TEG evaluation and multidisciplinary training. We shared transfusion algorithms to standardize the hemostatic management of surgical neonates exposed to increased bleeding risk and heterogeneous FFP transfusion practices.
Indeed, in pediatric and adult surgery, TEG has significantly reduced intra-operative FFP use while decreasing the risk of postoperative bleeding with a beneficial impact in terms of outcomes [10,11,12,13].
The purpose of this study is to evaluate the impact of a TEG-based Quality Improvement Project (here called intervention) on FFP transfusion of neonates undergoing surgery.
Materials and methods
This single-center retrospective pre-post implementation study was conducted in a level III NICU based in an academic hospital. All procedures were performed in accordance with the Helsinki Declaration. The Institutional Review Board approved the study protocol (Comitato Etico Milano Area 2 — n° 36_2021). Due to the retrospective nature of the study, the need for informed consent from the parents has been waived by our Institutional Review Board.
Quality improvement project
The project was based on a three-step intervention.
Step 1: training of the multidisciplinary team
The team included neonatologists and anesthesiologists, who routinely care for surgical neonates in the perioperative period. Education focused on neonatal hemostasis and diagnostic workup, including the basic principles of viscoelastic assays and evidence-based guidance for blood transfusion. Didactic sessions were accompanied by hands-on training on TEG 5000® (Hemoscope; Haemonetics, Niles, IL, USA) and Viscoelastic Coagulation Monitor (VCM®) System (Entegrion, Inc., Durham, NC), organized in small groups to acquire technical skills and traces’ interpretation clues, through the revision of real clinical cases.
Step 2: develop a multidisciplinary standard operating procedure (SOP) for blood products use in the surgical neonate
The team leader of this initiative (S. Gh.) developed an interdepartmental protocol based on current national and international guidelines [2, 14]. However, before this intervention, a specific procedure related to neonatal FFP transfusion management during the perioperative period was lacking. Therefore, decisions regarding the need and timing for FFP were at the discretion of the attending anesthesiologist, mainly based on the combined evaluation of (1) risk factors for bleeding, (2) active clinical bleeding, and (3) coagulopathy.
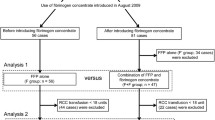
During the post-intervention period, both neonatologists and anesthesiologists followed a structured approach for FFP administration (Fig. 1). Coagulopathy was defined as PT and PTT above the 95th percentile, and fibrinogen below the 5th percentile, based on gestational age-dependent reference ranges by Christensen et al. [15]. In case of abnormally prolonged PT and PTT, the decision to administer FFP was made according to TEG-Reaction Time (R) values above the 95th percentile, referring to our institutional TEG normative intervals [5]. The evaluation of TEG-Maximum Amplitude (MA) below the 5th percentile contributed to the definition of hypocoagulability (Fig. 1). VCM-Clotting Time (CT) and Maximum Clotting Firmness (MCF) express the same clinical significance of R and MA, respectively. Therefore, VCM data were referred to internal reference ranges for neonates.
The Hospital Transfusion Center supplied the FFP units. As for the internal procedure, based on SIMTI (Italian Society of Immunohematology and Transfusional Medicine) recommendations, we administered ABO/AB compatible FFP in aliquotes of 15–20 ml/kg [16].
Step 3: implementation of TEG in routine care in 2018
We have chosen 12 months of perioperative TEG use for three reasons: (1) to allow time to gain familiarity with the coagulation monitoring devices; (2) to avoid potential confounding factors, such as technical issues; (3) to rely on institution-based TEG references ranges for different gestational and postnatal age classes.
Population
We retrospectively collected data from two cohorts of neonates exposed to major non-cardiac surgery born before (01/01/2017–31/12/2017) and after (01/01/2019 and 31/12/2019) the intervention.
We excluded patients with at least one of the following criteria: (1) congenital coagulopathy; (2) cardio-surgery, (3) minor surgery, (4) missing data related to perioperative transfusions.
We collected through the electronic patient charts Neocare® (GPI SpA), the following:
-
Perinatal data: gender, gestational age (GA), birth weight, delivery mode, Apgar score, resuscitation;
-
Pre- and intra-surgery data: American Society of Anesthesiologists score (ASA: grading of the anesthetic risk from 1-low to 5-high), hemostatic profile, blood products’ use, ventilatory and pharmacological management;
-
Post-surgery outcomes: mortality, hemostatic, respiratory, and renal morbidity.
Outcome measures
The main outcome measure was the number of neonates receiving FFP transfusion in the pre- and intra-operative period before and after the intervention.
As secondary outcomes, we evaluated across the two study periods: (1) the primary indications for FFP transfusion as reported in the patient charts; (2) the occurrence of abnormal coagulation defined as at least one prolonged PT or aPTT value exceeding 95°centile of the reference values [15]; (3) the number of thrombotic or major hemorrhagic events in the first 7 postoperative days; (4) the need for oxygen supplementation in the postoperative period compared to the pre-operative period; (5) onset of postoperative acute kidney injury (AKI) in the first 72 h after surgery; (6) need for intravenous diuretics in the 24 h following surgery; (7) postoperative weight gain; (8) mortality.
Bleeding (any site) was assessed through the NeoBAT score [17]. Cerebral hemorrhage was diagnosed by brain ultrasound and classified according to Papile et al. [18]. Major hemorrhage was defined as intraventricular hemorrhage ≥ grade 3, pulmonary hemorrhage defined as bleeding through an endotracheal tube with respiratory failure, and gastrointestinal hemorrhage defined as rectal bleeding.
AKI was defined as an increase in serum creatinine 24 h after surgery above 0.3 mg/dl with or without oliguria, diuresis rate below 1 ml/kg/h in the 24-h following surgery [19]. Diuresis was quantified as milliliters pro kilogram per hour; post-operative weight gain was estimated based on the increase in grams of post-operative daily weights (as a running total for postoperative days 0–5) referring to the pre-operative weight.
Data analysis
Demographic and clinical parameters were presented through descriptive statistics. The comparison between the two periods was performed using t-test or Mann–Whitney U test for continuous variables and Fisher’s Exact test for categorical variables.
A logistic regression model was used to estimate the effect of the intervention on pre- and intra-operative FFP transfusions, mortality, the incidence of AKI, and hemorrhagic and thrombotic events. In addition, the impact on respiratory function was studied using a multiple linear regression model. All models were corrected for gestational age and clinical conditions. The results are presented in terms of odds ratio or regression coefficient, confidence interval, and p-value. All tests performed are two-sided, and a p-value < 0.05 is considered significant. Data analyses were performed using the R software, version 4.0.1 or higher (R Foundation for Statistical Computing, Vienna, Austria).
Results
Overall population
We analyzed 139 neonates (72 in pre-intervention; 67 in post-intervention) with a mean (± SD) GA of 34.9 (± 5) weeks and birthweight 2265 (± 980) grams, which were exposed to 184 surgical interventions (91 in pre-intervention; 93 in post-intervention). Urgent surgeries were 61% (56/91) in 2017 and 69% (64/93) in 2019. Baseline characteristics did not vary between periods (Table 1). In the post-intervention, PT was longer (14.3 vs 13.2 s; p < 0.05), and fibrinogen was lower (229 vs 265 mg/dl; p < 0.05) if compared to the pre-intervention. In 2019, intraoperative FFP transfusions decreased (31% vs 60%, p < 0.001), while the pre-operative FFP exposure did not change (Fig. 1, Table 2, Supplementary Tables S1–S2). Moreover, logistic regression showed a significant reduction in FFP intraoperative transfusions between 2017 and 2019 (OR = 0.275; 95% CI: [0.143; 0.517]; p-value < 0.001). This result is confirmed (Table 2) also adjusting for GA at birth and clinical conditions (OR = 0.167; 95% CI: [0.070; 0.372]; p-value < 0.001). The reduction of intraoperative FFP transfusion did not impact mortality and morbidity, except for a mild improvement of diuresis in the post-intervention (Table 2).
Intra-operative FFP use: comparison of transfused neonates 2017 vs 2019
Drivers for intraoperative FFP prescription differed among study periods (Fig. 2, Table 3): in 2017, most transfusions (43.6%) were administered for volemic expansion, while coagulopathy was the main indication (58.6%) in 2019. Hypotension was reported as a determinant for FFP use only in the pre-intervention period. We could not retrieve the reason for FFP use in 27.3% and 10.3% of cases in the pre- and post-intervention period, respectively. If compared to the pre-intervention, PT was longer (15.8 vs 13.5 s; p < 0.003), postoperative platelet count (132 vs 205; p < 0.001) and fibrinogen were lower (203 vs 246 mg/dl; p = 0.048) in 2019. In the post-intervention, the neonates exposed to FFP transfusion had a mean TEG-R of 16 min and a mean TEG-MA of 51 mm. The clinical and hemostatic profile of neonates exposed to intraoperative FFP is shown in Table 3.
Intra-operative FFP use: comparison of non-transfused neonates 2017 vs 2019
In the pre-intervention, 36 out of 91 surgical interventions did not require FFP use. Of those, only one patient presented with a slightly prolonged aPTT, while the remaining neonates had a normal hemostatic profile. In the post-intervention, in 64 out of 93 surgical interventions, FFP was not necessary. The majority (55/64; 86%) had normal standard coagulation tests, while 9 out of 64 had prolonged PT or APTT. Among them, 8 out of 9 neonates had a normal TEG trace.
Subgroup and multivariate analysis
The comparison of transfusion management between pre- and post-intervention by stratifying patients based on GA (< 34 vs ≥ 34 weeks) and ASA score (< 4 vs ≥ 4) led to the main results from the overall population (Supplementary Table S3).
Discussion/conclusion
This study found that the Quality Improvement project based on TEG, staff training, and transfusion algorithm improved the hemostatic management of surgical neonates by reducing intraoperative FFP and supporting a more evidence-based FFP prescription. In addition, the reduced transfusion usage appeared to be safe as it did not impact the length of stay, mortality, bleeding events, and morbidity rates.
To our knowledge, this is the first attempt to improve FFP transfusion practice through a combined approach based on the viscoelastic assay, neonatal hemostatic training, and shared algorithms.
FFP is commonly administered in the NICU and, similarly to other blood products, it may be associated with (un)known risks, such as allergy, infections, transfusion-related acute lung injury (TRALI), and transfusion-associated cardiac overload (TACO) [20].
Indeed, under-estimation of transfusion-related adverse events is a major issue in neonatology, as transfused patients are usually sick. Data from UK hemovigilance showed that neonates are at higher risk of transfusion-related side effects than older children or adults [21]. Despite the availability of guidelines for neonatal blood products administration, adherence is still low for FFP, which is often administered prophylactically for prolonged PT and aPTT [3, 22].
The establishment of age-related reference ranges for standard coagulation tests and the shift from a routine admission coagulation screening to a clinically-oriented approach led to reduced FFP use [15, 23].
However, in selected neonatal categories, there is still a need for improvement in transfusion medicine. Surgical neonates illustrate this concept, as they usually undergo a coagulation screening before major procedures. The common multidisciplinary approach to these patients may include professionals that may be less familiar with neonatal hemostatic peculiarities.
In this context, the implementation of TEG, training, and shared transfusion algorithms increased compliance with FFP guidelines. Indeed, by comparing the reason for FFP transfusion across the two study periods, we may speculate a beneficial effect of training on a pre-existing knowledge gap in neonatal transfusion medicine. Furthermore, after the intervention, the number of transfusions with an off-guidelines indication declined, likely reflecting the increased awareness of healthcare providers of shared transfusion triggers. Nevertheless, our findings suggest that compliance with current guidelines can be further optimized.
In our opinion, the lack of effect of the intervention on the pre-surgery FFP use may rely on a “perceived” risk of bleeding in the most preterm, critical, and younger neonates. Indeed, Apgar scores, and gestational and postnatal age were lower and ASA scores higher in the pre-surgery transfused group, thus supporting this hypothesis.
The comparison of not-transfused neonates across the two study periods suggests a possible role for TEG. Exception made for those neonates presenting with normal standard coagulation tests, those with a prolonged PT or aPTT and a normal TEG trace did not receive FFP. We may speculate that in those cases, the attending physician was reassured by a normal viscoelastic test.
Moreover, the lack of effect of the reduced FFP use on mortality and primary morbidity outcomes may confirm the concept that FFP does not have a prophylactic effect in patients at higher risk of bleeding [15].
The improvement of diuresis observed in the post-intervention, although in the normal range in both study periods, further supports the concept that FFP transfusion may worsen cardiac and respiratory performances, namely TACO and TRALI [20, 24].
Indeed, FFP use has been identified as an independent risk factor for hemodynamically significant patent ductus arteriosus in preterm neonates due to a fluid management perturbation [25].
Interestingly, in this context, the reduction of FFP transfusion may partly reflect the increased awareness of neonatologists related to the emerging evidence that restrictive transfusion practices for red blood cells and platelets are beneficial, or at least non-inferior to liberal practices [26,27,28]. Nevertheless, the evidence for neonatal FFP use is still limited, and this study may provide a practical framework for resource optimization.
Potential limits require consideration. The study’s retrospective nature did not allow us to retrieve either the indication for intraoperative FFP transfusion in 18 out of 84 neonates or the eventual wastage of blood products. In addition, as this is a single-center project, the applicability of our findings to other NICUs may be somewhat limited. Furthermore, in our setting, the pre-intervention misuse of FFP was relevant, and this should be taken into account while evaluating the impact of the intervention itself. Lastly, although TEG and VCM are different devices, we deem our clinical experience adequate to evaluate the impact of this diagnostics on FFP use, further supported by institutional ranges.
In conclusion, our findings may inform future research showing that there is clinical equipoise to allow for larger studies to confirm the use of TEG in NICUs and to identify TEG cut-offs for transfusion practice. The implementation of viscoelastic assays combined to hemostatic training and shared algorithms should be encouraged to improve neonatal outcomes.
Availability of data and material
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Abbreviations
- aPTT:
-
Activated partial thromboplastin time
- ASA:
-
American Society of Anesthesiologists score
- CT:
-
Clotting time
- CFT:
-
Clotting formation time
- FFP:
-
Fresh frozen plasma
- GA:
-
Gestational age
- MA:
-
Maximum amplitude
- NICU:
-
Neonatal intensive care units
- PT:
-
Prothrombin time
- QI:
-
Quality improvement
- R:
-
Reaction time
- TEG:
-
Thrombo-elastography
- TACO:
-
Transfusion-associated cardiac overload
- TRALI:
-
Transfusion-related acute lung injury
- VLBW:
-
Very low birth weight
References
Keir AK, Stanworth SJ (2016) Neonatal plasma transfusion: an evidence-based review. Transfus Med Rev 30:174–182
Motta M, Del Vecchio A, Chirico G (2015) Fresh frozen plasma administration in the neonatal intensive care unit. Clin Perinatol 42:639–650
Motta M, Del Vecchio A, Perrone B, Ghirardello S, Radicioni M (2014) Fresh frozen plasma use in the NICU: a prospective, observational, multicentred study. Arch Dis Child Fetal Neonatal Ed 99(4):F303–F308
Tripodi A, Raffaeli G, Scalambrino E, Padovan L, Clerici M, Chantarangkul V, Cavallaro G, Peyvandi F, Mosca F, Ghirardello S (2020) Procoagulant imbalance in preterm neonates detected by thrombin generation procedures. Thromb Res 185:96–101
Raffaeli G, Tripodi A, Cavallaro G, Cortesi V, Scalambrino E, Pesenti N, Artoni A, Mosca F, Ghirardello S (2020) Thromboelastographic profiles of healthy very low birthweight infants serially during their first month. Arch Dis Child Fetal Neonatal Ed 105:412–418
Tripodi A, Ramenghi LA, Chantarangkul V, De Carli A, Clerici M, Groppo M, Mosca F, Mannucci PM (2008) Normal thrombin generation in neonates in spite of prolonged conventional coagulation tests. Haematologica 93:1256–1259
Whiting D, DiNardo JA (2014) TEG and ROTEM: technology and clinical applications. Am J Hematol 89:228–232
Sewell EK, Forman KR, Wong EC, Gallagher M, Luban NL, Massaro AN (2017) Thromboelastography in term neonates: an alternative approach to evaluating coagulopathy. Arch Dis Child Fetal Neonatal Ed 102:F79–F84
Ghirardello S, Raffaeli G, Scalambrino E, Chantarangkul V, Cavallaro G, Artoni A, Mosca F, Tripodi A (2018) The intra-assay reproducibility of thromboelastography in very low birth weight infants. Early Hum Dev 127:48–52
Dias JD, Sauaia A, Achneck HE, Hartmann J, Moore EE (2019) Thromboelastography-guided therapy improves patient blood management and certain clinical outcomes in elective cardiac and liver surgery and emergency resuscitation: a systematic review and analysis. J Thromb Haemost 17:984–994
Li C, Zhao Q, Yang K, Jiang L, Yu J (2019) Thromboelastography or rotational thromboelastometry for bleeding management in adults undergoing cardiac surgery: a systematic review with meta-analysis and trial sequential analysis. J Thorac Dis 11:1170
Nakayama Y, Nakajima Y, Tanaka K, Sessler D, Maeda S, Iida J, Ogawa S, Mizobe T (2015) Thromboelastometry-guided intraoperative haemostatic management reduces bleeding and red cell transfusion after paediatric cardiac surgery. Br J Anaesth 114:91–102
Faraoni D, Meier J, New HV, Van der Linden PJ, Hunt BJ (2019) Patient blood management for neonates and children undergoing cardiac surgery: 2019 NATA guidelines. J Cardiothorac Vasc Anesth 33:3249–3263
New HV, Berryman J, Bolton-Maggs PH, Cantwell C, Chalmers EA, Davies T, Gottstein R, Kelleher A, Kumar S, Morley SL (2016) Guidelines on transfusion for fetuses, neonates and older children. Br J Haematol 175:784–828
Christensen RD, Baer VL, Lambert DK, Henry E, Ilstrup SJ, Bennett ST (2014) Reference intervals for common coagulation tests of preterm infants (CME). Transfusion 54:627–632
Girelli G, Antoncecchi S, Casadei AM, Del Vecchio A, Isernia P, Motta M, Regoli D, Romagnoli C, Tripodi G, Velati C (2015) Recommendations for transfusion therapy in neonatology. Blood Transfus 13:484
Venkatesh V, Curley A, Khan R, Clarke P, Watts T, Josephson C, Muthukumar P, New H, Seeney F, Morris S (2013) A novel approach to standardised recording of bleeding in a high risk neonatal population. Arch Dis Child Fetal Neonatal Ed 98:F260–F263
Papile L-A, Burstein J, Burstein R, Koffler H (1978) Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 92:529–534
Selewski DT, Charlton JR, Jetton JG, Guillet R, Mhanna MJ, Askenazi DJ, Kent AL (2015) Neonatal acute kidney injury. Pediatrics 136:e463–e473
Parker RI (2014) Transfusion in critically ill children: indications, risks, and challenges. Crit Care Med 42:675–690
Stainsby D, Jones H, Wells A, Gibson B, Cohen H (2008) Adverse outcomes of blood transfusion in children: analysis of UK reports to the serious hazards of transfusion scheme 1996–2005. Br J Haematol 141:73–79
Stanworth SJ, Grant-Casey J, Lowe D, Laffan M, New H, Murphy MF, Allard S (2011) The use of fresh-frozen plasma in England: high levels of inappropriate use in adults and children. Transfusion 51:62–70
Houben NA, Heeger LE, Stanworth SJ, New HV, van der Bom JG, Fustolo-Gunnink S, Lopriore E (2020) Changes in the use of fresh-frozen plasma transfusions in preterm neonates: a single center experience. J Clin Med 9:3789
Semple JW, Rebetz J, Kapur R (2019) Transfusion-associated circulatory overload and transfusion-related acute lung injury. Blood 133:1840–1853
Huang CP, Hung YL, Hsieh WS, Shen CM (2021) Fresh frozen plasma transfusion: an independent risk factor for hemodynamically significant patent ductus arteriosus in premature infants. Am J Perinatol. https://doi.org/10.1055/s-0040-1722649
Franz AR, Engel C, Bassler D, Ruediger M, Thome UH, Maier RF, Kraegeloh-Mann I, Kron M, Essers J, Buehrer C (2020) Effects of liberal vs restrictive transfusion thresholds on survival and neurocognitive outcomes in extremely low-birth-weight infants: the ETTNO randomized clinical trial. JAMA 324:560–570
Kirpalani H, Bell EF, Hintz SR, Tan S, Schmidt B, Chaudhary AS, Johnson KJ, Crawford MM, Newman JE, Vohr BR (2020) Higher or lower hemoglobin transfusion thresholds for preterm infants. N Engl J Med 383:2639–2651
Curley A, Stanworth SJ, Willoughby K, Fustolo-Gunnink SF, Venkatesh V, Hudson C, Deary A, Hodge R, Hopkins V, Lopez Santamaria B (2019) Randomized trial of platelet-transfusion thresholds in neonates. N Engl J Med 380:242–251
Acknowledgements
We would like to acknowledge NICU staff of Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico.
Author information
Authors and Affiliations
Contributions
GR participated in the conceptualization and design of the work; she managed the acquisition of data; she gave substantial contributions to the analysis or interpretation of data. She wrote the first draft of the paper, gave final approval of the version published, and ensured that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. NP managed the data analysis and contributed to interpretation of data; he revised the work and gave final approval of the version published. GC participated in the conceptualization and design of the work; he gave substantial contribution to the interpretation of data; he critically revised the work and gave final approval of the version published. VC, FMa, GSA, and SGu gave substantial contribution to the acquisition and analysis of data, they revised the work and gave final approval of the version published. LN and FMa contributed to patients’ management, participated to the acquisition and interpretation of data; they revised the work and gave final approval of the version published. FMo participated in the conceptualization and design of the work; he gave substantial contribution to the interpretation of data; he critically revised the work and gave final approval of the version published. SGh participated in the conceptualization and design of the work; obtained the ethical local permissions and gave essential contribution to the interpretation of data. He revised the draft giving important intellectual contribution and gave final approval of the version published and ensured that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethics approval
This study protocol was reviewed and approved by The Institutional Review Board Comitato Etico Milano Area 2, approval number n° 36_2021. The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Consent to participate
Not applicable.
Consent for publication
The need for the written informed consent was waived by Comitato Etico Milano Area 2.
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Raffaeli, G., Pesenti, N., Cavallaro, G. et al. Optimizing fresh-frozen plasma transfusion in surgical neonates through thromboelastography: a quality improvement study. Eur J Pediatr 181, 2173–2182 (2022). https://doi.org/10.1007/s00431-022-04427-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-022-04427-6